Particle Size Effect on Optical and Gas-Sensing Properties of La0.67Ca0.2Ba0.13Fe0.97M0.03O3 (M = Ti4+, Mn3+, and Cr3+) Compounds
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
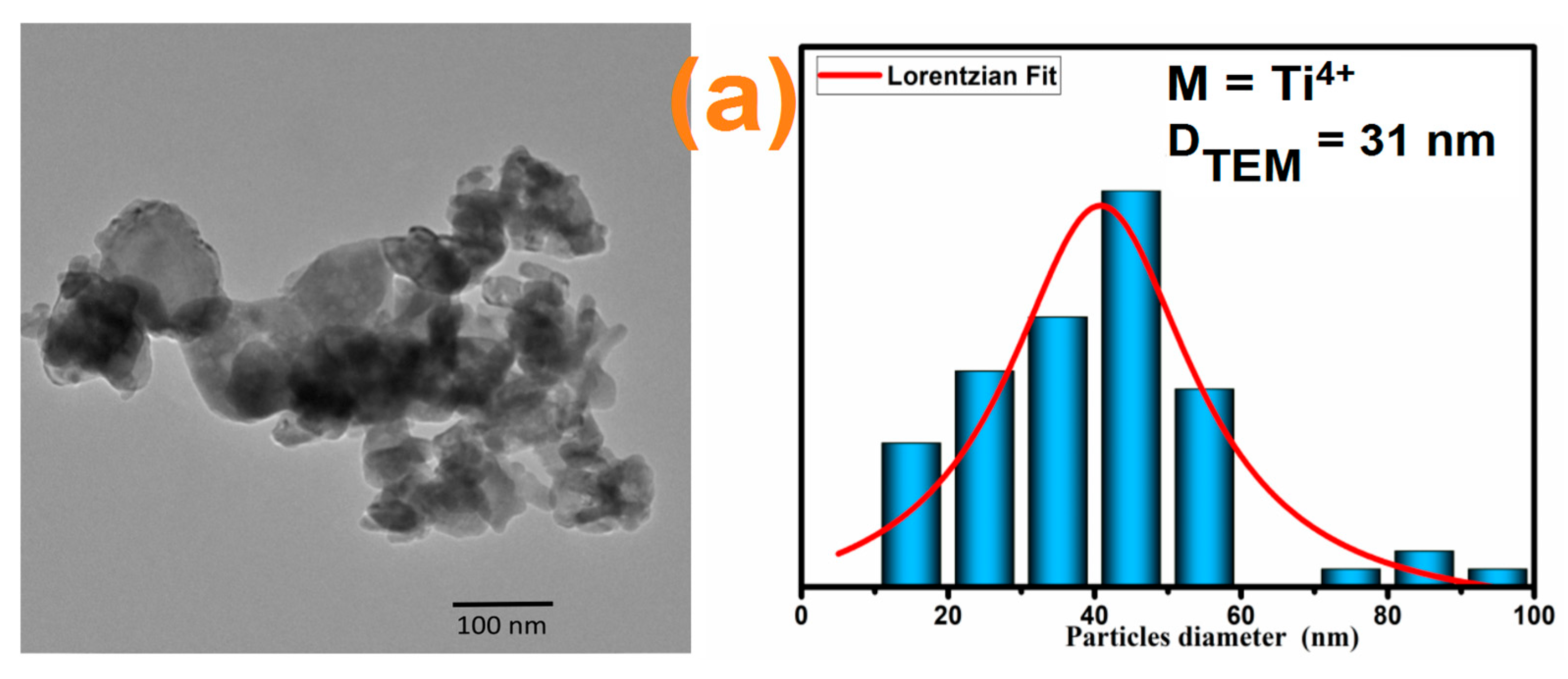
3.1. Morphological Study
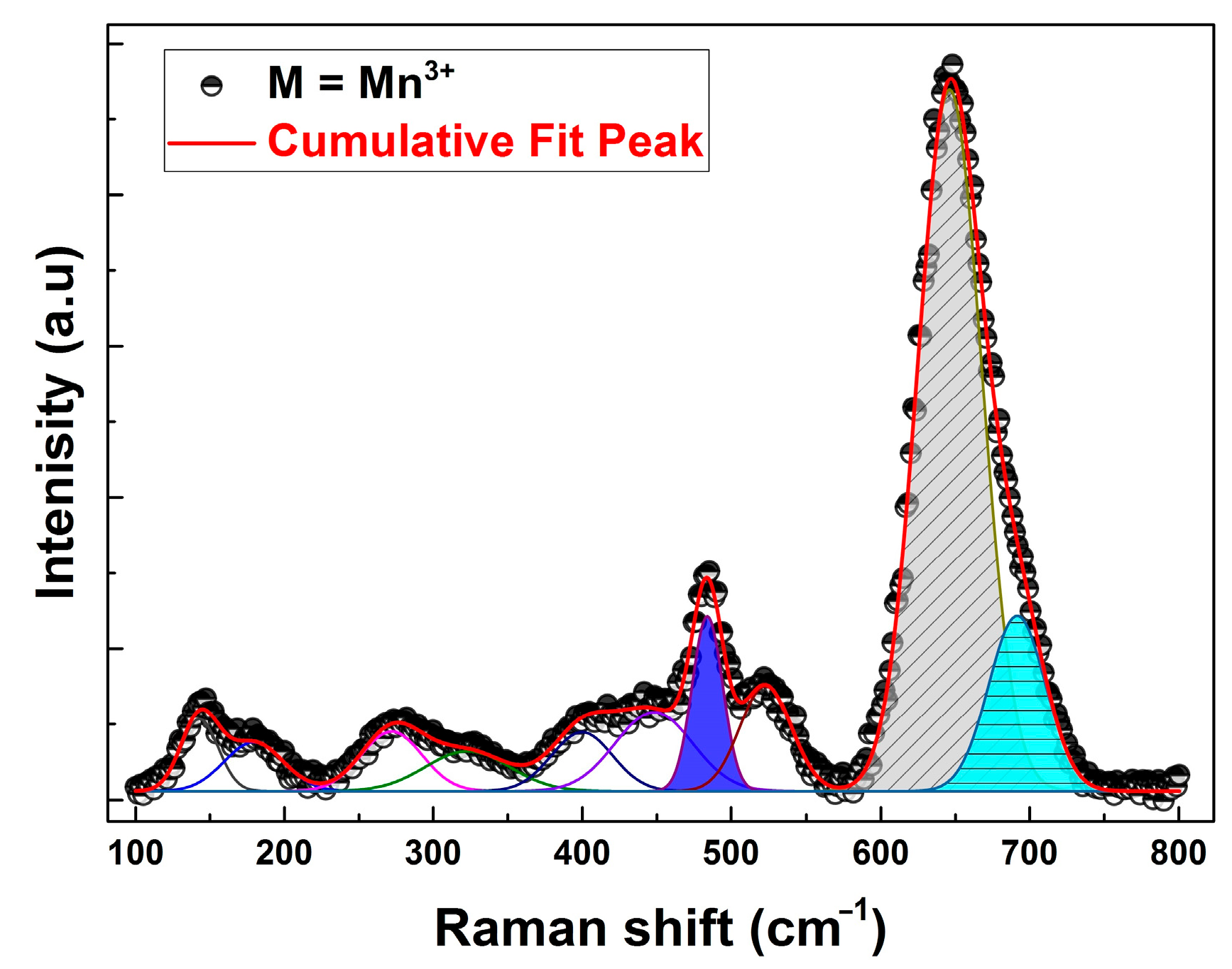
3.2. Raman Scattering Studies
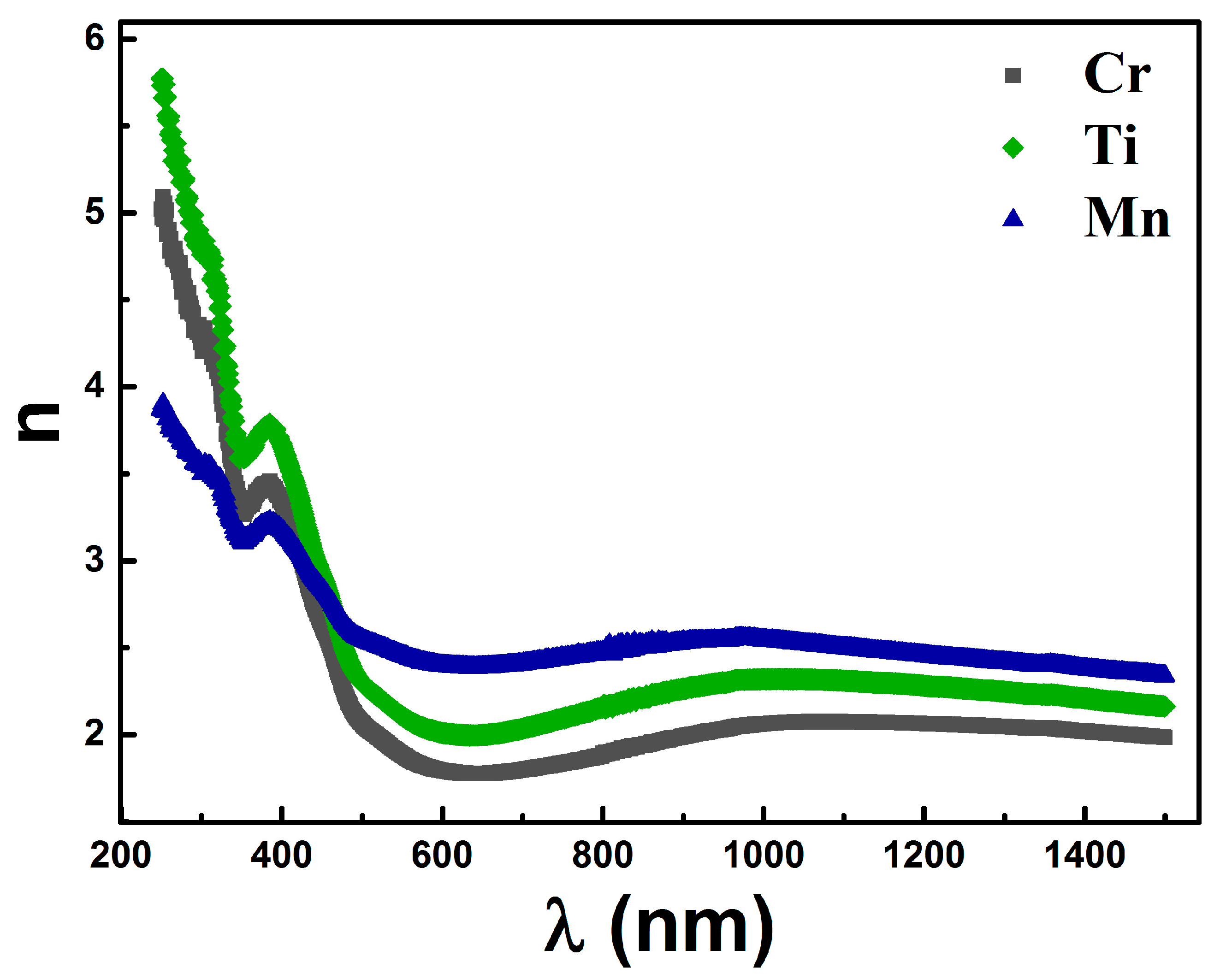
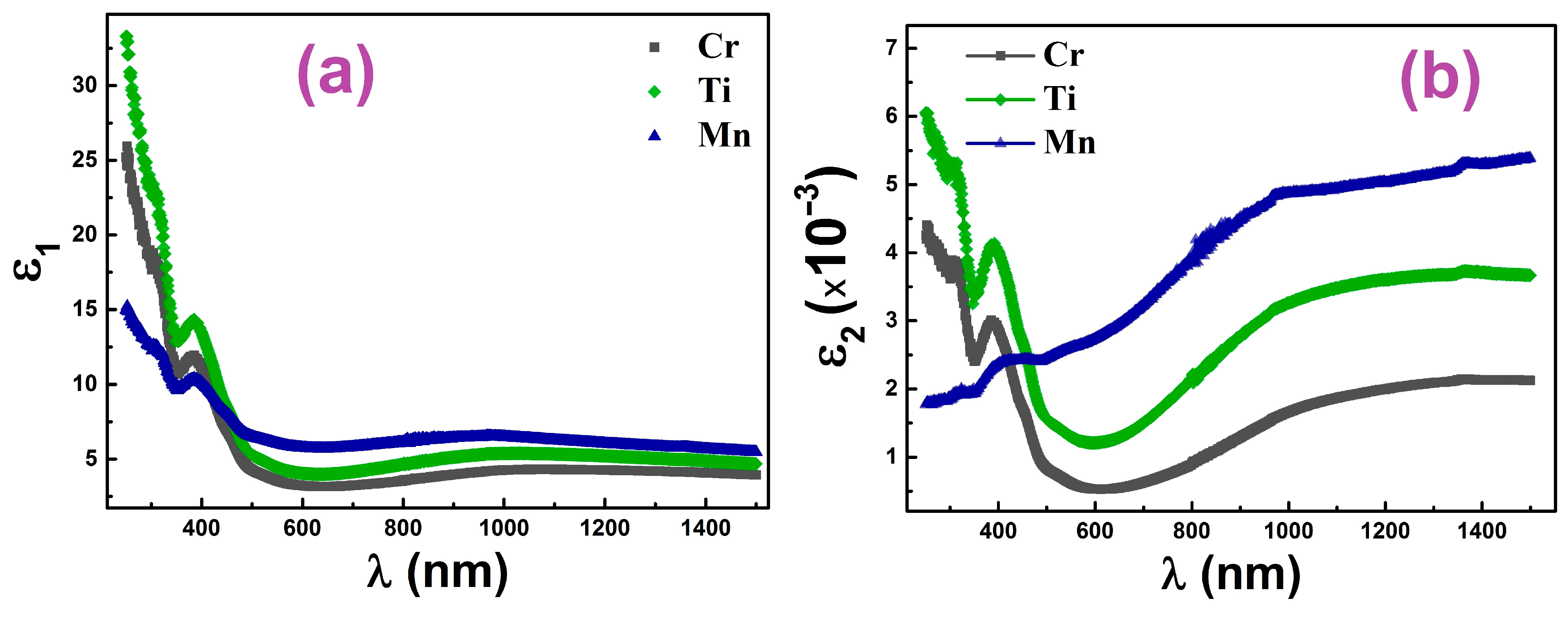
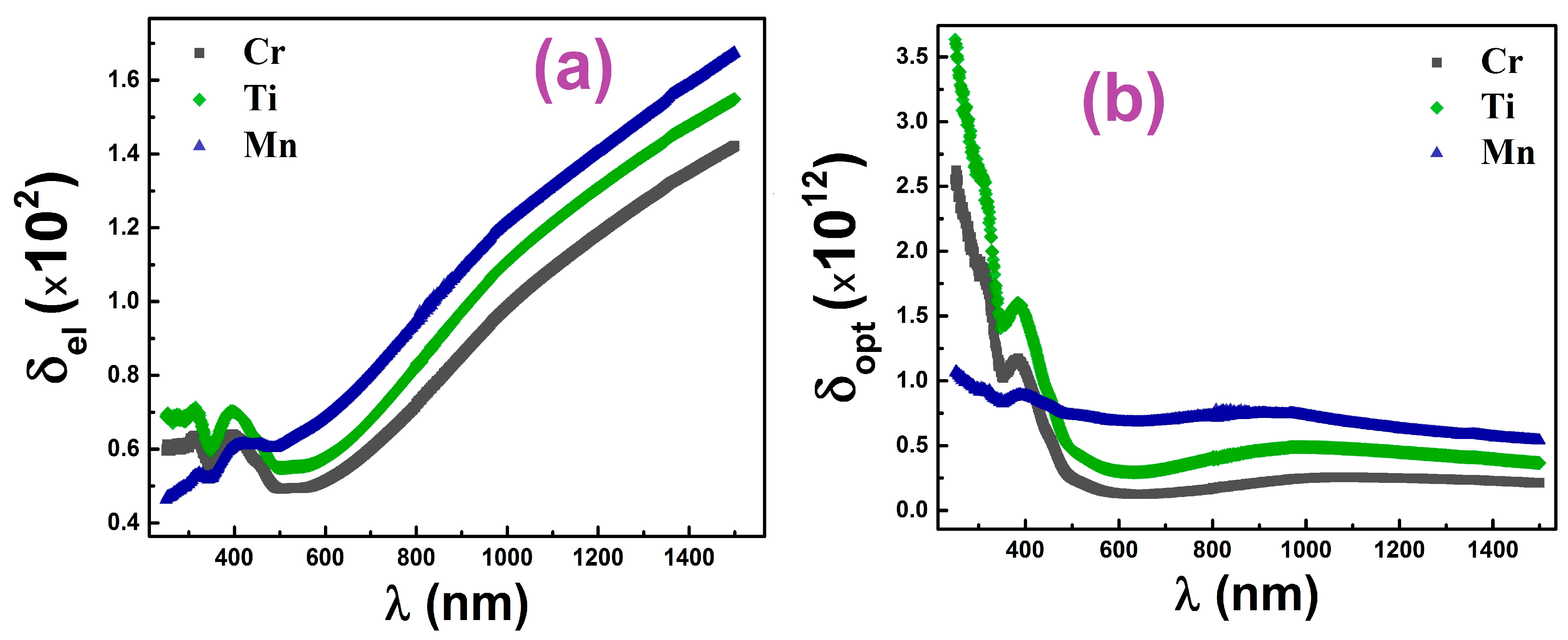
3.3. Optical Properties
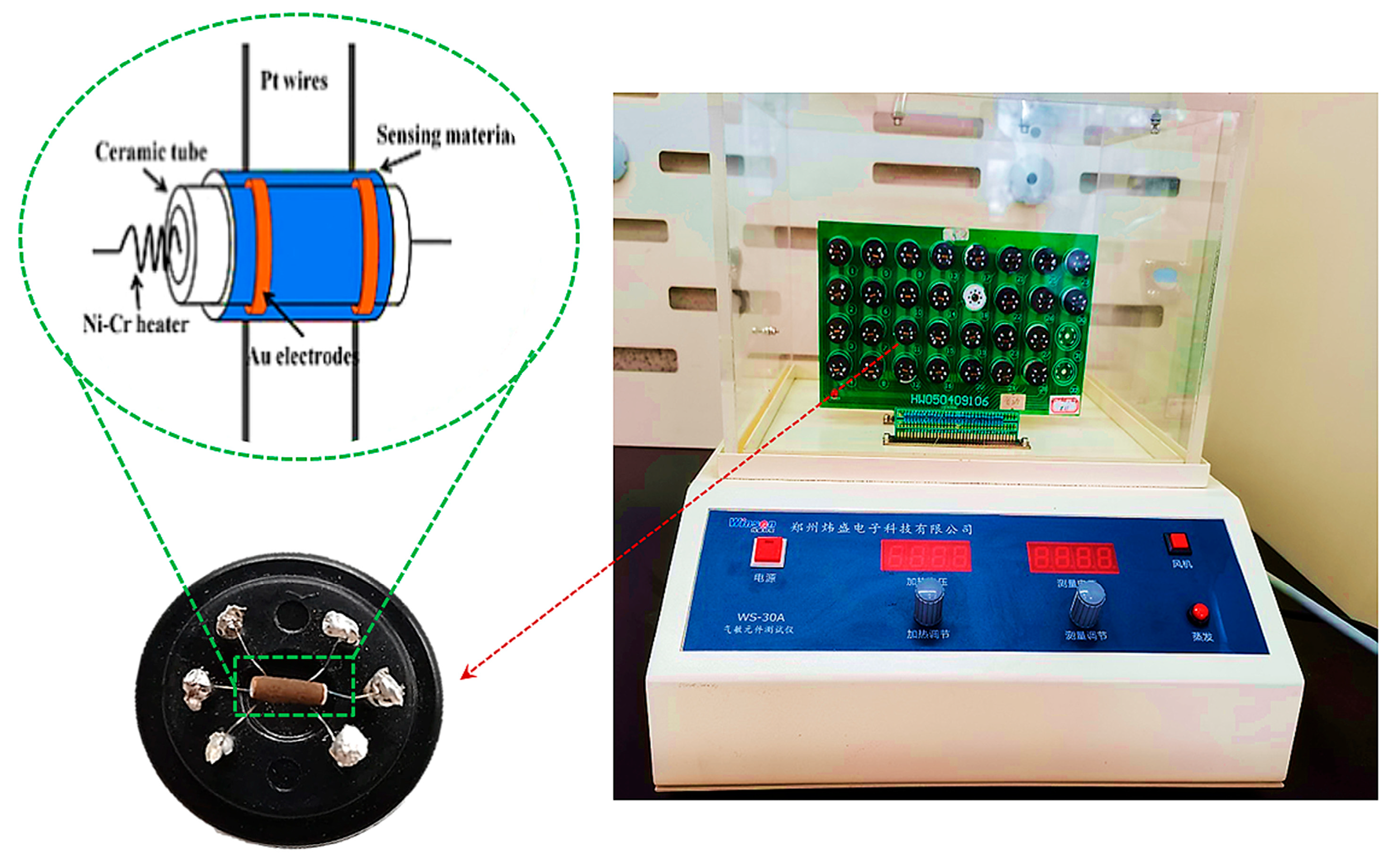
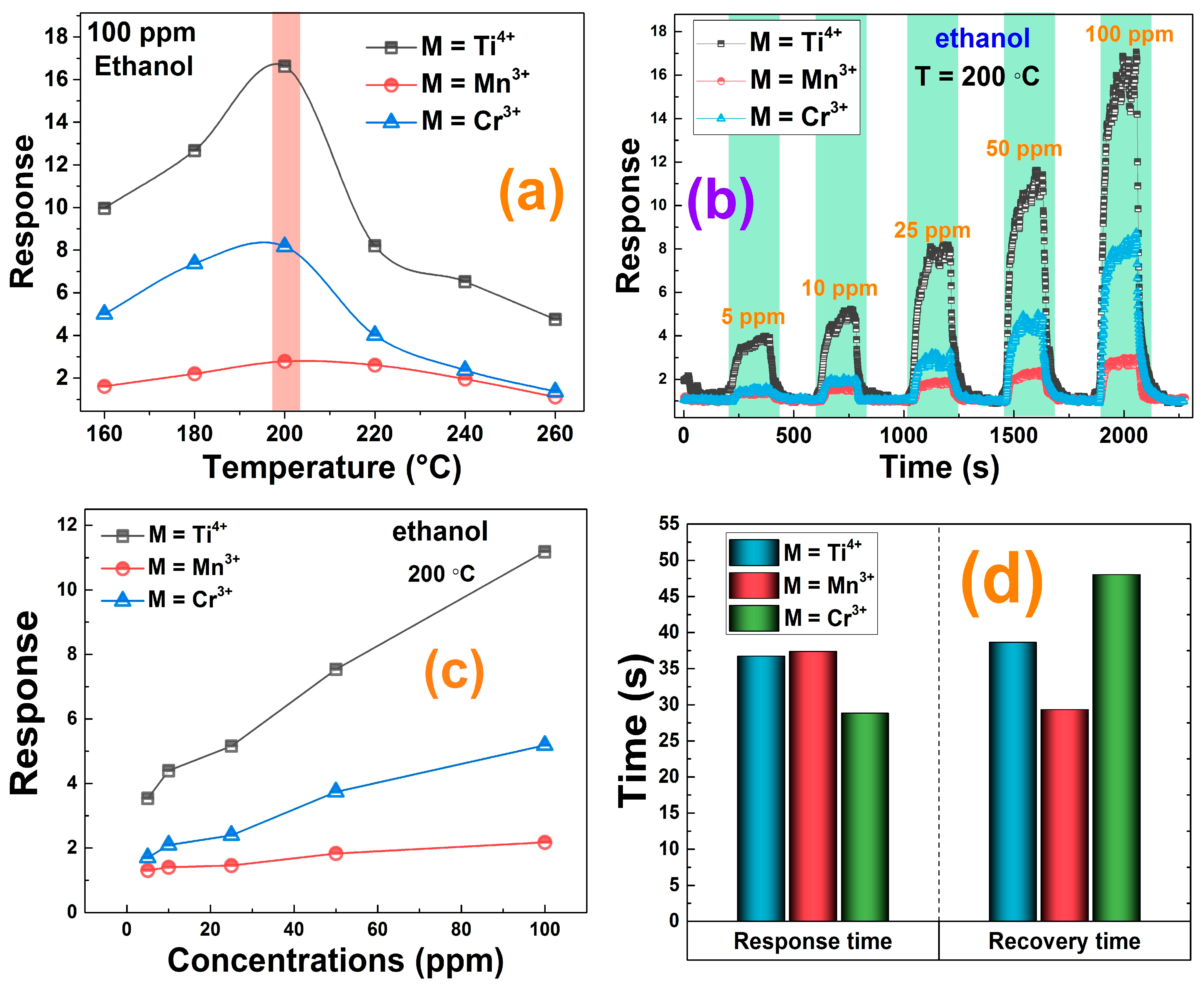
3.4. Gas-Sensing Measurements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Clements, A.L.; Griswold, W.G.; RS, A.; Johnston, J.E.; Herting, M.M.; Thorson, J.; Collier-Oxandale, A.; Hannigan, M. Low-Cost Air Quality Monitoring Tools: From Research to Practice (A Workshop Summary). Sensors 2017, 17, 2478. [Google Scholar] [CrossRef]
- Willett, M. Oxygen Sensing for Industrial Safety—Evolution and New Approaches. Sensors 2014, 14, 6084. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent Advances in Electrochemical Sensors for Detecting Toxic Gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Han, T.; Sun, B.; Kong, L.; Jin, Z.; Huang, X.; Liu, J.; Meng, F. Catalysis-Based Cataluminescent and Conductometric Gas Sensors: Sensing Nanomaterials, Mechanism, Applications and Perspectives. Catalysts 2016, 6, 210. [Google Scholar] [CrossRef]
- Zuruzi, S.; MacDonald, N.C. Facile Fabrication and Integration of Patterned Nanostructured TiO2 for Microsystems Applications. Adv. Funct. Mater. 2005, 15, 396. [Google Scholar] [CrossRef]
- Gibson, D.; MacGregor, C. A Novel Solid State Non-Dispersive Infrared CO2 Gas Sensor. Sensors 2013, 13, 7079. [Google Scholar] [CrossRef] [PubMed]
- Pijolat, C.; Pupier, C.; Sauvan, M.; Tournier, G.; Lalauze, R. Gas detection for automotive pollution control. Sens. Actuators B Chem. 1999, 59, 195–202. [Google Scholar] [CrossRef]
- Degler, D.; Barz, N.; Dettinger, U.; Peisert, H.; Chasse, T.; Weimar, U.; Barsan, N. Extending the toolbox for gas sensor research: Operando UV/vis diffuse reflectance, spectroscopy on SnO2-based gas sensors. Sens. Actuators B Chem. 2016, 224, 256–259. [Google Scholar] [CrossRef]
- Jildeh, Z.B.; Kirchner, P.; Oberlander, J.; Kremers, A.; Wagner, T.; Wagner, P.H.; Schöning, M.J. FEM-based modeling of a calorimetric gas sensor for hydrogen peroxide monitoring. Phys. Status Solidi 2017, 214, 1600912. [Google Scholar] [CrossRef]
- Yoo, R.; Güntner, A.T.; Park, Y.; Rim, H.J.; Lee, H.-S.; Lee, W. Sensing of acetone by Al-doped ZnO. Sens. Actuators B Chem. 2019, 283, 107–115. [Google Scholar] [CrossRef]
- Farida, A.A.; Rasmita, N.; Achary, P.G.R.; Mishra, D.K.; Sahoo, S.K.; Singh, U.P.; Nanda, B. Facile synthesis of porous LaFeO3 hexagons for acetone sensing. Mater. Today Proc. 2023, 74, 993–1001. [Google Scholar] [CrossRef]
- Righettoni, M.; Amann, A.; Pratsinis, S.E. Breath analysis by nanostructured metal oxides as chemo-resistive gas sensors. Mater. Today 2015, 18, 163–171. [Google Scholar] [CrossRef]
- Sharma, B.; Karuppasamy, K.; Vikraman, D.; Jo, E.B.; Sivakumar, P.; Kim, H.S. Porous, 3D-hierarchical α-NiMoO4 rectangular nanosheets for selective conductometric ethanol gas sensors. Sens. Actuators B Chem. 2021, 347, 130615. [Google Scholar] [CrossRef]
- Nie, Q.; Zhang, W.; Wang, L.; Guo, Z.; Li, C.; Yao, J.; Li, M.; Wu, D.; Zhou, L. Sensitivity enhanced, stability improved ethanol gas sensor based on multi-wall carbon nanotubes functionalized with Pt-Pd nanoparticles. Sens. Actuators B Chem. 2018, 270, 140–148. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Y.; Zhang, H.Y.; Chen, C. Design and evaluation of Cu-modified ZnO microspheres as a high-performance formaldehyde sensor based on density functional theory. Appl. Surf. Sci. 2020, 523, 147446. [Google Scholar] [CrossRef]
- Dutt, M.; Ratan, A.; Tomar, M.; Gupta, V.; Singh, V. Mesoporous metal oxide-α-Fe2O3 nanocomposites for sensing formaldehyde and ethanol at room temperature. J. Phys. Chem. Solids 2020, 145, 109536. [Google Scholar] [CrossRef]
- Li, Y.W.; Luo, N.; Sun, G.; Zhang, B.; Ma, G.Z.; Jin, H.H. Facile synthesis of ZnFe2O4/α-Fe2O3 porous microrods with enhanced TEA-sensing performance. J. Alloys Compd. 2018, 737, 255–262. [Google Scholar] [CrossRef]
- Han, B.Q.; Wang, J.H.; Yang, W.Y.; Chen, X.W.; Wang, H.R.; Chen, J.L. Hydrothermal synthesis of flower-like In2O3 as a chemiresistive isoprene sensor for breath analysis. Sens. Actuators B Chem. 2020, 309, 127788. [Google Scholar] [CrossRef]
- Yang, M.; Lu, J.Y.; Wang, X.; Zhang, H.; Chen, F.; Sun, J.B.; Yang, J.; Sun, Y.; Lu, G. Acetone sensors with high stability to humidity changes based on Ru-doped NiO flower-like microspheres. Sens. Actuators B Chem. 2020, 313, 127965. [Google Scholar] [CrossRef]
- Qiao, X.R.; Ma, C.; Chang, X.; Li, X.F.; Li, K.; Zhu, L.; Xia, F.; Xue, Q. 3D radial Co3O4 nanorod cluster derived from cobalt-based layered hydroxide metal salt for enhanced trace acetone detection. Sens. Actuators B Chem. 2021, 327, 128926. [Google Scholar] [CrossRef]
- Available online: https://pdf.directindustry.com/pdf/alphasense/p-type-metal-oxidesensor-overview/16860-704756.html (accessed on 15 December 2023).
- Moumen, A.; Kumarage, G.C.W.; Comini, E. P-Type Metal Oxide Semiconductor Thin Films: Synthesis and Chemical Sensor Applications. Sensors 2022, 22, 1359. [Google Scholar] [CrossRef] [PubMed]
- Barsan, N.; Weimar, U. Conduction Model of Metal Oxide Gas Sensors. J. Electroceramics 2001, 7, 143–167. [Google Scholar] [CrossRef]
- Xu, H.; Xu, J.; Li, H.; Zhang, W.; Zhang, Y.; Zhai, Z. Highly sensitive ethanol and acetone gas sensors with reduced working temperature based on Sr-doped BiFeO3 nanomaterial. J. Mater. Res. Technol. 2022, 17, 1955–1963. [Google Scholar] [CrossRef]
- Jamnani, S.R.; Moghaddam, H.M.; Leonardi, S.G.; Neri, G.; Ferlazzo, A. VOCs sensing properties of samarium oxide nanorods. Ceram. Int. 2024, 50, 403–411. [Google Scholar] [CrossRef]
- Jiang, B.; Zhou, T.; Zhang, L.; Yang, J.; Han, W.; Sun, Y.; Liu, F.; Sun, P.; Zhang, H.; Lu, G. Separated detection of ethanol and acetone based on SnO2-ZnO gas sensor with improved humidity tolerance. Sens. Actuators B Chem. 2023, 393, 134257. [Google Scholar] [CrossRef]
- Liua, T.; Xu, Y. Synthesis of nanocrystalline LaFeO3 powders via glucose sol–gel route. Mater. Chem. Phys. 2011, 129, 1047. [Google Scholar] [CrossRef]
- Zhu, C.; Nobuta, A.; Nakatsugawa, I.; Akiyama, T. Solution combustion synthesis of LaMO3 (M = Fe, Co, Mn) perovskite nanoparticles and the measurement of their electrocatalytic properties for air cathode. Int. J. Hydrogen Energy 2013, 38, 13238. [Google Scholar] [CrossRef]
- Qin, J.; Cui, Z.D.; Yang, X.J.; Zhu, S.L.; Li, Z.Y.; Liang, Y.Q. Three-dimensionally ordered macroporous La1−xMgxFeO3 as high-performance gas sensor to methanol. J. Alloys Compd. 2015, 635, 194. [Google Scholar] [CrossRef]
- Suhendi, E.; Ulhakim, M.T.; Setiawan, A.; Syarif, D.G. The Effect of SrO Doping on LaFeO3 using Yarosite Extraction based Ethanol Gas Sensors Performance Fabricated by Coprecipitation Method. Int. J. Nanoelectron. Mater. 2019, 12, 185. [Google Scholar]
- Benali, A.; Bejar, M.; Dhahri, E.; Graça, M.P.F.; Valente, M.A.; Radwan, A. High ethanol gas sensing property and modulation of magnetic and AC-conduction mechanism in 5% Mg-doped La0.8Ca0.1Pb0.1FeO3 compound. J. Mater. Sci. Mater. Electron. 2019, 30, 12389. [Google Scholar] [CrossRef]
- Song, P.; Qin, H.; Zhang, L.; Liu, X.; Huang, S.; Hu, J.; Jiang, M. Electrical and CO gas-sensing properties of perovskite-type La0.8Pb0.2Fe0.8Co0.2O3 semiconductive materials. Physica B Condens. Matter. 2005, 368, 204. [Google Scholar] [CrossRef]
- Li, L.; Qin, H.W.; Shi, C.M.; Zhang, L.; Chen, Y.P.; Hu, J.F. CO2 sensing properties of La1−xBaxFeO3 thick film and packed powder sensors. RSC Adv. 2015, 5, 103073. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, B.; Li, Y.; Xu, X.; Sun, G.; Cao, J.; Wang, Y. Synthesis of spindle-like Co-doped LaFeO3 porous microstructure for high performance n-butanol sensor. Sens. Actuators B Chem. 2021, 343, 130125. [Google Scholar] [CrossRef]
- Benali, E.M.; Benali, A.; Bejar, M.; Dhahri, E.; Khomchenko, V.A.; Peng, L.; Wu, J.; Costa, B.F.O. Structural, morphological and excellent gas sensing properties of La1−2xBaxBixFeO3 (0.00 ≤ x ≤ 0.20) nanoparticles. J. Alloys Compd. 2021, 883, 160856. [Google Scholar] [CrossRef]
- Li, F.; Wang, Z.F.; Wang, A.; Wu, S.M.; Zhang, L.J. N-type LaFe1−xMnxO3 prepared by sol-gel method for gas sensing. J. Alloys Compd. 2020, 816, 152647. [Google Scholar] [CrossRef]
- Ma, L.; Ma, S.Y.; Qiang, Z.; Xu, X.L.; Chen, Q.; Yang, H.M.; Chen, H.; Ge, Q.; Zeng, Q.Z.; Wang, B.Q. Preparation of Codoped LaFeO3 nanofibers with enhanced acetic acid sensing properties. Mater. Lett. 2017, 200, 47–50. [Google Scholar] [CrossRef]
- Ma, Z.; Yang, K.; Xiao, C.; Jia, L. C-doped LaFeO3 Porous Nanostructures for Highly Selective Detection of Formaldehyde. Sens. Actuators B Chem. 2021, 347, 130550. [Google Scholar] [CrossRef]
- Benali, E.M.; Benali, A.; Bejar, M.; Dhahri, E.; Graca, M.P.F.; Valente, M.A.; Sanguino, P.; Costa, B.F.O. Effect of annealing temperature on structural, morphological and dielectric properties of La0.8Ba0.1Ce0.1FeO3 perovskite. J. Mater. Sci. Mater. Electron. 2020, 31, 16220–16234. [Google Scholar] [CrossRef]
- Wu, J.; Gao, D.; Sun, T.; Bi, J.; Zhao, Y.; Ning, Z.; Fan, G.; Xie, Z. Highly selective gas sensing properties of partially inversed spinel zinc ferrite towards H2S. Sens. Actuators B Chem. 2016, 235, 258. [Google Scholar] [CrossRef]
- Peng, S.; Ma, M.; Yang, W.; Wang, Z.; Bi, J.; Wu, J. Acetone sensing with parts-per-billion limit of detection using a BiFeO3-based solid solution sensor at the morphotropic phase boundary. Sens. Actuators B Chem. 2020, 313, 128060. [Google Scholar] [CrossRef]
- Law, D.P.; Blakeney, A.B.; Tkachuk, R. The Kubelka–Munk Equation: Some Practical Considerations. J. Near Infrared Spectrosc. 1996, 4, 189–193. [Google Scholar] [CrossRef]
- Feng, J.; Liu, T.; Xu, Y.; Zhao, J.; He, Y. Effects of PVA content on the synthesis of LaFeO3 via sol–gel route. Ceram. Int. 2011, 37, 1203–1207. [Google Scholar] [CrossRef]
- Dhahri, A.; Moualhi, Y.; Henchiri, C.; Benali, A.; Sanguino, P.; Graça, M.P.F.; Valente, M.A.; Abdelmoula, N.; Rahmouni, H.; Costa, B.F.O. Study of structural properties and conduction mechanisms of La0.67Ca0.2Ba0.13Fe0.97Ti0.03O3 perovskite. Inorg. Chem. Commun. 2022, 140, 109435. [Google Scholar] [CrossRef]
- Smirnova, I.S. Normal modes of the LaMnO3 Pnma phase: Comparison with La2CuO4 Cmca phase. Physica B 1999, 262, 247–261. [Google Scholar] [CrossRef]
- Romero, M.; Gomez, R.W.; Marquina, V.; Perez-Mazariego, J.L.; Escamilla, R. Synthesis by molten salt method of the AFeO3 system (A = La, Gd) and its structural, vibrational and internal hyperfine magnetic field characterization. Physica B 2014, 443, 90–94. [Google Scholar] [CrossRef]
- Popa, M.; Frantti, J.; Kakihana, M. Lanthanum Ferrite LaFeO3+d nanopowders obtained by the polymerizable complex method. Solid State Ion. 2002, 154–155, 437–445. [Google Scholar] [CrossRef]
- Rachid, F.Z.; Omari, L.H.; Lassri, H.; Lemziouka, H.; Derkaoui, S.; Haddad, M.; Lamhasni, T.; Sajieddine, M. Synthesis, structural and optical properties of LaFe1−xCrxO3 nanoparticles. Opt. Mat. 2020, 109, 110332. [Google Scholar] [CrossRef]
- Saha, S.; Sinha, T.; Mookerjee, A. Electronic structure, chemical bonding, and optical properties of paraelectric BaTiO3. Phys. Rev. B 2000, 62, 8828. [Google Scholar] [CrossRef]
- Khan, M.T.; Shkir, M.; Almohammedi, A.; AlFaify, S. Fabrication and characterization of La doped PbI2 nanostructured thin films for opto-electronic applications. Solid State Sci. 2019, 90, 95–101. [Google Scholar] [CrossRef]
- Omari, L.H.; Lemziouka, H.; Haddad, M.; Lamhasni, T. Structural, optical and electrical properties of 0.97(PbTiO3)-0.03(LaFeO3) solid solutions. Mater. Today Proc. 2019, 13, 1205–1214. [Google Scholar] [CrossRef]
- Ganesh, V.; Haritha, L.; Anis, M.; Shkir, M.; Yahia, I.S.; Singh, A.; AlFaify, S. Structural, morphological, optical and third order nonlinear optical response of spin-coated NiO thin films: An effect of N doping. Solid State Sci. 2018, 86, 98–106. [Google Scholar] [CrossRef]
- Millis, A.J.; Zimmers, A.; Lobo, R.P.S.M.; Bontemps, N.; Homes, C.C. Mott physics and the optical conductivity of electron-doped cuprates. Phys. Rev. B 2005, 72, 224517. [Google Scholar] [CrossRef]
- Omari, L.H.; Lemziouka, H.; Moubah, R.; Haddad, M.; Lassri, H. Structural and optical properties of Fe-doped Ruddlesden-Popper Ca3Ti2−xFexO7−δ nanoparticles. Mat. Chem. Phys. 2020, 246, 122810. [Google Scholar] [CrossRef]
- Benali, A.; Azizi, S.; Bejar, M.; Dhahri, E.; Graça, M.F.P. Structural, electrical and ethanol sensing properties of double-doping LaFeO3 perovskite oxides. Ceram. Int. 2014, 40, 14367. [Google Scholar] [CrossRef]
- Smiy, S.; Bejar, M.; Dhahri, E.; Fiorido, T.; Aguir, K.; Bendahan, M. Preparation of double-doping LaFeO3 thin film for ethanol sensing application. J. Mol. Struct. 2022, 1267, 133543. [Google Scholar] [CrossRef]
- Xiang, J.; Chen, X.; Zhang, X.; Gong, L.; Zhang, Y.; Zhang, K. Preparation and characterization of Ba-doped LaFeO3 nanofibers by electrospinning and their ethanol sensing properties. Mater. Chem. Phys. 2018, 213, 122. [Google Scholar] [CrossRef]
- Ambardekar, V.; Bandyopadhyay, P.P.; Majumder, S.B. Understanding on the ethanol sensing mechanism of atmospheric plasma sprayed 25 wt. % WO3-75 wt. % SnO2 coating. Sens. Actuators B Chem. 2019, 290, 414–425. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T. Design of WO3-SnO2 core-shell nanofibers and their enhanced gas sensing performance based on different work function. Appl. Surf. Sci. 2018, 442, 30–37. [Google Scholar] [CrossRef]
- Malik, R.; Tomer, V.K.; Chaudhary, V.; Dahiya, M.S.; Nehra, S.P.; Rana, P.S.; Duhan, S. Ordered mesoporous In-(TiO2/WO3) nanohybrid: An ultrasensitive n-butanol sensor. Sens. Actuators B Chem. 2017, 239, 364–373. [Google Scholar] [CrossRef]
- Murade, P.A.; Sangawar, V.S.; Chaudhari, G.N.; Kapse, V.D.; Bajpeyee, A.U. Acetone gas-sensing performance of Sr-doped nanostructured LaFeO3 semiconductor prepared by citrate sol–gel route. Curr. Appl. Phys. 2011, 11, 451–456. [Google Scholar] [CrossRef]
- Zhang, L.; Qin, H.; Song, P.; Hu, J.; Jiang, M. Electric properties and acetone-sensing characteristics of La1−xPbxFeO3 perovskite system. Mater. Chem. Phys. 2006, 8, 358–362. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Zhang, J.; Chen, J.L.; Zhu, Z.Q.; Liu, Q.J. Improvement of response to formaldehyde at Ag–LaFeO3 based gas sensors through incorporation of SWCNTs. Sens. Actuators B Chem. 2014, 195, 509–514. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, T.M.; Liu, D.J. Formaldehyde gas sensing property and mechanism of TiO2–Ag nanocomposite. Physica B 2010, 405, 4235–4239. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B Chem. 2014, 192, 607. [Google Scholar] [CrossRef]
- Ding, J.-C.; Li, H.-Y.; Cai, Z.-X.; Wang, X.-X.; Guo, X. Near room temperature CO sensing by mesoporous LaCoO3 nanowires functionalized with Pd nanodots. Sens. Actuators B Chem. 2016, 222, 517. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, B.; Hu, J.; Qin, H.; Jiang, M. Semiconducting gas sensor for ethanol based on LaMgxFe1−xO3 nanocrystals. Sens. Actuators B Chem. 2008, 129, 53. [Google Scholar] [CrossRef]
- Liu, X.; Cheng, B.; Qin, H.; Song, P.; Huang, S.; Zhang, R.; Hu, J.; Jiang, M. Preparation, electrical and gas-sensing properties of perovskite-type La1−xMgxFeO3 semiconductor materials. Phys. Chem. Solids 2007, 68, 511. [Google Scholar] [CrossRef]
- Xiangfeng, C.; Xingqin, L.; Guangyao, M. Effects of CdO dopant on the gas sensitivity properties of ZnFe2O4 semiconductors. Sens. Actuators B Chem. 2000, 65, 64. [Google Scholar] [CrossRef]
- Balamurugan, C.; Lee, D.W. Perovskite hexagonal YMnO3 nanopowder as p-type semiconductor gas sensor for H2S detection. Sens. Actuators B Chem. 2015, 221, 857. [Google Scholar] [CrossRef]





| Sensor | S | Cg (ppm) | Topr (°C) | Reference |
|---|---|---|---|---|
| WO3–SnO2 composite | 1.73 | 300 (ethanol) | 250 | [58] |
| Core–shell WO3–SnO2 nanofibers | 5.09 | 10 (ethanol) | 280 | [59] |
| NiO nanosheets | 4.09 | 500 (ethanol) | 200 | [60] |
| LaFeO3 (Sol-gel) | 0.55 | 500 (acetone) | 275 | [61] |
| La0.7Sr0.3FeO3 (Sol-gel) | 0.7 | |||
| La0.68Pb0.32FeO3 (Sol-gel) | 50 | 7 | 240 | [62] |
| La0.67Ca0.2Ba0.13Fe0.97Ti0.03O3 | 3.91 | 5 (ethanol) | 200 | This work |
| 3.82 | 5 (acetone) | 250 |
| Gas | Time | M = Ti4+ | M = Mn3+ | M = Cr3+ |
|---|---|---|---|---|
| Ethanol | Response time | 40.74 | 37.39 | 28.84 |
| Recovery time | 43.67 | 26.35 | 48.03 | |
| Acetone | Response time | 50.39 | 57.387 | 45.549 |
| Recovery time | 56.32 | 49.347 | 59.739 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhahri, A.; Saoudi, H.; Gavinho, S.R.; Benali, A.; Abdelmoula, N.; Dhahri, R.; Peng, L.; Wu, J.; Pina, J.; Costa, B.F.O. Particle Size Effect on Optical and Gas-Sensing Properties of La0.67Ca0.2Ba0.13Fe0.97M0.03O3 (M = Ti4+, Mn3+, and Cr3+) Compounds. Crystals 2024, 14, 173. https://doi.org/10.3390/cryst14020173
Dhahri A, Saoudi H, Gavinho SR, Benali A, Abdelmoula N, Dhahri R, Peng L, Wu J, Pina J, Costa BFO. Particle Size Effect on Optical and Gas-Sensing Properties of La0.67Ca0.2Ba0.13Fe0.97M0.03O3 (M = Ti4+, Mn3+, and Cr3+) Compounds. Crystals. 2024; 14(2):173. https://doi.org/10.3390/cryst14020173
Chicago/Turabian StyleDhahri, Ahmed, H. Saoudi, S. R. Gavinho, A. Benali, N. Abdelmoula, R. Dhahri, Lin Peng, Jiangtao Wu, J. Pina, and B. F. O. Costa. 2024. "Particle Size Effect on Optical and Gas-Sensing Properties of La0.67Ca0.2Ba0.13Fe0.97M0.03O3 (M = Ti4+, Mn3+, and Cr3+) Compounds" Crystals 14, no. 2: 173. https://doi.org/10.3390/cryst14020173





