New Data on Crystal Phases in the System MgSO4–OC(NH2)2–H2O
Abstract
:1. Introduction
2. Materials and Methods
2.1. Syntheses Conditions
2.2. Syntheses Approaches
2.3. Powder X-ray Diffraction (PXRD)
2.4. Single Crystal X-ray Diffraction
2.5. Thermal Analyses
2.6. IR and Raman Spectroscopy
3. Results and Discussion
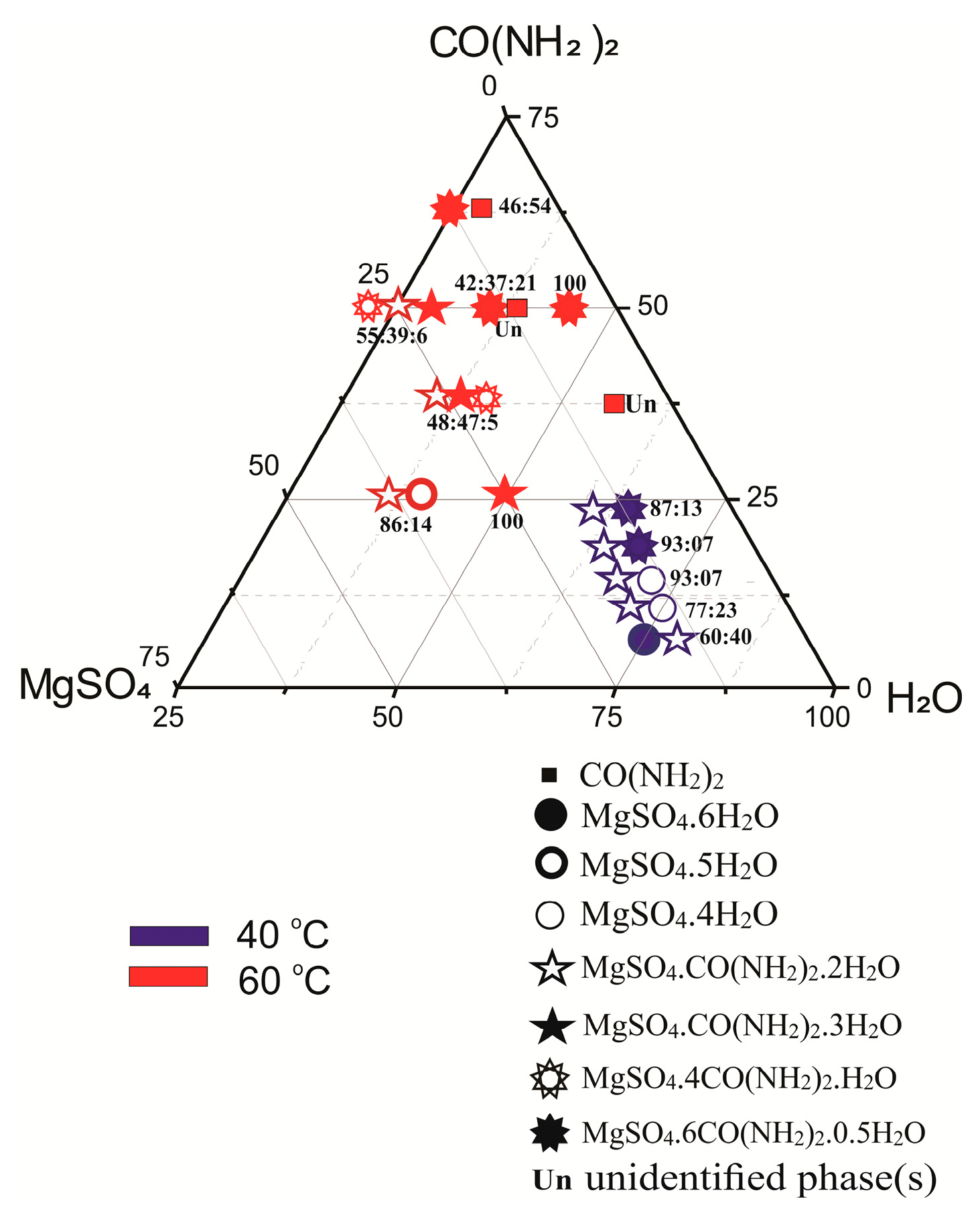
3.1. Synthesis of Crystal Phases in the System MgSO4–OC(NH2)2–H2O
- (i)
- nearly 100% MgSO4·6OC(NH2)2·0.5H2O—initial ratio MgSO4:OC(NH2)2:H2O~1:6:4 at 60 °C;
- (ii)
- nearly 100% MgSO4·OC(NH2)2·3H2O—initial ratio MgSO4:OC(NH2)2:H2O~1:1:2 at 60 °C;
- (iii)
- about 93% MgSO4·OC(NH2)2·2H2O—initial ratio MgSO4:OC(NH2)2:H2O~1:2:5 at 40 °C; and about 55% MgSO4·OC(NH2)2·H2O—initial ratio MgSO4:OC(NH2)2:H2O~1:2:1 at 60 °C.
- (i)
- MgSO4·OC(NH2)2·2H2O—MgSO4·7H2O:OC(NH2)2 = 1:1 at 80 °C;
- (ii)
- MgSO4·OC(NH2)2·3H2O—MgSO4·7H2O:OC(NH2)2 = 1:1 at 60 °C;
- (iii)
- MgSO4·4OC(NH2)2·H2O—MgSO4·7H2O:OC(NH2)2 = 1:4 at 60 °C;
- (iv)
- MgSO4·6OC(NH2)2·0.5H2O—MgSO4·7H2O:OC(NH2)2 = 1:6 at 60 °C.
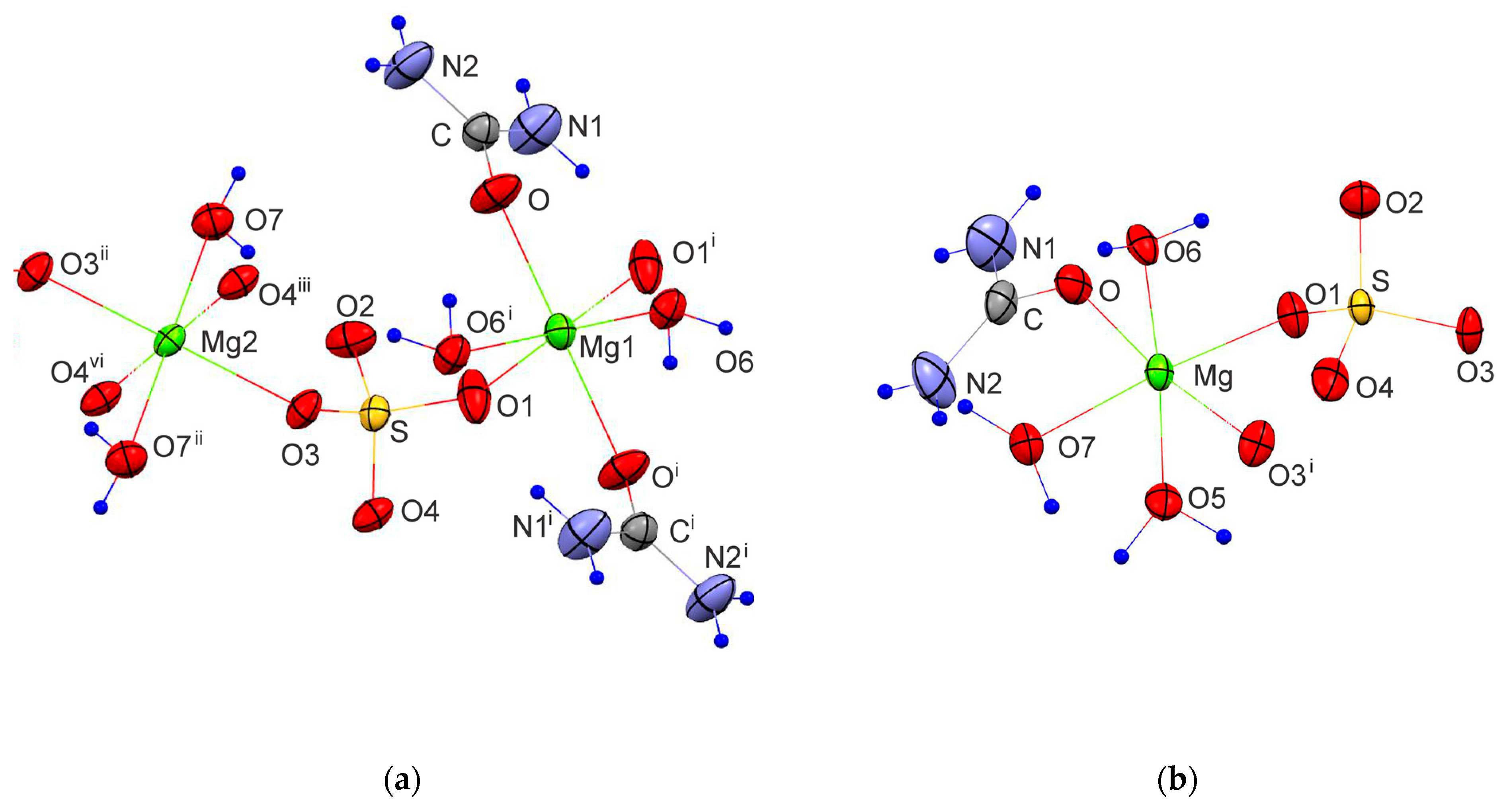
3.2. Single Crystal Analyses
3.3. Thermal Data
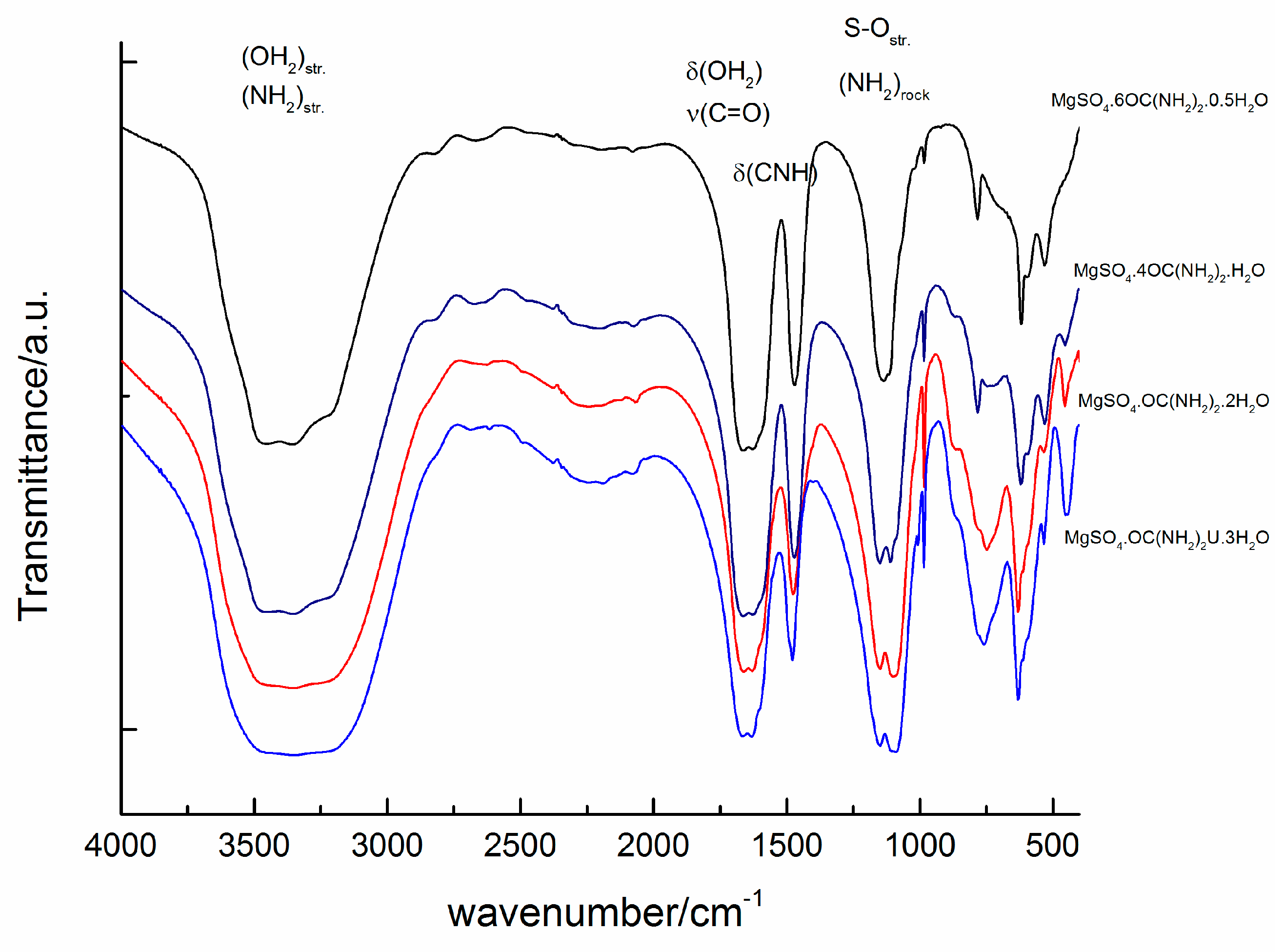
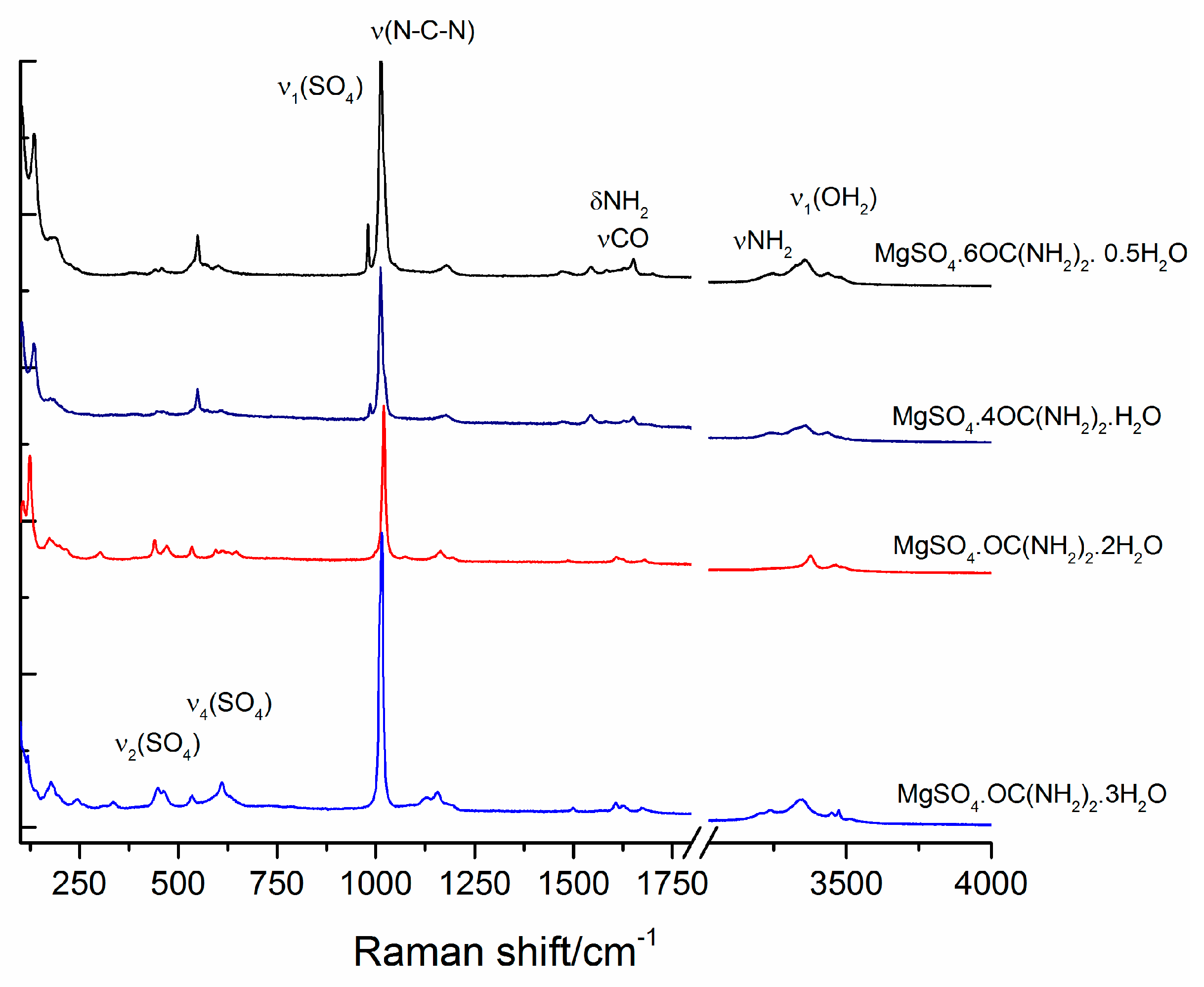
3.4. IR and Raman Spectra
4. Conclusions
- (i)
- Conditions for preparation of pure MgSO4·OC(NH2)2·2H2O, MgSO4·OC(NH2)2·3H2O, MgSO4·4OC(NH2)2·H2O and MgSO4·6OC(NH2)2·0.5H2O are specified (evaporation from dilute aqueous solutions) and of pure MgSO4·OC(NH2)2·3H2O and MgSO4·6OC(NH2)2·0.5H2O (upon mixing MgSO4·nH2O and urea in appropriate ratios and by applying mechanoactivation);
- (ii)
- The crystal structures of MgSO4·OC(NH2)2·2H2O and MgSO4·OC(NH2)2·3H2O are determined and their features are compared with previously published structures of MgSO4·4OC(NH2)2·H2O and MgSO4·6OC(NH2)2·0.5H2O;
- (iii)
- Thermal analysis establishes a direct relationship between the thermal stability of the studied compounds, as well as the decomposition temperature of urea and the OC(NH2)2:H2O ratio in the octahedral environment of the magnesium ion in the structures of the respective compounds;
- (iv)
- Certain spectroscopic characteristics (IR and Raman) of the studied compounds are reported for the first time and the results have been analysed with respect to the specific properties of the chemical composition.
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Patnaik, P. Handbook of Inorganic Chemicals, 1st ed.; The McGraw-Hill: New York, NY, USA, 2003; pp. 510–515. [Google Scholar]
- Bowen, H.J.M. Trace Elements in Biochemistry, 1st ed.; Academic Press: New York, NY, USA, 1996; pp. 241–250. [Google Scholar]
- Rogolino, D.; Carcelli, M.; Sechi, M.; Neamati, N. Viral enzymes containing magnesium: Metal binding as a successful strategy in drug design. Coord. Chem. Rev. 2012, 256, 3063–3086. [Google Scholar] [CrossRef]
- Seelig, M.S. Magnesium Deficiency in the Pathogenesis of Disease: Early Roots of Cardiovascular, Skeletal, and Renal Abnormalities, 1st ed.; Springer Science & Business Media: New York, NY, USA, 2012; pp. 3–20. [Google Scholar]
- Hsiao, C.; Tannenbaum, E.; VanDeusen, H.; Hershkovitz, E.; Perng, G.; Tannenbaum, A.; Williams, L.D. Chapter 1: Complexes of Nucleic Acids with Group I and Group II Cations. In Nucleic Acid-Metal Ion Interactions, 1st ed.; The RSC Publishihg: London, UK, 2008; pp. 1–38. [Google Scholar]
- Sanchez, P.A. Soil fertility and hunger in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef]
- Hawkesford, M.J.; Çakmak, İ.; Coskun, D.; De Kok, L.J.; Lambers, H.; Schjoerring, J.K.; White, P.J. Chapter 6: Functions of macronutrients. In Marschner’s Mineral Nutrition of Plants, 4th ed.; Academic Press: London, UK, 2023; pp. 201–281. [Google Scholar]
- Asa’ad, I.A. Magnesium and Drug Interactions. Iraqi Natl. J. Nurs. Spec. 2011, 24, 25–36. [Google Scholar]
- Remick, K.A.; Helmann, J.D. The elements of life: A biocentric tour of the periodic table. In Advances in Microbial Physiology, 1st ed.; Academic Press: London, UK, 2023; Volume 82, pp. 1–127. [Google Scholar]
- Hennings, E.; Schmidt, H.; Voigt, W. Crystal structures of hydrates of simple inorganic salts. I. Water-rich magnesium halide hydrates MgCl2·8H2O, MgCl2·12H2O, MgBr2·6H2O, MgBr2·9H2O, MgI2·8H2O and MgI2·9H2O. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2013, 69, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Rusev, R.; Tsvetanova, L.; Shivachev, B.; Kossev, K.; Nikolova, R. Ureates and hydrates of magnesium chloride, nitrate and tetrafluoroborate. Bulg. Chem. Commun. 2018, 50, 79–89. [Google Scholar]
- Kosev, K.; Petrova, N.; Georgieva, I.; Titorenkova, R.; Nikolova, R. Crystalline adducts of urea with magnesium iodide. J. Mol. Struct. 2021, 1224, 129009. [Google Scholar] [CrossRef]
- Lebioda, Ł.; Stadnicka, K.; Sliwinski, J. Hexakis (urea) magnesium bromide–urea (1/4). Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1979, 35, 157–158. [Google Scholar] [CrossRef]
- Lebioda, Ł.; Lewiński, K. Structure of diaquatetrakis (urea) magnesium bromide. Acta Crystallogr. Sect. B Struct. Crystallogr. Cryst. Chem. 1980, 36, 693–695. [Google Scholar] [CrossRef]
- Kossev, K.; Tsvetanova, L.; Dimowa, L.; Nikolova, R.; Shivachev, B. Synthesis and crystal structure of magnesium chlorate dihydrate and magnesium chlorate hexahydrate. Bulg. Chem. Commun. 2013, 45, 543–548. [Google Scholar]
- Todorov, T.; Petrova, R.; Kossev, K.; Macícek, J.; Angelova, O. Hexakis (urea-O) magnesium Dichlorate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 927–929. [Google Scholar] [CrossRef]
- Yamagata, K.; Achiwa, N.; Hashimoto, M.; Koyano, N.; Iwata, Y.; Shibuya, I. Magnesium formate-urea (1/2). Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1992, 48, 793–795. [Google Scholar] [CrossRef]
- Hayden, T.D.; Kim, E.E.; Eriks, K. Crystal structures of bis (urea) bis(dihydrogenphosphato) calcium-bis (urea) and its isomorphous magnesium analog, M[OC(NH2)2]2(H2PO4)2.2CO(NH2)2 (M = Ca, Mg). Inorg. Chem. 1982, 21, 4054–4058. [Google Scholar] [CrossRef]
- Angelova, O.; Macíček, J.; Petrova, R.; Todorov, T.; Mihailova, B. Structure of molecular adducts of inorganic salts. IV. Comparison of the Cd (ReO4)2·2Urea and Cd (ReO4)2·2H2O structures 1. Z. Für Krist. -Cryst. Mater. 1996, 211, 163–169. [Google Scholar] [CrossRef]
- Georgieva, I.; Kossev, K.; Titorenkova, R.; Petrova, N.; Zahariev, T.; Nikolova, R. Effect of urea on arrangement of novel Mg (II) perrhenate crystal structures and their optical properties: Experimental and theoretical insight. J. Solid State Chem. 2022, 312, 123263. [Google Scholar] [CrossRef]
- Whittaker, C.W.; Lundstrom, F.O.; Shim, J.H. The system magnesium sulfate-urea-water at 30°. J. Am. Chem. Soc. 1936, 58, 1975–1977. [Google Scholar] [CrossRef]
- Yee, J.Y.; Davis, R.O.E.; Hendricks, S.B. Double Compounds of Urea with Magnesium Nitrate and Magnesium Sulfate. J. Am. Chem. Soc. 1937, 59, 570–571. [Google Scholar] [CrossRef]
- Sulaimankulov, K.; Druzhinin, I.G.; Bergman, A.G. Interaction of urea with sulfates of divalent metals. i. isotherms 0, 30 and 45° systems: Water-urea-magnesium sulfate. J. Inorg. Chem. 1957, 2, 2668. (In Russian) [Google Scholar]
- Sulaimankulov, K. Compounds of Urea with Inorganic Salts, 1st ed.; Ilim: Frunse, Russia, 1971; p. 224. (In Russian) [Google Scholar]
- Zhang, F.; Wei, X.; Guo, Z.; Shi, Q. A study on the isothermal solubility of MgSO4-CO(NH2)2-H2O ternary system at 25 °C. Chin. J. Inorg. Chem. 1997, 13, 375–379. [Google Scholar]
- Mahadevan, C.K. Thermal parameters of MgSO4·7H2O and NiSO4·7H2O crystals added with urea and thiourea. Phys. B Condens. Matter 2008, 403, 3164–3167. [Google Scholar] [CrossRef]
- Honer, K.; Kalfaoglu, E.; Pico, C.; McCann, J.; Baltrusaitis, J. Mechanosynthesis of magnesium and calcium salt–urea ionic cocrystal fertilizer materials for improved nitrogen management. ACS Sustain. Chem. Eng. 2017, 5, 8546–8550. [Google Scholar] [CrossRef]
- Honer, K.; Pico, C.; Baltrusaitis, J. Reactive mechanosynthesis of urea ionic cocrystal fertilizer materials from abundant low solubility magnesium-and calcium-containing minerals. ACS Sustain. Chem. Eng. 2018, 6, 4680–4687. [Google Scholar] [CrossRef]
- Adlim, M.; Ramayani, R.F.I.; Khaldun, I.; Muzdalifah, F.; Sufardi, S.; Rahmaddiansyah, R. Fertilizing Properties of Urea-Magnesium Slowrelease Fertilizer Made of Rice-Husk-Ash Natural-Rubber Chitosan Composite. Rasayan J. Chem. 2021, 14, 1851–1859. [Google Scholar] [CrossRef]
- Maynard-Casely, H.E.; Brand, H.E.; Wilson, S.; Wallwork, K.S. Mineral diversity on Europa: Exploration of phases formed in the MgSO4–H2SO4–H2O ternary. ACS Earth Space Chem. 2021, 5, 1716–1725. [Google Scholar] [CrossRef]
- Adassooriya, N.M.; Mahanta, S.P.; Thakuria, R. Mechanochemistry as an emerging tool for the preparation of sustained release urea cocrystals as a nitrogen source. CrystEngComm 2022, 24, 1679–1689. [Google Scholar] [CrossRef]
- Todorov, T.; Petrova, R.; Kossev, K.; Macicek, J.; Angelova, O. Magnesium sulfate tetraurea monohydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 456–458. [Google Scholar] [CrossRef]
- Todorov, T.; Petrova, R.; Kossev, K.; Macicek, J.; Angelova, O. Magnesium sulfate hexaurea hemihydrate. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 1998, 54, 1758–1760. [Google Scholar] [CrossRef]
- Degen, T.; Sadki, M.; Bron, E.; König, U.; Nénert, G. The HighScore suite. Powder Diffr. 2014, 29, S13–S18. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A Program for the Representation and Manipulation of Crystal Structures and Calculation of the Resulting X-Ray Powder Patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- Chukanov, N.V. Infrared spectra of mineral species, 1st ed.; Extended Library, Springer: Dordrecht, The Netherlands, 2014; pp. 21–1701. [Google Scholar]
- Buzgar, N.; Apopei, A.; Buzatu, A. Romanian Database of Raman Spectroscopy. Available online: http://rdrs.uaic.ro (accessed on 26 July 2023).
- Makreski, P.; Jovanovski, G.; Dimitrovska, S. Minerals from Macedonia: XIV. Identification of some sulfate minerals by vibrational (infrared and Raman) spectroscopy. Vib. Spectrosc. 2005, 39, 229–239. [Google Scholar] [CrossRef]
- Frost, R.; Kristof, J.; Rintoul, L.; Kloprogge, T. Raman spectroscopy of urea and urea-intercalated kaolinites at 77 K. Spectrochim. Acta Part A 2000, 56, 1681–1691. [Google Scholar] [CrossRef]



| Compound | Synthesis | Identification | Structural Data | References |
|---|---|---|---|---|
| MgSO4·OC(NH2)2·2H2O | Solution, MgSO4·7H2O + nUrea + mH2O/25 °C | Chem. analyses of obtained solid, IR | Powder diffraction pattern | [25,27] |
| MgSO4·OC(NH2)2·3H2O | Solution, MgSO4·7H2O + nUrea + mH2O/30 °C | Chem. analyses of obtained solid | Powder diffraction pattern | [21,23,24] |
| MgSO4·4OC(NH2)2·H2O | Solution, MgSO4·7H2O + 4Urea + mH2O/22 °C (RT) | Single crystal and powder XRD Crystal structure determined | P21/n a = 9.345(2), b = 14.683(4), c = 11.244(3) β = 93.07(2) | [24,32] |
| MgSO4·6OC(NH2)2·0.5H2O | Mechanoactivation, MgSO4·7H2O + 6Urea/22 °C (RT) Solution, MgSO4·7H2O + 6Urea + mH2O/22 °C (RT) | Single crystal and powder XRD Crystal structure determined | Pnma a = 15.316(5), b = 19.798(5), c = 14.484(2) | [28,33] |
| MgSO4·5OC(NH2)2·2H2O | Solution, MgSO4·7H2O + nUrea + 95% methyl alcohol/30 °C | Single crystal and powder XRD Unit cell parameters only | XRD Pnma a = 17.32, b = 11.40, c = 9.61 | [22] |
| MgSO4·6OC(NH2)2·2H2O | Solution, MgSO4·7H2O + nUrea + 95% methyl alcohol/25 °C | Single crystal and powder XRD Unit cell parameters only | XRD Pccn a = 16.20, b = 19.97, c = 14.38 | [22] |
| Chemical Formula | Mg(SO4)·OC(NH2)2·2H2O | Mg(SO4)·OC(NH2)2·3H2O |
|---|---|---|
| Formula weight | 216.46 | 234.48 |
| Temperature (K) | 290(2) | 290(2) |
| Crystal system | Triclinic | Monoclinic |
| Space group | P-1 | P 21 |
| a (Å) | 5.2140(8) | 5.2452(11) |
| b (Å) | 7.7014(9) | 7.7080(14) |
| c (Å) | 10.4275(16) | 10.230(2) |
| α (°) | 68.758(13) | 90 |
| β (°) | 77.177(13) | 94.291(8) |
| γ (°) | 71.498(12) | 90 |
| Volume (Å3) | 367.38(10) | 412.43(14) |
| Z | 2 | 2 |
| ρcalc (g/cm3) | 1.957 | 1.888 |
| μ (mm−1) | 0.534 | 0.492 |
| Crystal size (mm3) | 0.03 × 0.02 × 0.02 | 0.03 × 0.03 × 0.01 |
| Radiation, λ (Å) | MoKα λ = 0.71073 | MoKα λ = 0.71073 |
| Θ range for data collection (°) | 2.111 to 25.345 | 3.313 to 26.004 |
| Limiting indices | −6 ≤ h ≤ 6, −9 ≤ k ≤ 9, −12 ≤ l ≤ 12 | −6 ≤ h ≤ 6, −9 ≤ k ≤ 9, −12 ≤ l ≤ 12 |
| Reflections collected /unique | 10667/1347 (R(int) = 0.0564) | 6912/1618 (R(int) = 0.0608) |
| Data/restraints/parameters | 1347/0/112 | 1476/1/119 |
| Goodness-of-fit on F2 | 1.239 | 0.914 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0673, wR2 = 0.1474 | R1 = 0.0371 wR2 = 0.0994 |
| Final R indexes [all data] | R1 = 0.0762 wR2 = 0.1511 | R1 = 0.0379 wR2 = 0.1003 |
| Largest diff. peak/hole (e Å−3) | 0.870/−0.448 | 0.372/−0.590 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikolova, R.; Kostov-Kytin, V.; Petrova, N.; Kossev, K.; Titorenkova, R.; Velyanova, G. New Data on Crystal Phases in the System MgSO4–OC(NH2)2–H2O. Crystals 2024, 14, 227. https://doi.org/10.3390/cryst14030227
Nikolova R, Kostov-Kytin V, Petrova N, Kossev K, Titorenkova R, Velyanova G. New Data on Crystal Phases in the System MgSO4–OC(NH2)2–H2O. Crystals. 2024; 14(3):227. https://doi.org/10.3390/cryst14030227
Chicago/Turabian StyleNikolova, Rositsa, Vladislav Kostov-Kytin, Nadia Petrova, Krasimir Kossev, Rositsa Titorenkova, and Gergana Velyanova. 2024. "New Data on Crystal Phases in the System MgSO4–OC(NH2)2–H2O" Crystals 14, no. 3: 227. https://doi.org/10.3390/cryst14030227







