The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Starting Materials
2.2. Calcium Oxalate Extraction Protocols
2.3. Experimental Techniques Applied for Phytolith Characterization
2.3.1. Flame Atomic Absorption Spectroscopy (FAAS)
2.3.2. X-ray Fluorescence (XRF)
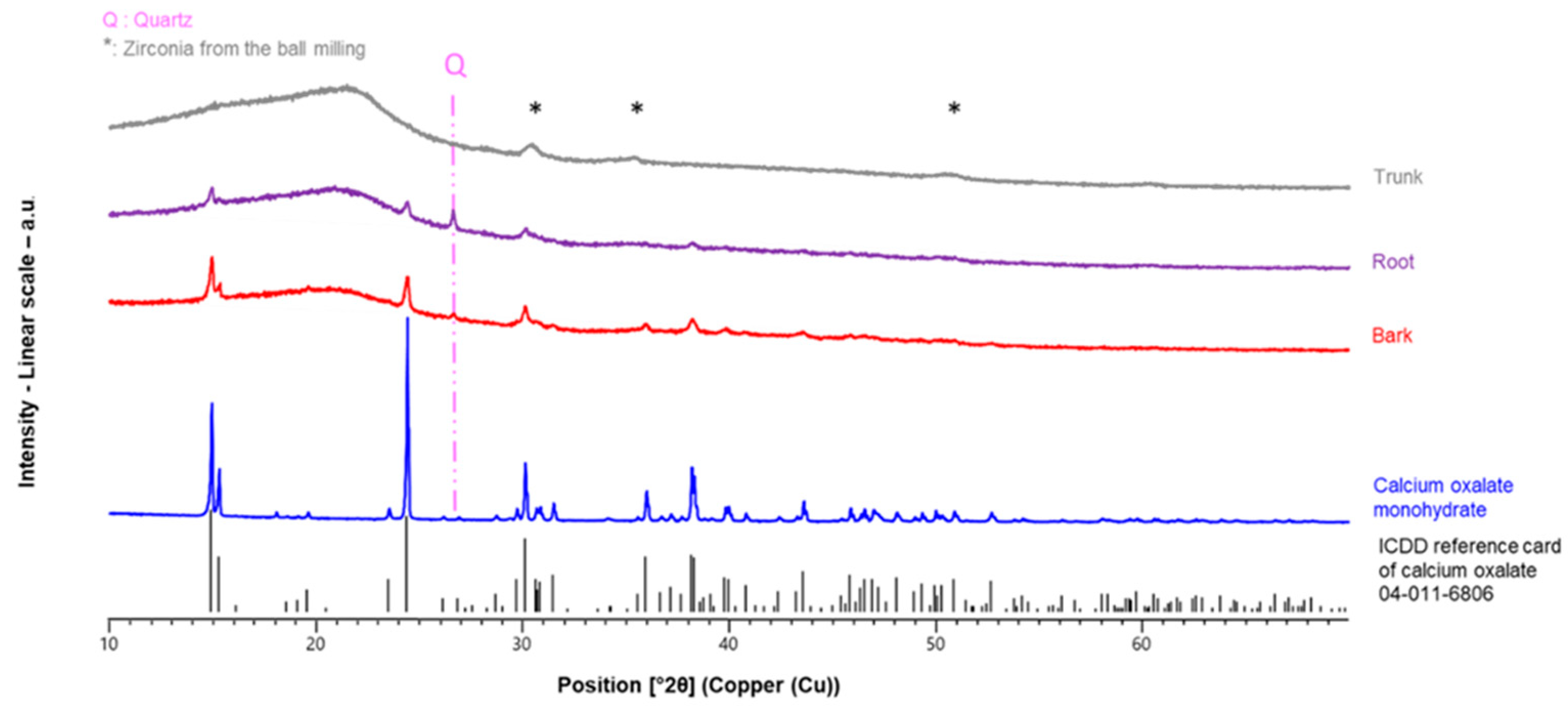
2.3.3. X-ray Diffraction (XRD)
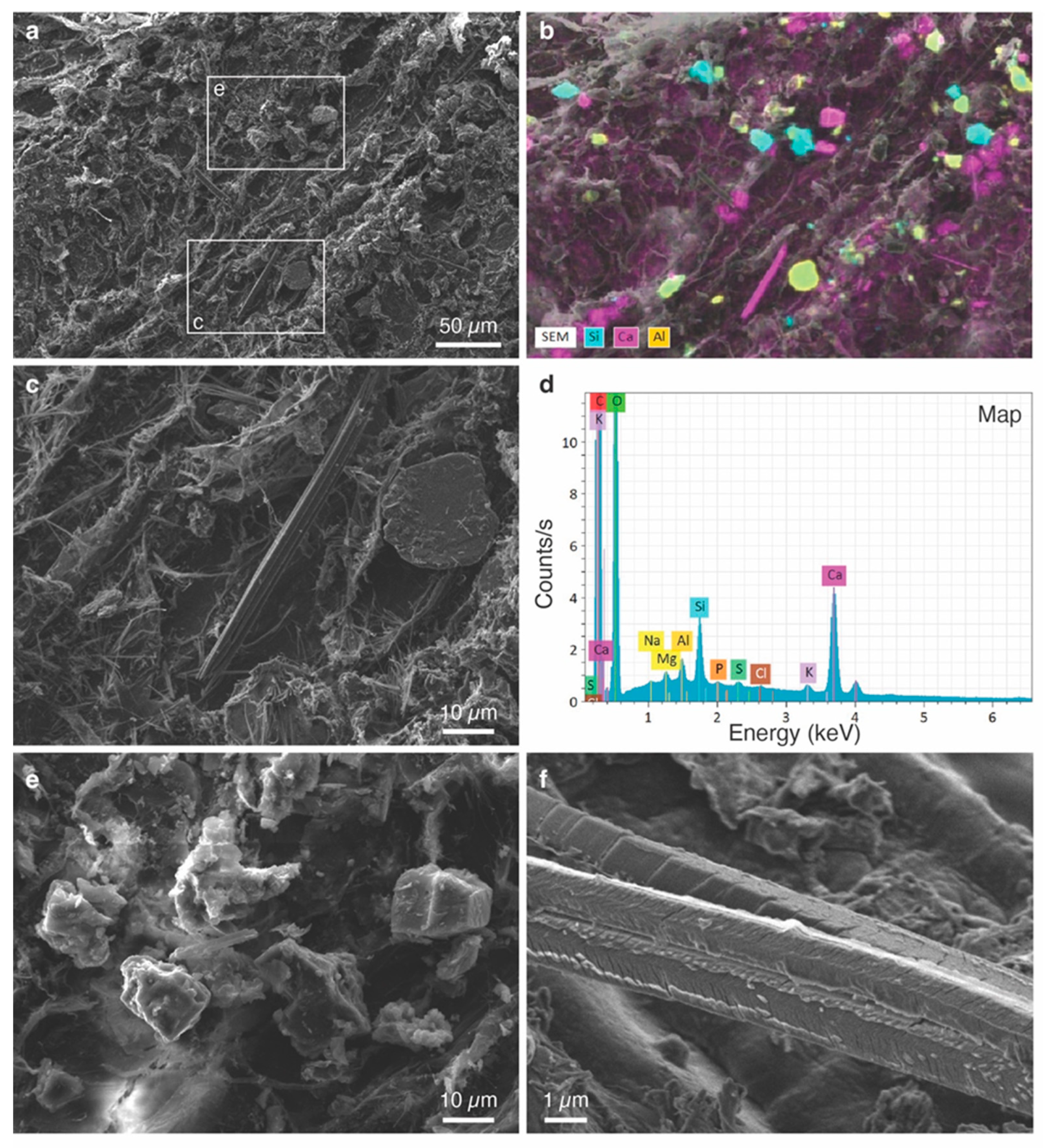
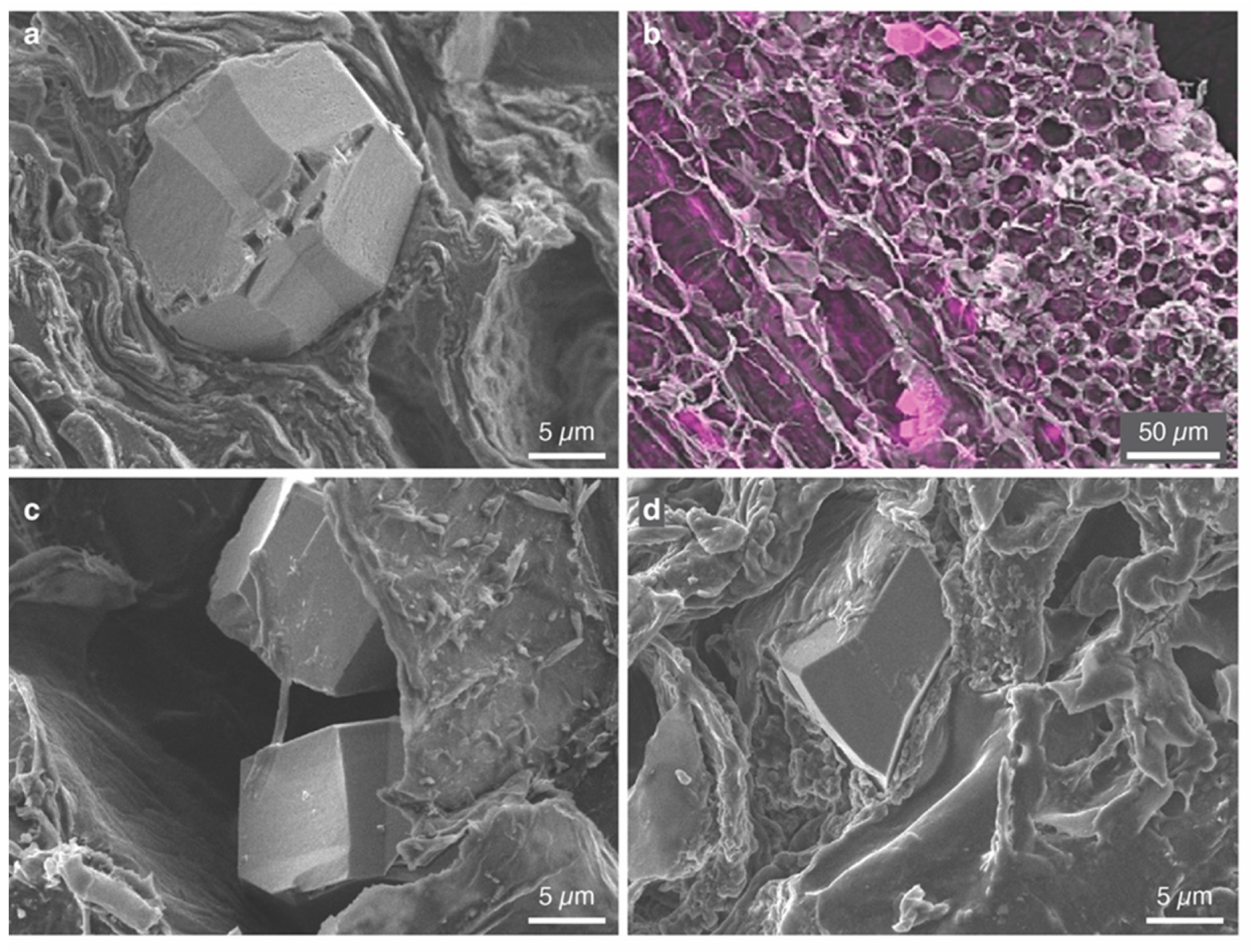
2.3.4. Scanning Electron Microscopy (SEM)
2.3.5. 13C-Nuclear Magnetic Resonance (NMR)
3. Results and Discussion
3.1. Bulk Chemical Composition of Plant Materials
3.2. Mineralogical Composition of Plant Materials
3.3. Characterization of Individual Phytoliths
3.4. 13C-NMR Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tang, R.-J.; Luan, S. Regulation of Calcium and Magnesium Homeostasis in Plants: From Transporters to Signaling Network. Curr. Opin. Plant Biol. 2017, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Broadley, M.R. Calcium in Plants. Ann. Bot. 2003, 92, 487–511. [Google Scholar] [CrossRef]
- Franceschi, V.R.; Nakata, P.A. CALCIUM OXALATE IN PLANTS: Formation and Function. Annu. Rev. Plant Biol. 2005, 56, 41–71. [Google Scholar] [CrossRef] [PubMed]
- Pérez Cuadra, V.; Hermann, P. Characterization and Macropattern of Calcium Oxalate Phytoliths in Argentinean Endemic Species of Chenopodioideae (Amaranthaceae). Quat. Int. 2013, 287, 83–88. [Google Scholar] [CrossRef]
- Crutcher, E.; Crutcher, H. Calcium Oxalate Phytoliths in Environmental Samples 1. Microscope 2020, 67, 3–11. [Google Scholar]
- Franceschi, V.R.; Horner, H.T. Calcium Oxalate Crystals in Plants. Bot. Rev. 1980, 46, 361–427. [Google Scholar] [CrossRef]
- Gębura, J.; Winiarczyk, K. A Study on Calcium Oxalate Crystals in Tinantia Anomala (Commelinaceae) with Special Reference to Ultrastructural Changes during Anther Development. J. Plant Res. 2016, 129, 685–695. [Google Scholar] [CrossRef]
- Stephens, W.E. Whewellite and Its Key Role in Living Systems. Geol. Today 2012, 28, 180–185. [Google Scholar] [CrossRef]
- Vigliaturo, R.; Kehrli, D.; Garra, P.; Dieterlen, A.; Trouvé, G.; Dietze, V.; Wilson, J.P.; Gieré, R. Opaline Phytoliths in Miscanthus Sinensis and Its Cyclone Ash from a Biomass-Combustion Facility. Ind. Crops Prod. 2019, 139, 111539. [Google Scholar] [CrossRef]
- DataCite Search. Available online: https://search.datacite.org/works/10.17632/w86x7fbdhz (accessed on 30 May 2023).
- Golyeva, A. Biomorphic Analysis as a Part of Soil Morphological Investigations. CATENA 2001, 43, 217–230. [Google Scholar] [CrossRef]
- Madella, M.; Lancelotti, C.; Osterrieth, M. Comprehensive Perspectives on Phytolith Studies in Quaternary Research. Quat. Int. 2013, 287, 1–2. [Google Scholar] [CrossRef]
- Pető, Á. Studying Modern Soil Profiles of Different Landscape Zones in Hungary: An Attempt to Establish a Soil-Phytolith Identification Key. Quat. Int. 2013, 287, 149–161. [Google Scholar] [CrossRef]
- Leszczuk, A.; Wydrych, J.; Szczuka, E. The Occurrence of Calcium Oxalate Crystals and Distribution of Arabinogalactan Proteins (AGPs) in Ovary Cells During Fragaria x Ananassa (Duch.) Development. J. Plant Growth Regul. 2019, 38, 1028–1036. [Google Scholar] [CrossRef] [Green Version]
- Tooulakou, G.; Giannopoulos, A.; Nikolopoulos, D.; Bresta, P.; Dotsika, E.; Orkoula, M.G.; Kontoyannis, C.G.; Fasseas, C.; Liakopoulos, G.; Klapa, M.I.; et al. Alarm Photosynthesis: Calcium Oxalate Crystals as an Internal CO2 Source in Plants. Plant Physiol. 2016, 171, 2577–2585. [Google Scholar] [CrossRef] [Green Version]
- Tütüncü Konyar, S.; Öztürk, N.; Dane, F. Occurrence, Types and Distribution of Calcium Oxalate Crystals in Leaves and Stems of Some Species of Poisonous Plants. Bot. Stud. 2014, 55, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calmés, J.; Piquemal, M. Variation Saisonnière Des Cristaux d’oxalate de Calcium Des Tissus de Vigne Vierge. Can. J. Bot. 1977, 55, 2075–2078. [Google Scholar] [CrossRef]
- Webb, M.A. Cell-Mediated Crystallization of Calcium Oxalate in Plants. Plant Cell 1999, 11, 751–761. [Google Scholar] [CrossRef] [Green Version]
- Arnott, H.J.; Webb, M.A. Twinned Raphides of Calcium Oxalate in Grape (Vitis): Implications for Crystal Stability and Function. Int. J. Plant Sci. 2000, 161, 133–142. [Google Scholar] [CrossRef]
- Huang, C.; Li, Y.; Wu, J.; Huang, Z.; Chang, S.X.; Jiang, P. Intensive Management Increases Phytolith-Occluded Carbon Sequestration in Moso Bamboo Plantations in Subtropical China. Forests 2019, 10, 883. [Google Scholar] [CrossRef] [Green Version]
- Rajendiran, S.; Coumar, M.V.; Kundu, S.; Dotaniya, M.; Rao, A. Role of Phytolith Occluded Carbon of Crop Plants for Enhancing Soil Carbon Sequestration in Agro-Ecosystems. Curr. Sci. 2012, 103, 911–920. [Google Scholar]
- Verrecchia, E.P.; Braissant, O.; Cailleau, G. The Oxalate–Carbonate Pathway in Soil Carbon Storage: The Role of Fungi and Oxalotrophic Bacteria. In Fungi in Biogeochemical Cycles; Gadd, G.M., Ed.; British Mycological Society Symposia; Cambridge University Press: Cambridge, UK, 2006; pp. 289–310. ISBN 978-0-521-84579-3. [Google Scholar]
- Sun, X.; Liu, Q.; Zhao, G.; Chen, X.; Tang, T.; Xiang, Y. Comparison of Phytolith-Occluded Carbon in 51 Main Cultivated Rice (Oryzasativa) Cultivars of China. RSC Adv. 2017, 7, 54726–54733. [Google Scholar] [CrossRef] [Green Version]
- Minocha, R.; Chamberlain, B.; Long, S.; Turlapati, S.A.; Quigley, G. Extraction and Estimation of the Quantity of Calcium Oxalate Crystals in the Foliage of Conifer and Hardwood Trees. Tree Physiol. 2015, 35, 574–580. [Google Scholar] [CrossRef] [PubMed]
- Parr, J.F.; Dolic, V.; Lancaster, G.; Boyd, W.E. A Microwave Digestion Method for the Extraction of Phytoliths from Herbarium Specimens. Rev. Palaeobot. Palynol. 2001, 116, 203–212. [Google Scholar] [CrossRef]
- Webb, M.A.; Cavaletto, J.M.; Carpita, N.C.; Lopez, L.E.; Arnott, H.J. The Intravacuolar Organic Matrix Associated with Calcium Oxalate Crystals in Leaves of Vitis. Plant J. 1995, 7, 633–648. [Google Scholar] [CrossRef]
- Subashini, S.; Sathishkumar, K. Physico-Chemical Characteristics and Thermal Stability of Calcium Oxalate Crystals Isolated from Beta Vulgaris Root. J. Environ. Biol. 2019, 40, 775–783. [Google Scholar] [CrossRef]
- Ragland, K.W.; Aerts, D.J.; Baker, A.J. Properties of Wood for Combustion Analysis. Bioresour. Technol. 1991, 37, 161–168. [Google Scholar] [CrossRef]
- Yu, C.; Thy, P.; Wang, L.; Anderson, S.N.; VanderGheynst, J.S.; Upadhyaya, S.K.; Jenkins, B.M. Influence of Leaching Pretreatment on Fuel Properties of Biomass. Fuel Process. Technol. 2014, 128, 43–53. [Google Scholar] [CrossRef]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Dhahak, A.; Bounaceur, R.; Le Dreff-Lorimier, C.; Schmidt, G.; Trouve, G.; Battin-Leclerc, F. Development of a Detailed Kinetic Model for the Combustion of Biomass. Fuel 2019, 242, 756–774. [Google Scholar] [CrossRef] [Green Version]
- Orfão, J.J.M.; Antunes, F.J.A.; Figueiredo, J.L. Pyrolysis Kinetics of Lignocellulosic Materials—Three Independent Reactions Model. Fuel 1999, 78, 349–358. [Google Scholar] [CrossRef]
- El May, Y.; Jeguirim, M.; Dorge, S.; Trouvé, G.; Said, R. Study on the Thermal Behavior of Different Date Palm Residues: Characterization and Devolatilization Kinetics under Inert and Oxidative Atmospheres. Energy 2012, 44, 702–709. [Google Scholar] [CrossRef]
- Popova, E.; Chernov, A.; Maryandyshev, P.; Brillard, A.; Kehrli, D.; Trouvé, G.; Lyubov, V.; Brilhac, J.-F. Thermal Degradations of Wood Biofuels, Coals and Hydrolysis Lignin from the Russian Federation: Experiments and Modeling. Bioresour. Technol. 2016, 218, 1046–1054. [Google Scholar] [CrossRef]
- Likar, M.; Vogel-Mikuš, K.; Potisek, M.; Hančević, K.; Radić, T.; Nečemer, M.; Regvar, M. Importance of Soil and Vineyard Management in the Determination of Grapevine Mineral Composition. Sci. Total Environ. 2015, 505, 724–731. [Google Scholar] [CrossRef]
- Rabaçal, M.; Fernandes, U.; Costa, M. Combustion and Emission Characteristics of a Domestic Boiler Fired with Pellets of Pine, Industrial Wood Wastes and Peach Stones. Renew. Energy 2013, 51, 220–226. [Google Scholar] [CrossRef]
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Beech (#797). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Beech (#2142). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Fir (#791). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Spruce (#163). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Phyllis2 Database. Database for the Physico-Chemical Composition of (Treated) Lignocellulosic Biomass, Micro- and Macroalgae, Various Feedstocks for Biogas Production and Biochar. Wood, Spruce (#2402). Available online: https://phyllis.nl (accessed on 5 September 2022).
- Scurfield, G.; Michell, A.J.; Silva, S.R. Crystals in Woody Stems. Bot. J. Linn. Soc. 1973, 66, 277–289. [Google Scholar] [CrossRef]
- Weiner, S.; Pinkas, I.; Kossoy, A.; Feldman, Y. (Isai) Calcium Sulfate Hemihydrate (Bassanite) Crystals in the Wood of the Tamarix Tree. Minerals 2021, 11, 289. [Google Scholar] [CrossRef]
- Hourlier, D. Thermal Decomposition of Calcium Oxalate: Beyond Appearances. J. Therm. Anal. Calorim. 2019, 136, 2221–2229. [Google Scholar] [CrossRef]
- Szekely, A.; Varhegyi, G.; Till, F.; Szabo, P.; Jakab, E. The effects of Heat and mass Transport on the Results of Thermal Decomposition Studies. J. Anal. Appl. Pyrolysis 1987, 11, 83–92. [Google Scholar] [CrossRef]
- Aquilano, D.; Franchini-Angela, M. Twin Laws of Whewellite, CaC2O4·H2O. A Structural and Growth Approach. Phys. Chem. Miner. 1981, 7, 124–129. [Google Scholar] [CrossRef]
- Wooten, J.B. 13C CPMAS NMR of Bright and Burley Tobaccos. J. Agric. Food Chem. 1995, 43, 2858. [Google Scholar] [CrossRef]





| Trunk | Root | Bark | Bark Calcined at 250 °C | Bark Calcined at 300 °C | Bark Calcined at 350 °C | |
|---|---|---|---|---|---|---|
| Organic component | 98.0 | 96.6 | 95.0 | 89.1 | 82.7 | 49.8 |
| Na2O | b.d.a | 0.05 | b.d.a | 0.16 | 0.57 | 1.62 |
| MgO | 0.08 | 0.08 | 0.17 | 1.08 | 1.77 | 4.61 |
| Al2O3 | 0.02 | 0.23 | 0.08 | 0.91 | 3.51 | 10.46 |
| SiO2 | 0.04 | 0.41 | 0.17 | 0.92 | 3.71 | 11.68 |
| P2O5 | 0.04 | 0.2 | 0.02 | 0.08 | 0.55 | 1.55 |
| K2O | 0.44 | 0.20 | 0.31 | 1.00 | 1.15 | 2.89 |
| CaO | 0.34 | 1.33 | 3.81 | 6.89 | 6.29 | 18.48 |
| TiO2 | 0.03 | 0.07 | b.d.a | b.d.a | 0.05 | 0.18 |
| Cr2O3 | b.d.a | b.d. | b.d.a | b.d.a | 0.08 | b.d.a |
| MnO | b.d.a | b.d. | b.d.a | 0.10 | 0.09 | 0.28 |
| Fe2O3 | b.d.a | 0.11 | 0.06 | 0.20 | 1.06 | 2.26 |
| Ni | b.d.a | b.d. | b.d.a | b.d.a | b.d.a | 0.02 |
| Cu | b.d.a | 0.008 | b.d.a | 0.03 | 0.04 | 0.08 |
| Zn | 0.003 | 0.009 | 0.006 | 0.02 | 0.08 | 0.23 |
| Rb | b.d.a | b.d.a | b.d.a | b.d.a | 0.005 | 0.009 |
| Sr | b.d.a | 0.004 | 0.009 | 0.02 | 0.02 | 0.05 |
| Pb | b.d.a | b.d. | b.d. | b.d.a | 0.006 | 0.008 |
| Zr | 0.78 | 0.36 | 0.30 | b.d.a | b.d.a | b.d.a |
| S | 0.02 | 0.08 | 0.04 | 0.14 | 0.27 | 0.71 |
| Cl | 0.01 | 0.009 | 0.02 | 0.04 | b.d.a | 0.08 |
| Calcium oxalate monohydrate b | 0.9 | 3.5 | 9.9 | 17.9 | 16.4 | 48.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trouvé, G.; Michelin, L.; Kehrli, D.; Josien, L.; Rigolet, S.; Lebeau, B.; Gieré, R. The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination. Crystals 2023, 13, 967. https://doi.org/10.3390/cryst13060967
Trouvé G, Michelin L, Kehrli D, Josien L, Rigolet S, Lebeau B, Gieré R. The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination. Crystals. 2023; 13(6):967. https://doi.org/10.3390/cryst13060967
Chicago/Turabian StyleTrouvé, Gwenaëlle, Laure Michelin, Damaris Kehrli, Ludovic Josien, Séverinne Rigolet, Bénédicte Lebeau, and Reto Gieré. 2023. "The Multi-Analytical Characterization of Calcium Oxalate Phytolith Crystals from Grapevine after Treatment with Calcination" Crystals 13, no. 6: 967. https://doi.org/10.3390/cryst13060967







