Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering
Abstract
:1. Introduction
2. Experiment
3. Results
4. Discussion
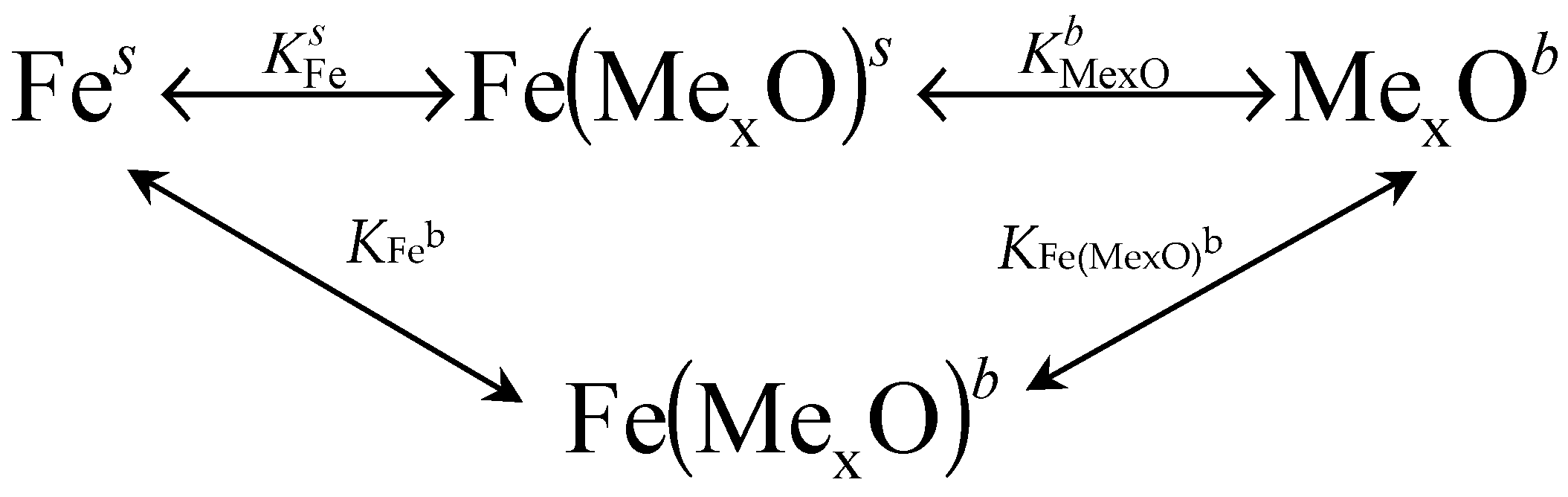
5. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mathaudhu, S.N.; Boyce, B.L. Thermal Stability: The Next Frontier for Nanocrystalline Materials. JOM 2015, 67, 2785–2787. [Google Scholar] [CrossRef]
- Gertsman, V.Y.; Birringer, R. On the room-temperature grain growth in nanocrystalline copper. Scr. Metall. Mater. 1994, 30, 577–581. [Google Scholar] [CrossRef]
- Rupert, T.J.; Gianola, D.S.; Gan, Y.; Hemker, K.J. Experimental Observations of Stress-Driven Grain Boundary Migration. Science 2009, 326, 1686–1690. [Google Scholar] [CrossRef]
- Zhang, K.; Weertman, J.R.; Eastman, J.A. The influence of time, temperature, and grain size on indentation creep in high-purity nanocrystalline and ultrafine grain copper. Appl. Phys. Lett. 2004, 85, 5197–5199. [Google Scholar] [CrossRef]
- Weertman, J.R. Retaining the Nano in Nanocrystalline Alloys. Science 2012, 337, 921–922. [Google Scholar] [CrossRef] [PubMed]
- Natter, H.; Schmelzer, M.; Löffler, M.-S.; Krill, C.E.; Fitch, A.; Hempelmann, R. Grain-Growth Kinetics of Nanocrystalline Iron Studied in Situ by Synchrotron Real-Time X-ray Diffraction. J. Phys. Chem. B 2000, 104, 2467–2476. [Google Scholar] [CrossRef]
- Smith, C.S. Grains, phases, and interphases: An interpretation of microstructure. Trans. Metall. Soc. AIME 1948, 175, 15–51. [Google Scholar]
- Burke, J.E. Some factor affecting the rate of grain growth in metals. Trans. Metall. Soc. AIME 1949, 180, 73–91. [Google Scholar]
- Burke, J.E.; Turnbull, D. Recrystallization and grain growth. Prog. Met. Phys. 1952, 3, 220–292. [Google Scholar] [CrossRef]
- Hösel, M.; Krebs, F.C. Large-scale roll-to-roll photonic sintering of flexo printed silver nanoparticle electrodes. J. Mater. Chem. 2012, 22, 15683–15688. [Google Scholar] [CrossRef]
- Layani, M.; Magdassi, S. Flexible transparent conductive coatings by combining self-assembly with sintering of silver nanoparticles performed at room temperature. J. Mater. Chem. 2011, 21, 15378–15382. [Google Scholar] [CrossRef]
- Asoro, M.A.; Kovar, D.; Shao-Horn, Y.; Allard, L.F.; Ferreira, P. Coalescence and sintering of Pt nanoparticles: In situ observation by aberration-corrected HAADF STEM. Nanotechnology 2010, 21, 025701. [Google Scholar] [CrossRef]
- Cao, Y.; Denny, M.S., Jr.; Caspar, J.V.; Farneth, W.E.; Guo, Q.; Ionkin, A.S.; Johnson, L.K.; Lu, M.; Malajovich, I.; Radu, D.; et al. High-Efficiency Solution-Processed Cu2ZnSn(S, Se)4 Thin-Film Solar Cells Prepared from Binary and Ternary Nanoparticles. J. Am. Chem. Soc. 2012, 134, 15644–15647. [Google Scholar] [CrossRef]
- Luding, S.; Manetsberger, K.; Müllers, J. A discrete model for long time sintering. J. Mech. Phys. Solids 2005, 53, 455–491. [Google Scholar] [CrossRef]
- Braginsky, M.; Tikare, V.; Olevsky, E. Numerical simulation of solid state sintering. Int. J. Solids Struct. 2005, 42, 621–636. [Google Scholar] [CrossRef]
- Pan, J.; Huang, R. Multi-Scale Modelling of Sintering. Key Eng. Mater. 2008, 368–372, 1668–1672. [Google Scholar] [CrossRef]
- Wakai, F.; Yoshida, M.; Shinoda, Y.; Akatsu, T. Coarsening and grain growth in sintering of two particles of different sizes. Acta Mater. 2005, 53, 1361–1371. [Google Scholar] [CrossRef]
- Panigrahi, B.B. Sintering and grain growth kinetics of ball milled nanocrystalline nickel powder. Mater. Sci. Eng. A 2007, 460–461, 7–13. [Google Scholar] [CrossRef]
- Gorshkov, V.; Kuzmenko, V.; Privman, V. Nonequilibrium Kinetic Modeling of Sintering of a Layer of Dispersed Nanocrystals. CrystEngComm 2014, 16, 10395–10409. [Google Scholar] [CrossRef]
- Shkatov, V.; Mazur, I. Modeling the Dynamic Recrystallization and Flow Curves Using the Kinetics of Static Recrystallization. Materials 2019, 12, 3024. [Google Scholar] [CrossRef]
- Zhu, K.N.; Ruan, Q.; Godfrey, A. The kinetics of grain growth in near-micrometre grain size copper produced by spark plasma sintering. IOP Conf. Ser. Mater. Sci. Eng. 2015, 89, 012060. [Google Scholar] [CrossRef]
- Arabczyk, W.; Jasińska, I.; Lendzion-Bieluń, Z. Kinetics studies of recrystallization process of metallic catalysts for ammonia synthesis. Catal. Today 2011, 169, 93–96. [Google Scholar] [CrossRef]
- Nielsen, A. (Ed.) Ammonia Catalysis and Manufacture; Springer: Berlin, Germany, 1995. [Google Scholar]
- Arabczyk, W.; Pelka, R.; Jasińska, I. Extended Surface of Materials as a Result of Chemical Equilibrium. J. Nanomater. 2014, 2014, 159. [Google Scholar] [CrossRef]
- Janecki, Z.; Gołębiowski, A.; Kałucki, K.; Arabczyk, W.; Szmidt-Szałowski, K.; Kowalczyk, Z.; Śpiewak, Z.; Ludwiczak, S. The state of research on catalysts for ammonia synthesis. Przem. Chem. 1988, 67, 479. [Google Scholar]
- Humphreys, F.J.; Hatherly, M. Recrystallization and Related Annealing Phenomena; Pergamon Press: Oxford, UK, 2017. [Google Scholar]
- Barański, A.; Dziembaj, R.; Kotarba, A.; Łagan, J.M.; Łojewska, J.; Pieprzyk, E.; Mleczko, L.; Baerns, M. A new approach to kinetic study of wet atmosphere activation of fused iron catalyst. Appl. Catal. A 1997, 162, 133–148. [Google Scholar] [CrossRef]
- Jasińska, I.; Arabczyk, W. Kinetic Studies of the Recrystallization Process of Iron Catalyst for Ammonia Synthesis. Chem. Pap. 2005, 59, 496–499. [Google Scholar]
- Lendzion-Bieluń, Z.; Gleń, M. Studies on the recrystallization of nanocrystalline metals. Pol. J. Chem. Technol. 2007, 9, 5–7. [Google Scholar] [CrossRef]
- Kuznetsov, L.D.; Lachinov, S.S. The Effect Of Al2O3 And K2O Promoters on the activity of iron catalyst in the synthesis of ammonia. Zh. Fiz. Khim. 1959, 33, 2542. [Google Scholar]
- Boudart, M. Ammonia synthesis: The bellwether reaction in heterogeneous catalysis. Top. Catal. 1994, 1, 405–414. [Google Scholar] [CrossRef]
- Jennings, J.R. (Ed.) Catalytic Ammonia Synthesis, Fundamentals and Practice; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Schlögl, R. Handbook of Heterogeneous Catalysis; WILEY-VCH Verlag GmbH & Co., KgaA: Weinheim, Germany, 2008. [Google Scholar]
- Liu, H.Z. Ammonia Synthesis Catalysts: Innovation and Practice; World Sci Publishing Co., Ltd.: Singapore, 2013. [Google Scholar]
- Nørskov, J.K. From quantum physics to heterogeneous catalysis. Top. Catal. 1994, 1, 385–403. [Google Scholar] [CrossRef]
- Somorjai, G.A. Introduction to Surface Chemistry and Catalysis; John Wiley & Sons: New York, NY, USA, 1994. [Google Scholar]
- Ozaki, A.; Aika, K. Catalysis—Science and Technology; Anderson, J.R., Boudart, M., Eds.; Springer: Berlin, Germany, 1982; Volume 1. [Google Scholar]
- Van de Voorde, M.; Sels, B. (Eds.) Nanotechnology in Catalysis: Applications in the Chemical Industry, Energy Development, and Environment Protection; Wiley-VCH: Weinheim, Germany, 2017. [Google Scholar]
- Zhou, B.; Han, S.; Raja, R.; Somorjai, G.A. Nanotechnology in Catalysis; Springer: New York, NY, USA, 2007. [Google Scholar]
- Gleiter, H. Nanostructured materials: Basic concepts and microstructure. Acta Mater. 2000, 48, 1–29. [Google Scholar] [CrossRef]
- Ertl, G. Surface Science and Catalysis—Studies on the Mechanism of Ammonia Synthesis: The P. H. Emmett Award Address. Catal. Rev. 1980, 21, 201–223. [Google Scholar] [CrossRef]
- Ertl, G. Physical characterization of industrial catalysts: The mechanism of ammonia synthesis. Stud. Surf. Sci. Catal. 1989, 44, 315–320. [Google Scholar]
- Somorjai, G.A.; Materer, N. Surface structure in ammonia synthesis. Top. Catal. 1994, 1, 215–231. [Google Scholar] [CrossRef]
- Ertl, G. Elementary Steps in Ammonia Synthesis. In Catalytic Ammonia Synthesis, Fundamentals and Practice; Jennings, J.R., Ed.; Plenum Press: New York, NY, USA, 1991. [Google Scholar]
- Paal, Z.; Ertl, G.; Lee, S.B. Interactions of potassium, oxygen and nitrogen with polycrystalline iron surfaces. Appl. Surf. Sci. 1981, 8, 231–249. [Google Scholar] [CrossRef]
- Aika, K.; Tamaru, K. Ammonia Synthesis over Non-Iron Catalysts and Related Phenomena, Ammonia Catalysis and Manufacture; Nielsen, A., Ed.; Springer: Berlin, Germany, 1995. [Google Scholar]
- Ertl, G. Reactions at Solid Surfaces; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2009. [Google Scholar]
- Arabczyk, W.; Müssig, H.J. Nitrogen bonding states on iron studied by PES and AES. Vacuum 1987, 37, 137–140. [Google Scholar] [CrossRef]
- Schlögl, R.; Schoonmaker, R.C.; Muhler, M.; Ertl, G. Bridging the “material gap” between single crystal studies and real catalysis. Catal. Lett. 1988, 1, 237–241. [Google Scholar] [CrossRef]
- Strongin, D.R.; Bare, S.R.; Somorjai, G.A. The effects of aluminum oxide in restructuring iron single crystal surfaces for ammonia synthesis. J. Catal. 1987, 103, 289–301. [Google Scholar] [CrossRef]
- Strongin, D.R.; Somorjai, G.A. Ammonia-pretreatment-induced restructuring of iron single-crystal surfaces: Its effects on ammonia synthesis and on coadsorbed aluminum oxide and potassium. J. Catal. 1989, 118, 99–110. [Google Scholar] [CrossRef]
- Arabczyk, W.; Narkiewicz, U.; Kałucki, K. Model of active surface of iron catalyst for ammonia synthesis. Vacuum 1994, 45, 267–269. [Google Scholar] [CrossRef]
- Arabczyk, W.; Narkiewicz, U. Growth of iron oxides on the Fe(111) surface precovered with sulphur and/or potassium. Appl. Surf. Sci. 1997, 108, 379–384. [Google Scholar] [CrossRef]
- Arabczyk, W.; Kałucki, K. New model of deactivation of iron catalysts for ammonia-synthesis. Stud. Surf. Sci. Catal. 1993, 75, 2539–2542. [Google Scholar] [CrossRef]
- Arabczyk, W. Untersuchung der Segregation Nichtmetallischer Elemente und Ihres Einflusses auf die Oxydation der Eisen-(111) Oberflache mit Hilfe Elektronspektrometrischer Methoden; Technische Universität Dresden: Dresden, Germany, 1987. [Google Scholar]
- Arabczyk, W.; Narkiewicz, U.; Moszyński, D. Double-Layer Model of the Fused Iron Catalyst for Ammonia Synthesis. Langmuir 1999, 15, 5785–5789. [Google Scholar] [CrossRef]
- Arabczyk, W.; Jasińska, I.; Lubkowski, K. The surface properties of iron catalyst for ammonia synthesis. React. Kinet. Catal. Lett. 2004, 83, 385–392. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z.; Arabczyk, W. Method for determination of the chemical composition of phases of the iron catalyst precursor for ammonia synthesis. Appl. Catal. A 2001, 207, 37–41. [Google Scholar] [CrossRef]
- Arabczyk, W.; Ekiert, E.; Pelka, R. Size-dependent transformation of α-Fe into γ′-Fe4N in nanocrystalline the Fe-NH3-H2 system. J. Phys. Chem. C 2016, 120, 17989–17995. [Google Scholar] [CrossRef]
- Arabczyk, W.; Ekiert, E.; Pelka, R. Hysteresis phenomenon in the reaction system of nanocrystalline iron with mixture of ammonia and hydrogen. Phys. Chem. Chem. Phys. 2016, 18, 25796–25800. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Wilk, B. Studies of phase transitions occurring in the system of nanocrystalline Fe/NH3/H2. Mater. Chem. Phys. 2019, 237, 121853. [Google Scholar] [CrossRef]
- Moszyńska, I.; Moszyński, D.; Arabczyk, W. Hysteresis in nitriding and reduction in the nanocrystalline iron-ammonia-hydrogen system. Przem. Chem. 2009, 88, 526–529. [Google Scholar]
- Wilk, B.; Arabczyk, W. Investigation of nitriding and reduction processes in a nanocrystalline iron–ammonia–hydrogen system at 350 °C. Phys. Chem. Chem. Phys. 2015, 17, 20185–20193. [Google Scholar] [CrossRef]
- Moszyński, D.; Moszyńska, I.; Arabczyk, W. Iron nitriding and reduction of iron nitrides in nanocrystalline Fe-N system. Mater. Lett. 2012, 78, 32–34. [Google Scholar] [CrossRef]
- Moszyński, D. Nitriding of nanocrystalline iron in the atmospheres with variable nitriding potential. J. Phys. Chem. C 2014, 118, 15440–15447. [Google Scholar] [CrossRef]
- Moszyński, D.; Moszyńska, I.; Arabczyk, W. The transformation of alpha-Fe into gamma’-Fe4N in nanocrystalline Fe-N system. Appl. Phys. Lett. 2013, 103, 253108. [Google Scholar] [CrossRef]
- Wilk, B.; Pelka, R.; Arabczyk, W. Study of the iron catalyst for ammonia synthesis by chemical potential programmed reaction method. J. Phys. Chem. C 2017, 121, 8548–8556. [Google Scholar] [CrossRef]
- Pelka, R.; Arabczyk, W. Studies of the Kinetics of Reaction Between Iron Catalysts and Ammonia—Nitriding of Nanocrystalline Iron with Parallel Catalytic Ammonia Decomposition. Top. Catal. 2009, 52, 1506–1516. [Google Scholar] [CrossRef]
- Pelka, R.; Kiełbasa, K.; Arabczyk, W. The effect of iron nanocrystallites’ size in catalysts for ammonia synthesis on nitriding reaction and catalytic ammonia decomposition. Centr. Eur. J. Chem. 2011, 9, 240–244. [Google Scholar] [CrossRef]
- Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z. Reaction model taking into account the catalyst morphology and its active specific surface in the process of catalytic ammonia decomposition. Materials 2021, 14, 7229. [Google Scholar] [CrossRef] [PubMed]
- Pelka, R. A method of determining nanoparticle size distribution in iron ammonia synthesis catalyst by measuring mass changes during the nitriding process. Catal. Today 2017, 286, 118–123. [Google Scholar] [CrossRef]
- Pelka, R.; Arabczyk, W. A new method for determining the nanocrystallite size distribution in systems where chemical reaction between solid and a gas phase occurs. J. Nanomater. 2013, 2013, 645050. [Google Scholar] [CrossRef]
- Rogowski, J. TOF-SIMS study of morphology and chemical composition of wustite-based precursor and iron catalyst for ammonia synthesis. Appl. Surf. Sci. 2019, 469, 82–89. [Google Scholar] [CrossRef]
- Arabczyk, W.; Narkiewicz, U.; Moszyński, D. Influence of potassium/oxygen layer on properties of iron surfaces. Appl. Catal. 1999, 182, 379–384. [Google Scholar] [CrossRef]
- Spencer, M.J.S.; Hung, A.; Snook, I.K.; Yarovsky, I. Density Functional Theory Study of the Relaxation and Energy of Iron Surfaces. Surf. Sci. 2002, 513, 389–398. [Google Scholar] [CrossRef]
- Tyson, W.R.; Miller, W.A. Surface Free Energies of Solid Metals. Estimation from Liquid Surface Tension Measurements. Surf. Sci. 1977, 62, 267–276. [Google Scholar] [CrossRef]
- Lendzion-Bieluń, Z. A comparison of the distribution of promoters in reduced and oxidized form of iron catalyst for ammonia synthesis. Pol. J. Chem. 2007, 81, 433–440. [Google Scholar]
- Jasińska, I.; Lubkowski, K.; Arabczyk, W. The surface properties of iron catalyst for ammonia synthesis. Ann. Pol. Chem. Soc. 2003, 2, 1205–1209. [Google Scholar]
- Arabczyk, W.; Jasińska, I.; Pelka, R. Measurements of the relative number of active sites on iron catalyst for ammonia synthesis by hydrogen desorption. Catal. Today 2011, 169, 97–101. [Google Scholar] [CrossRef]
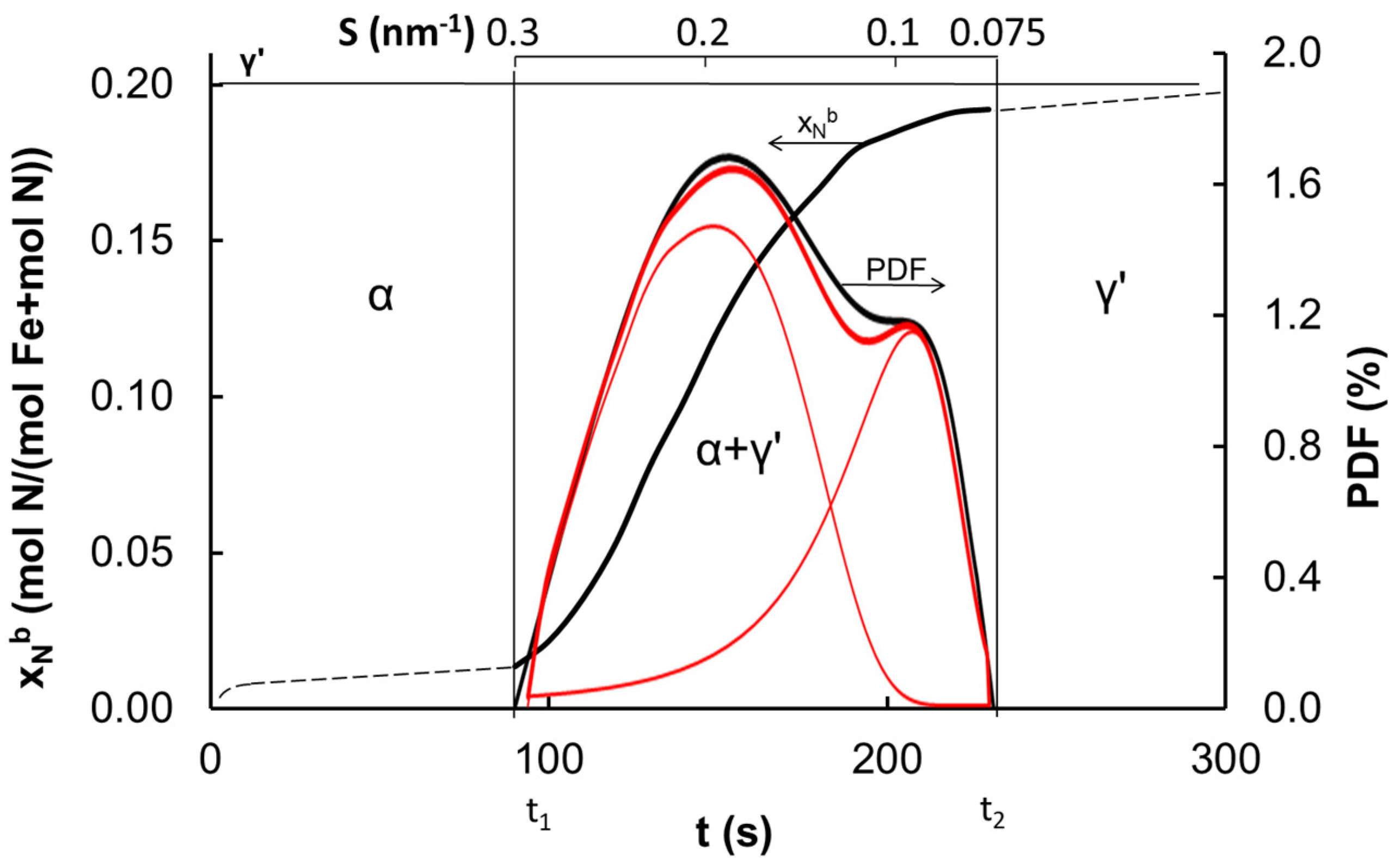
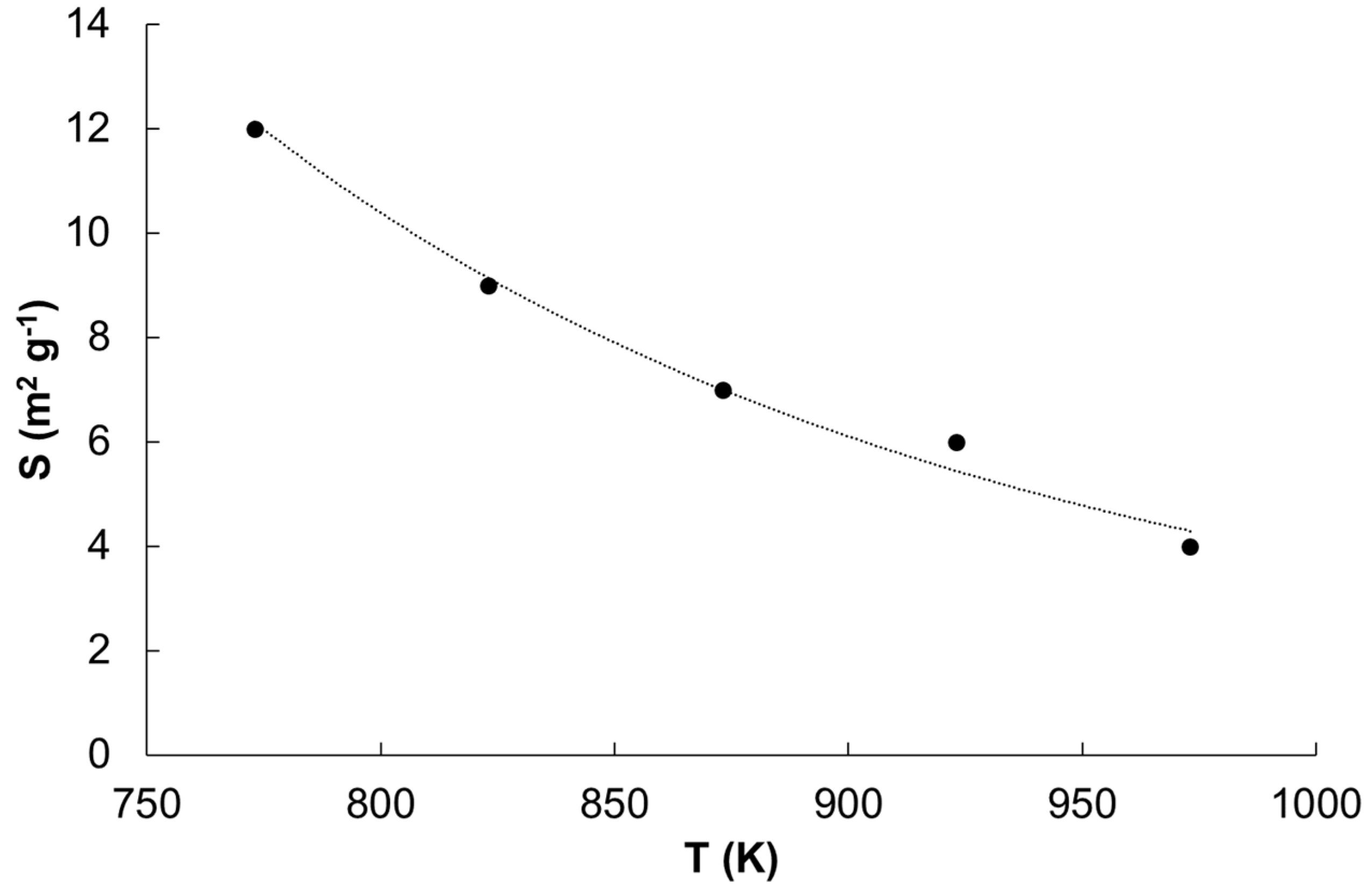

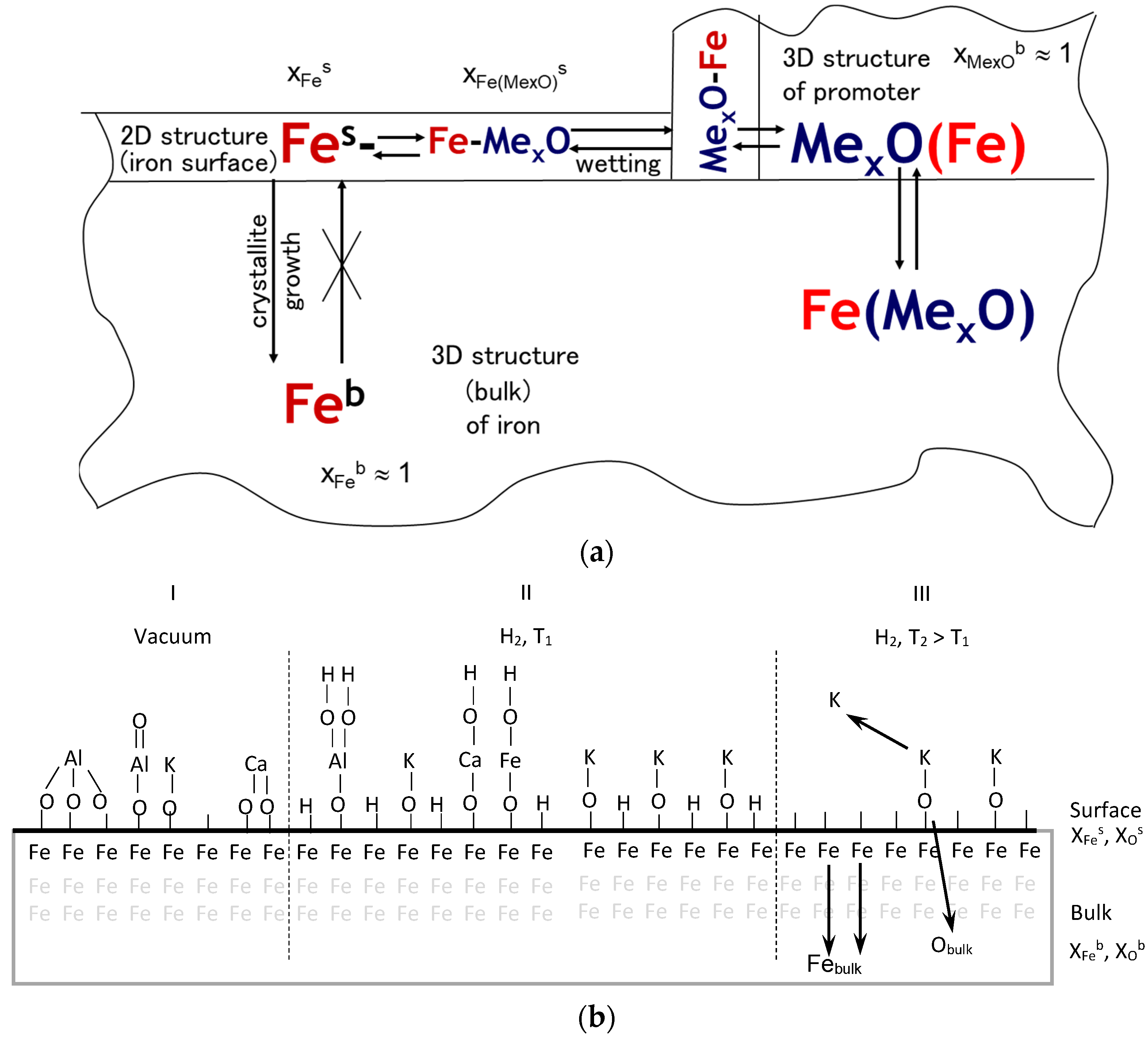
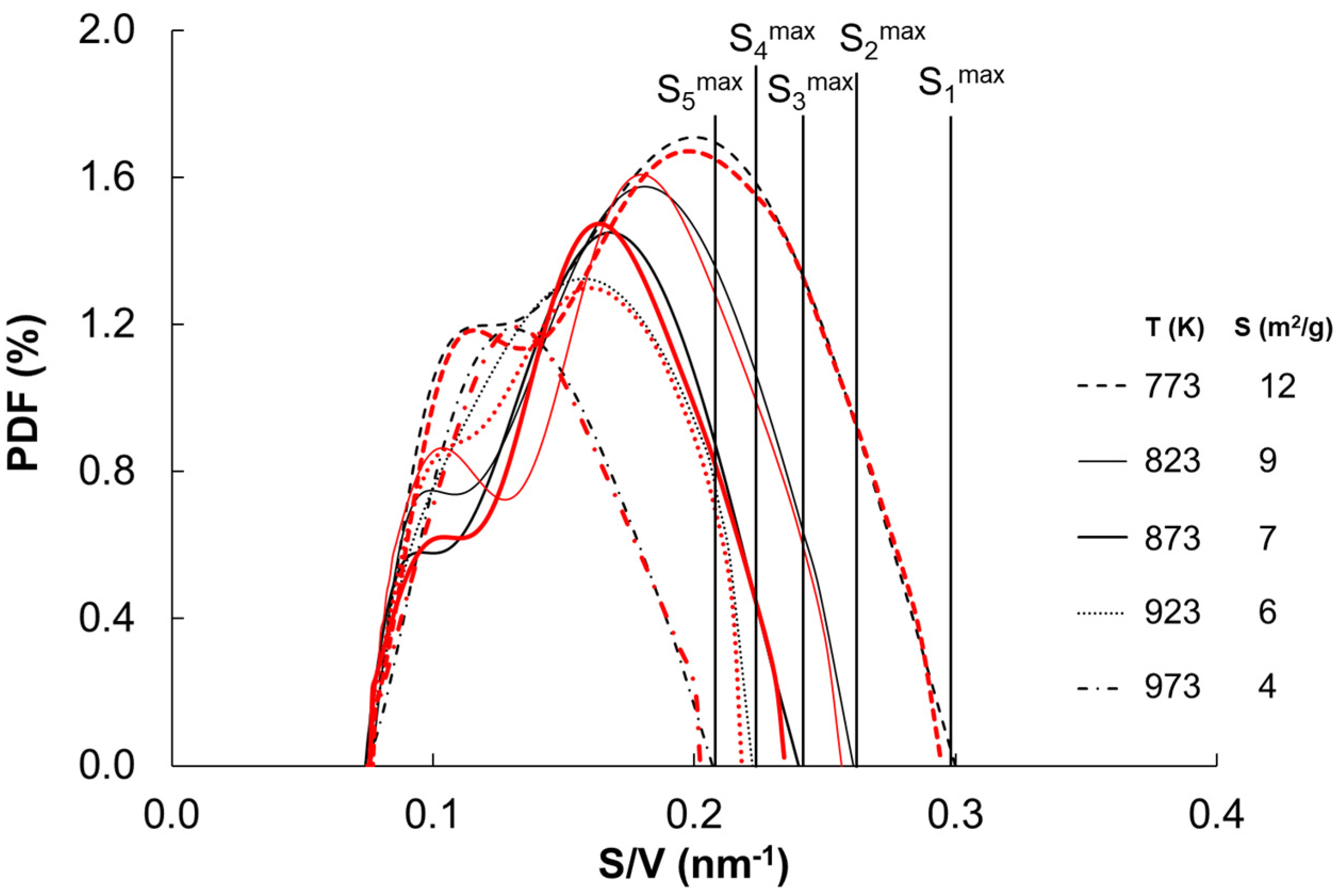
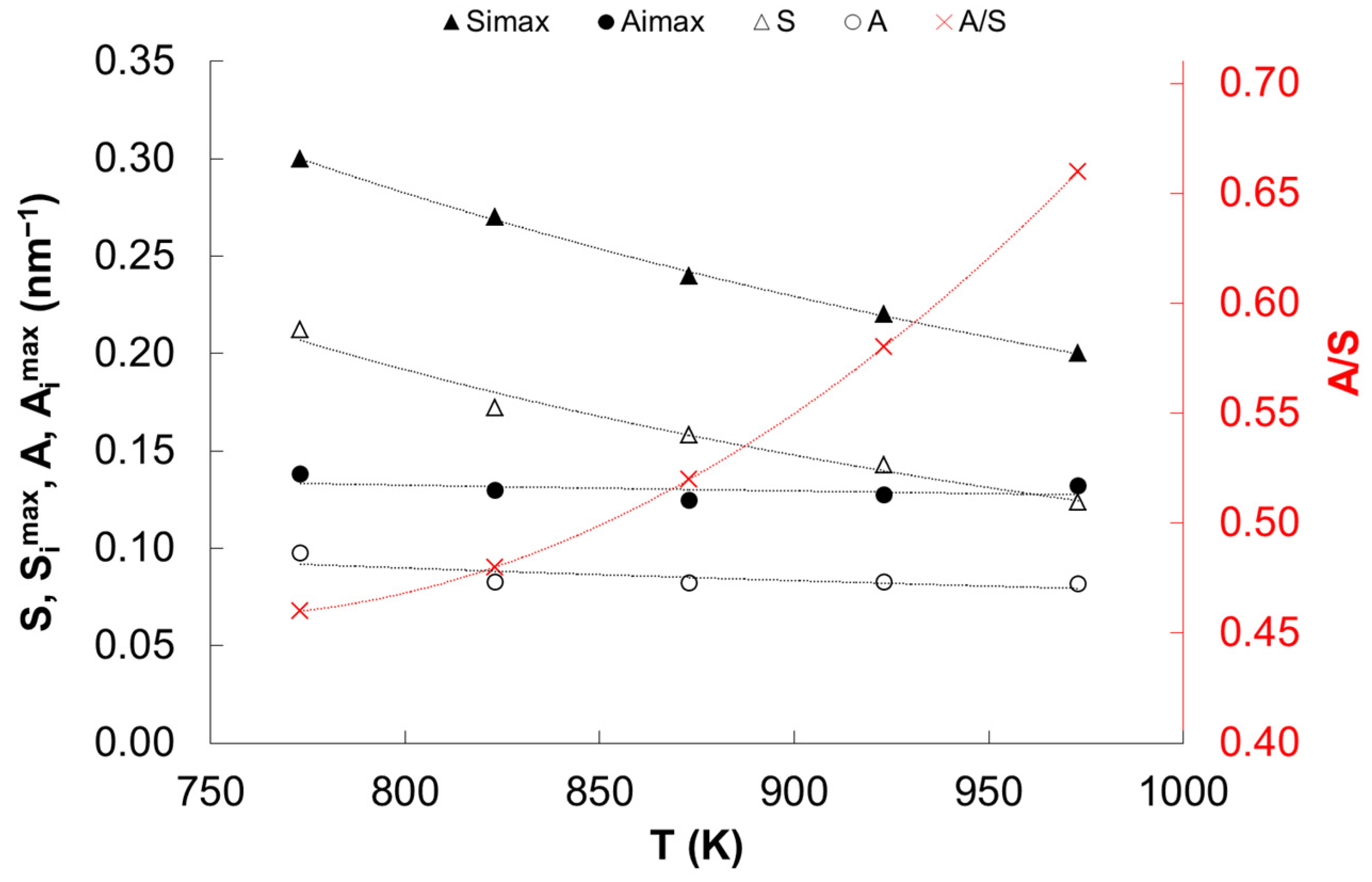
| Temperature (K) | Specific Surface Area (m2/g) | Average Size of Iron Nanocrystallites (nm) |
|---|---|---|
| 773 | 12.9 | 17 |
| 813 | 8.8 | 23 |
| 843 | 6.4 | 27 |
| 933 | 5.9 | 35 |
| 993 | 3.2 | 43 |
| Substance | Enthalpy of Formation, ΔHform (kJ/mol) | Enthalpy of Formation with Respect to One Me-O Bond, ΔH*form (kJ/mol) | Wetting Enthalpy ΔHwet + ΔHFe(MexO)s (kJ/mol) |
|---|---|---|---|
| K2O | −363 | −182 | −50 |
| Fe3O4 | −1118 | −140 | 0 |
| Al2O3 | −1676 | −280 | 232 |
| CaO | −635 | −317 | 269 |
| Temperature | ΔG(Simax) | ΔG(S) | ΔH(Simax) | ΔH(S) | ΔS(Simax) | ΔS(S) |
|---|---|---|---|---|---|---|
| (K) | (kJ mol–1) | (kJ mol–1 K–1) | ||||
| 773 | −55 | −28 | 0.7 | 33.4 | 0.072 | 0.078 |
| 823 | −58 | −30 | ||||
| 873 | −60 | −33 | ||||
| 923 | −67 | −37 | ||||
| 973 | −71 | −45 | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arabczyk, W.; Pelka, R.; Jasińska, I.; Lendzion-Bieluń, Z. Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering. Crystals 2024, 14, 188. https://doi.org/10.3390/cryst14020188
Arabczyk W, Pelka R, Jasińska I, Lendzion-Bieluń Z. Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering. Crystals. 2024; 14(2):188. https://doi.org/10.3390/cryst14020188
Chicago/Turabian StyleArabczyk, Walerian, Rafał Pelka, Izabella Jasińska, and Zofia Lendzion-Bieluń. 2024. "Thermodynamics of Iron Ammonia Synthesis Catalyst Sintering" Crystals 14, no. 2: 188. https://doi.org/10.3390/cryst14020188





