Electrochemical and Optical Properties of Magnesium-Alloy Hydrides Reviewed
Abstract
:1. Introduction


| Medium | Hydrogen Content | Volume Density* (H atoms l−1) | Energy Density** | |
|---|---|---|---|---|
| (wt. %) | (10−19) | MJ kg−1 | mJ I−1 | |
| H2 (g) (150 atm) | 100.00 | 0.5 | 141.90 | 1.02 |
| H2 (l) (−253°C) | 100.00 | 4.2 | 141.90 | 9.92 |
| MgH2 | 7.65 | 6.7 | 9.92 | 14.32 |
| VH2 | 2.10 | 11.4 | - | - |
| Mg2NiH4 | 3.60 | 5.9 | 4.48 | 11.49 |
| TiFeH1.95 | 1.95 | 5.5 | 2.47 | 13.56 |
| LaNi5H6.7 | 1.50 | 7.6 | 1.94 | 12.77 |
| ZrMn2H3.6 | 1.75 | 6.0 | - | - |
| ZrMn2Fe0.8H3.4 | 1.38 | 4.8 | - | - |
2. Gas Phase versus Electrochemical Hydrogen Storage


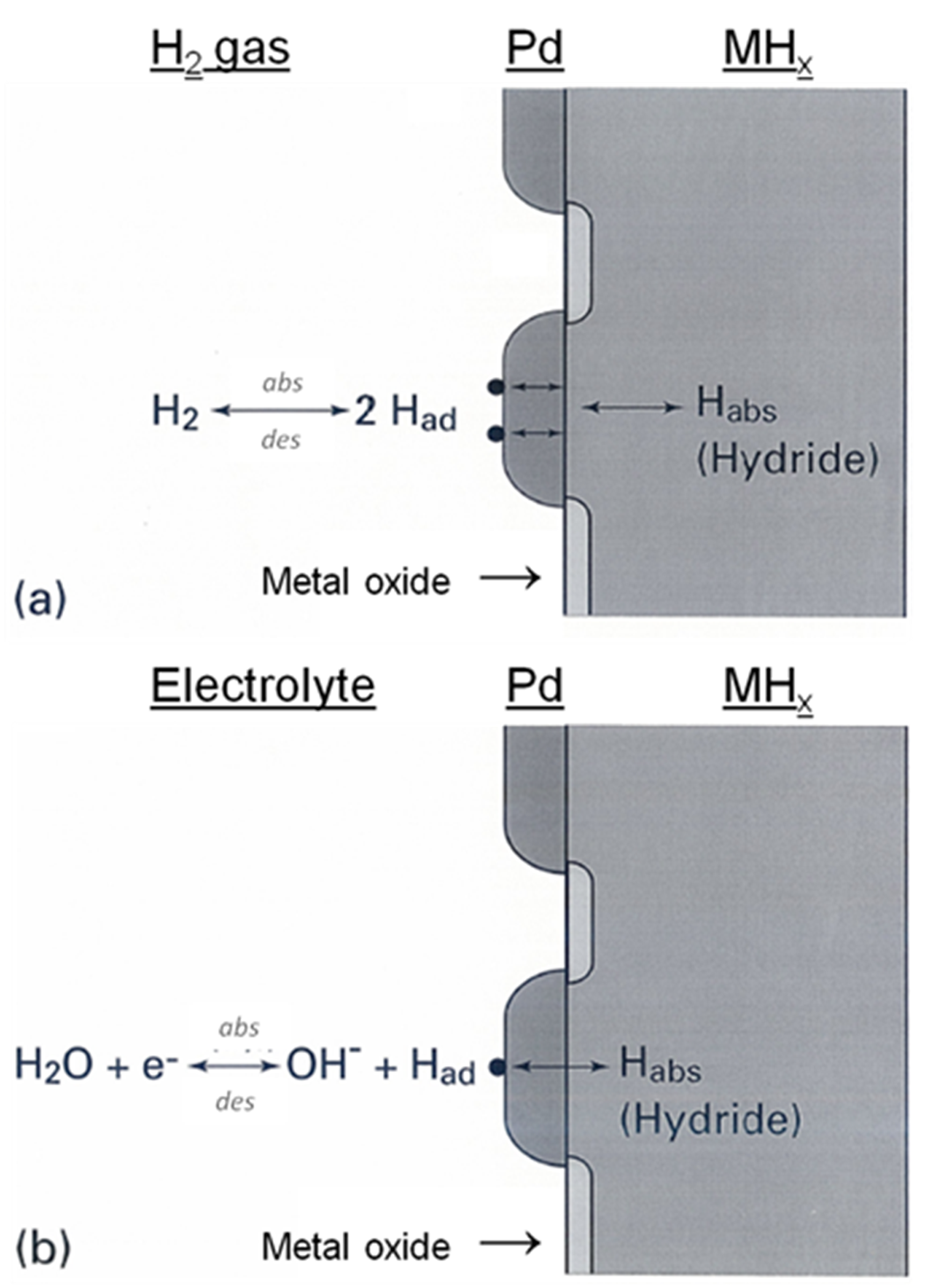

 and
and  are equilibrium and standard pressures, respectively,
are equilibrium and standard pressures, respectively,  is the standard enthalpy change,
is the standard enthalpy change,  the gas constant,
the gas constant,  the temperature and
the temperature and  is the entropy change. The operating temperatures of the metal hydrides are determined by the equilibrium pressure and the overall reaction kinetics.
is the entropy change. The operating temperatures of the metal hydrides are determined by the equilibrium pressure and the overall reaction kinetics.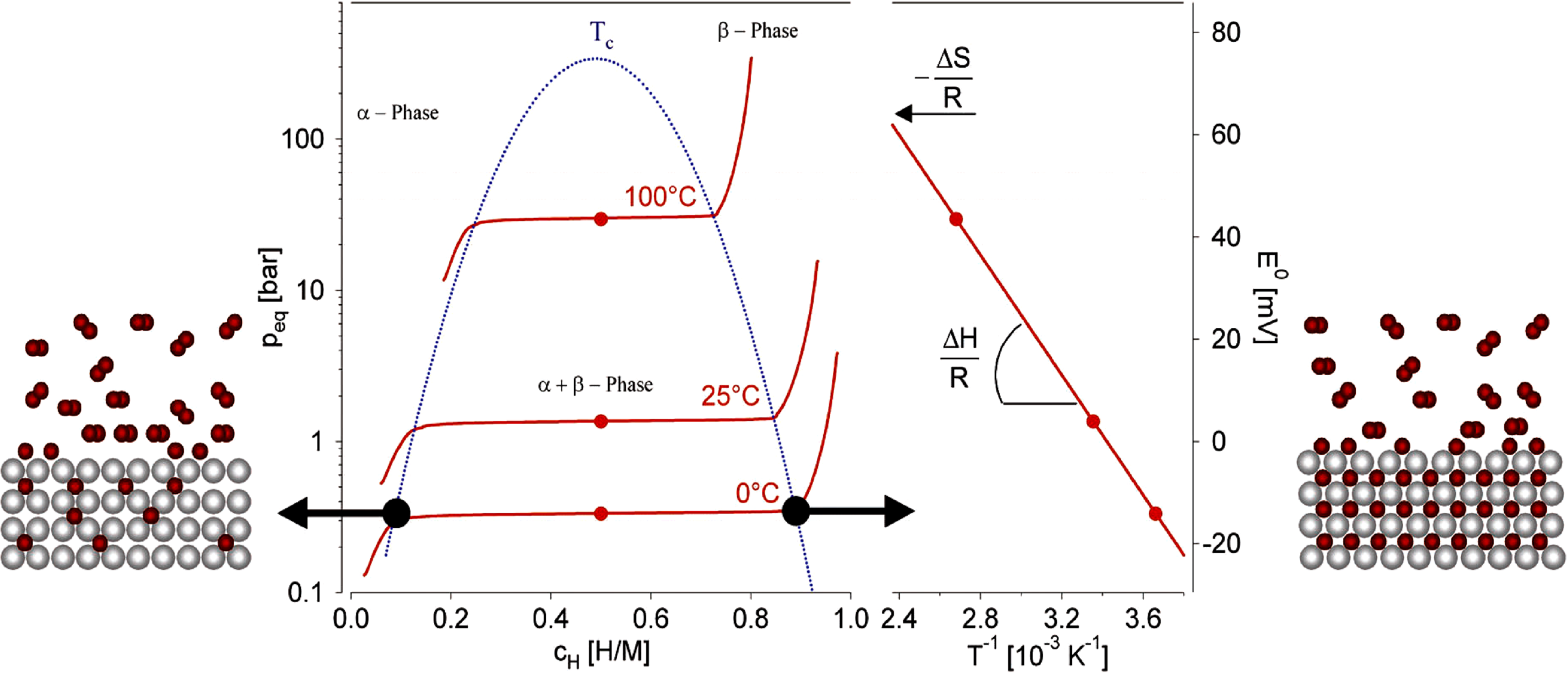




 ).
).
3. Applications of Metal-Hydrides
3.1. NiMH Battery
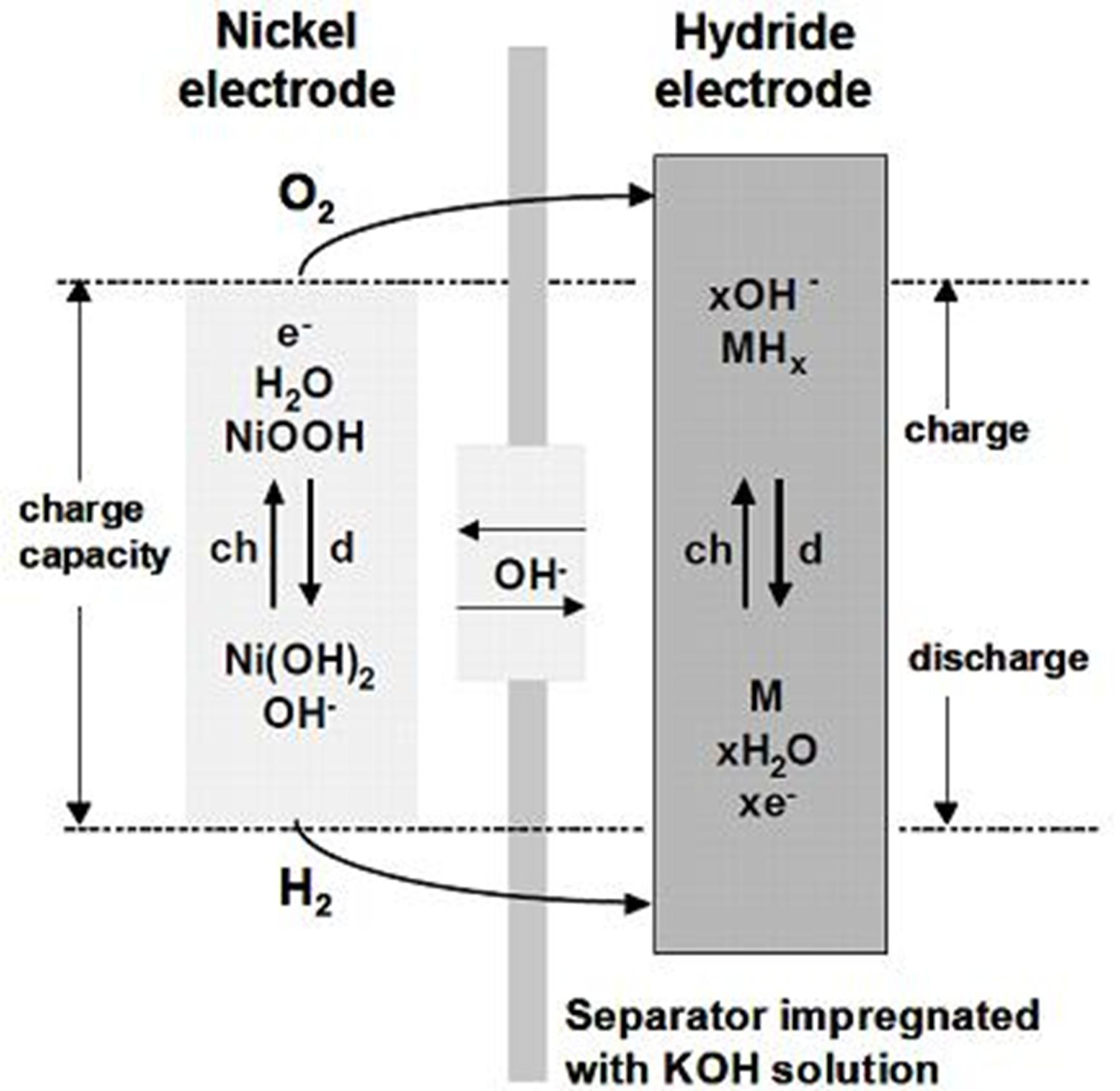


3.2. Electrochromic Applications

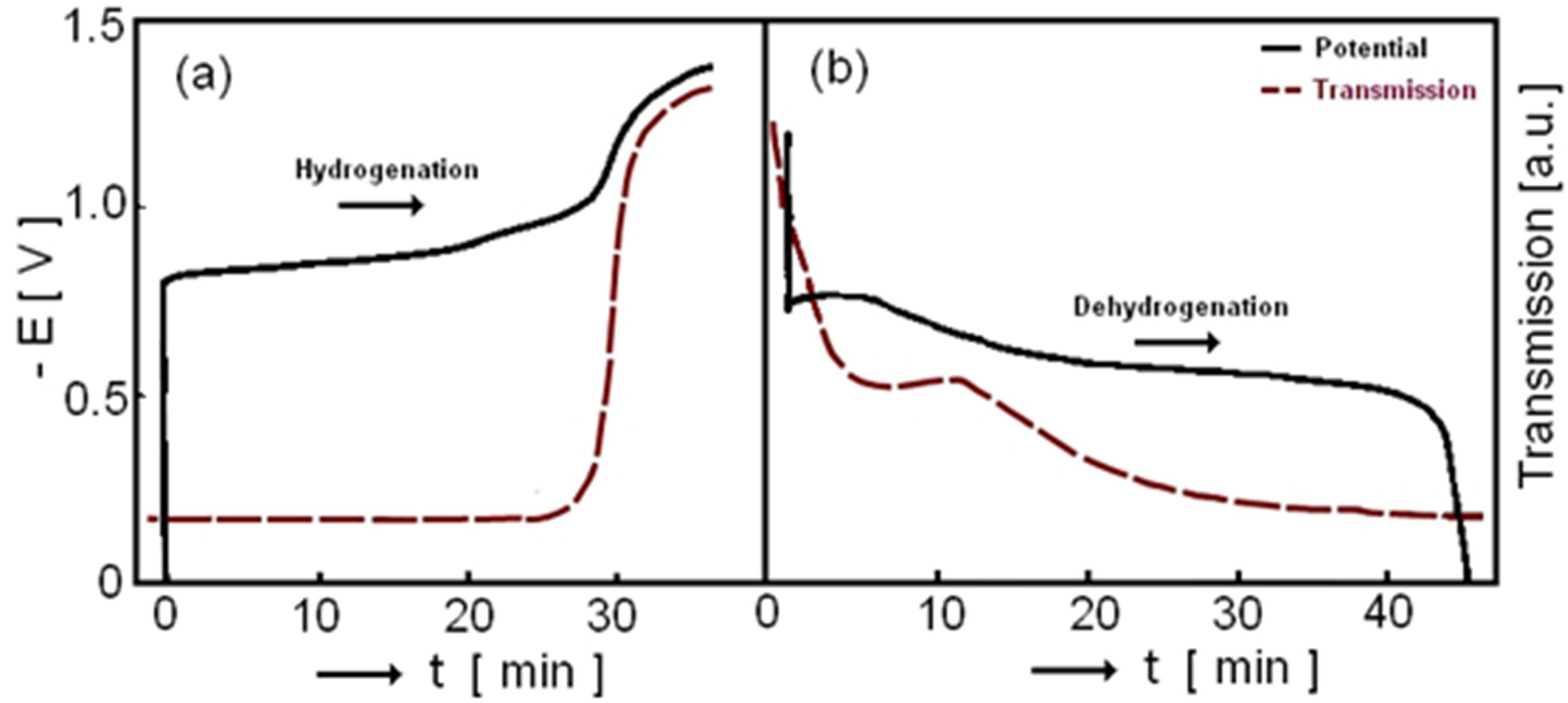
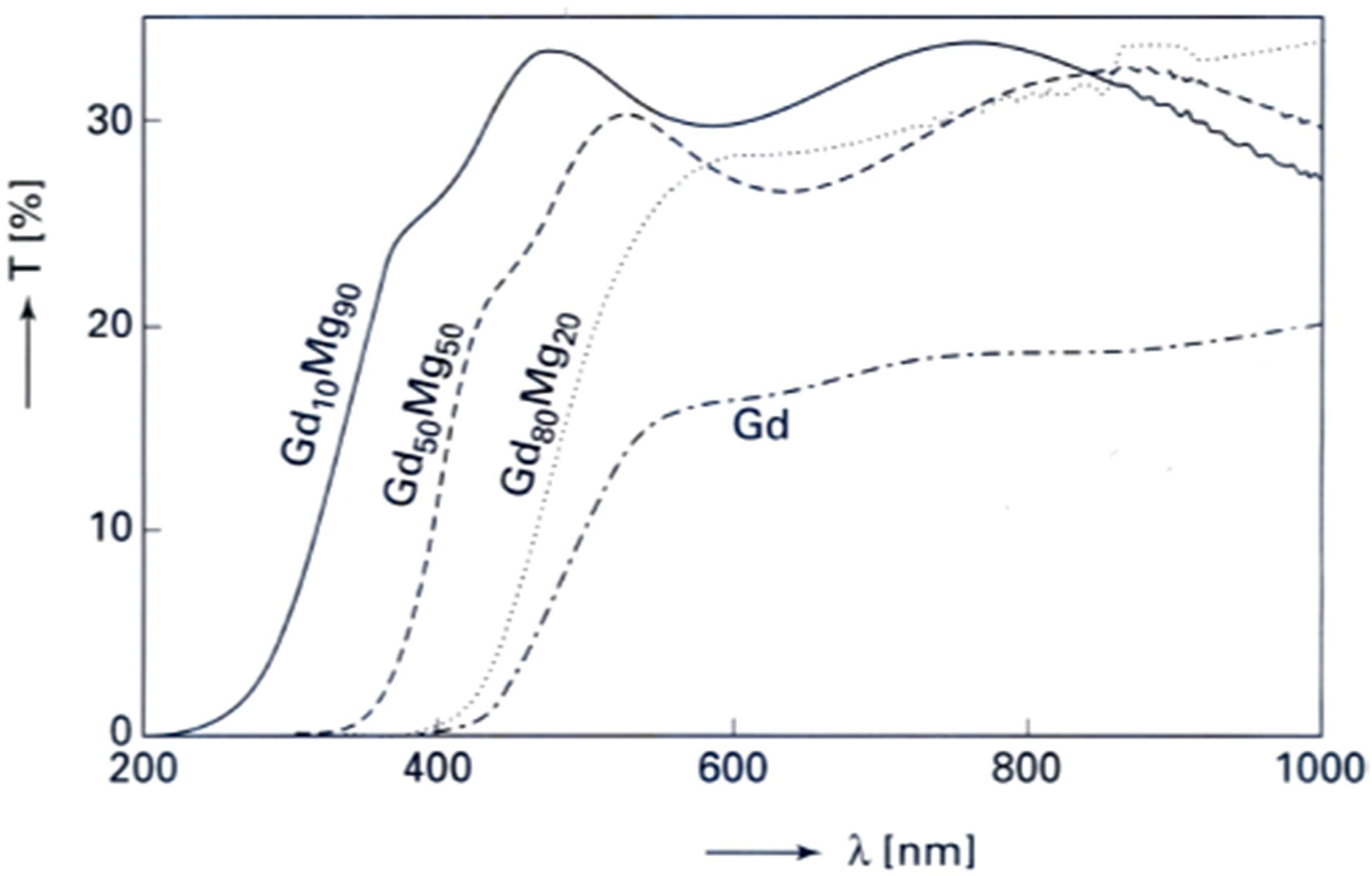
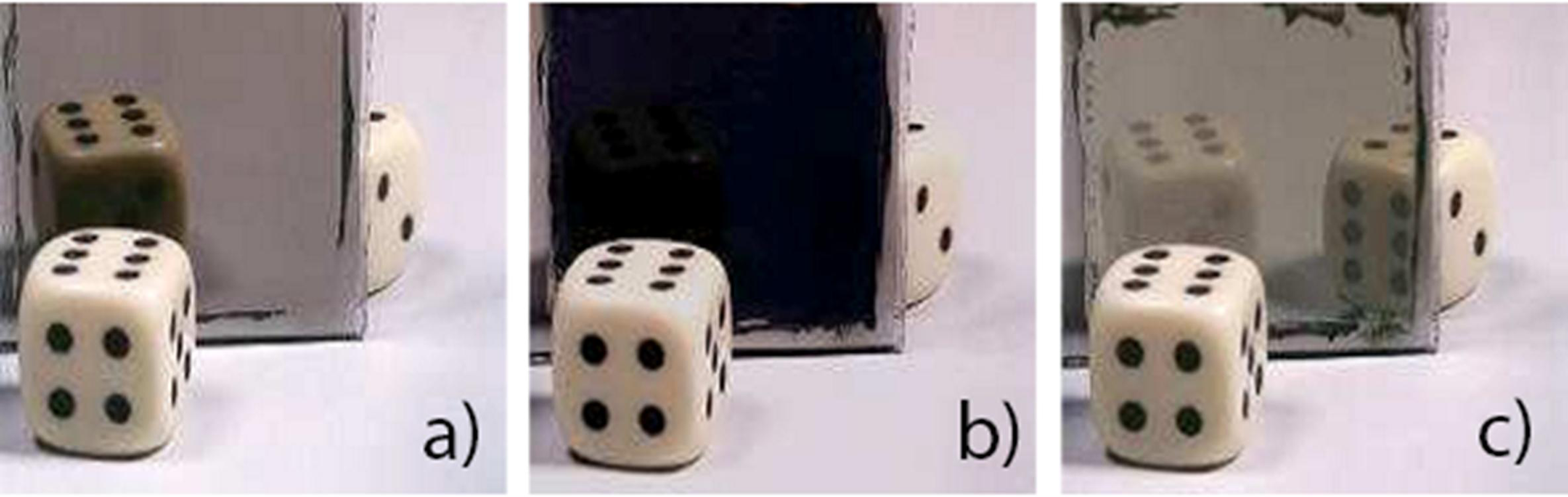
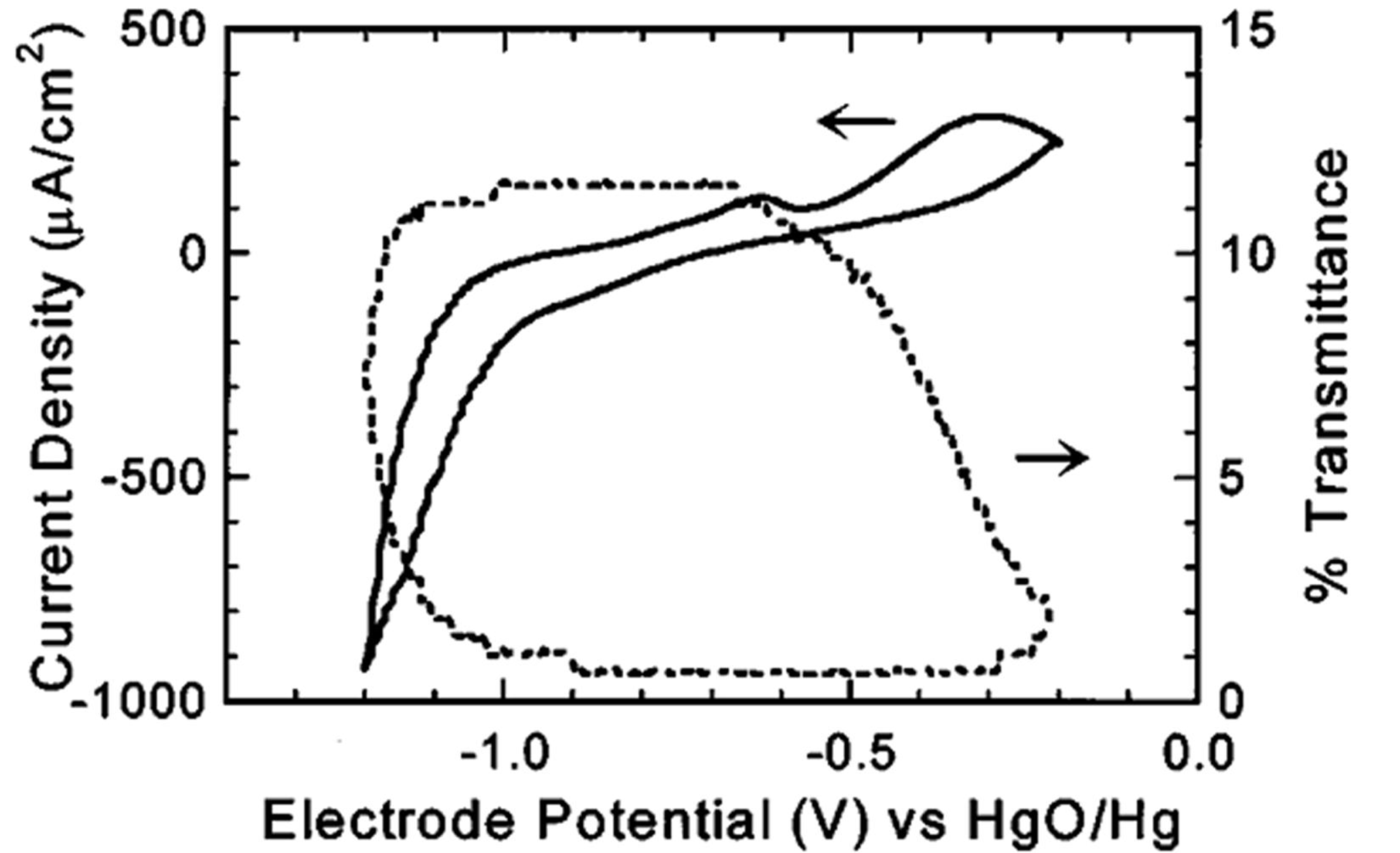

4. Magnesium-Based Binary Alloys
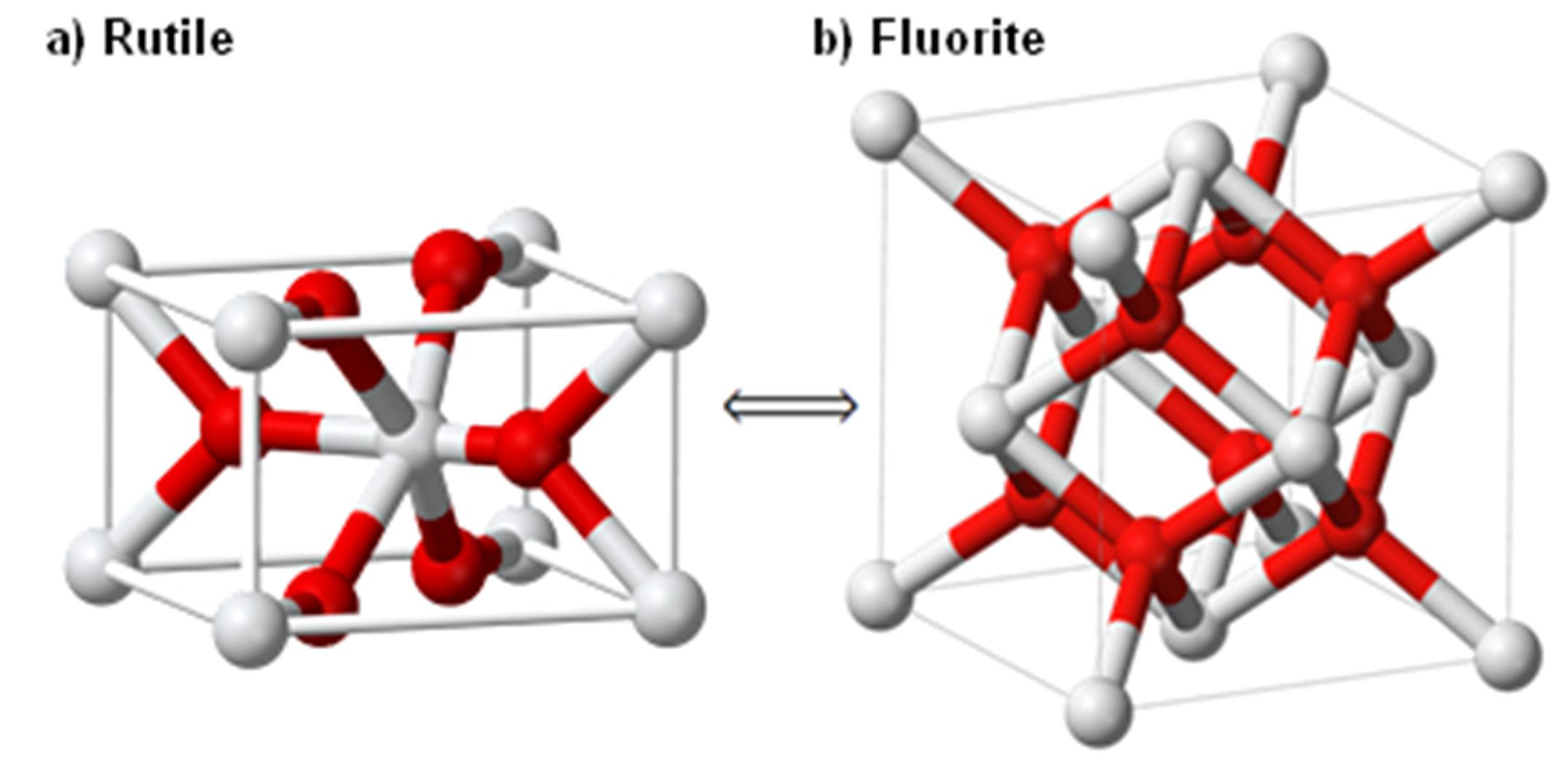
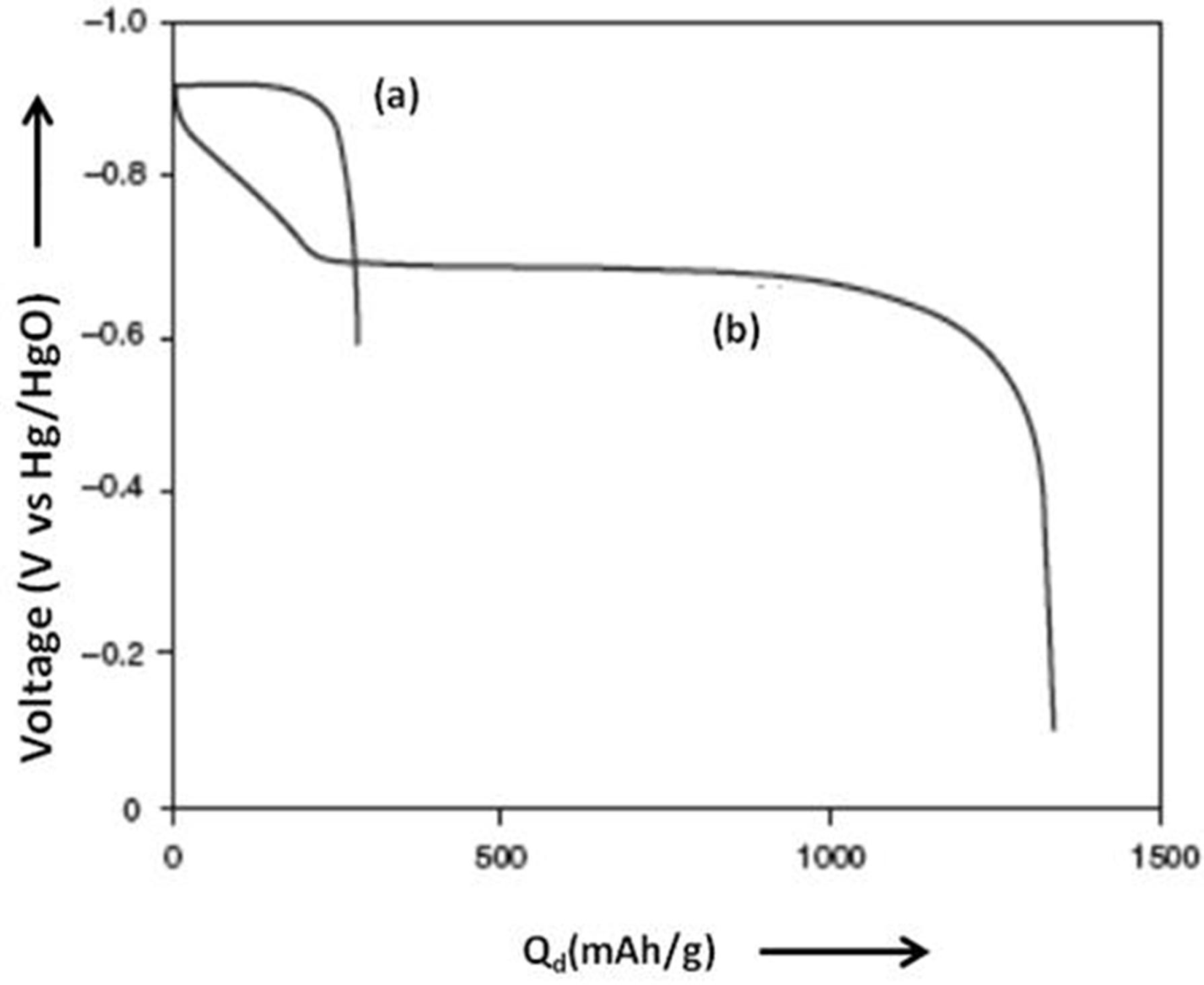
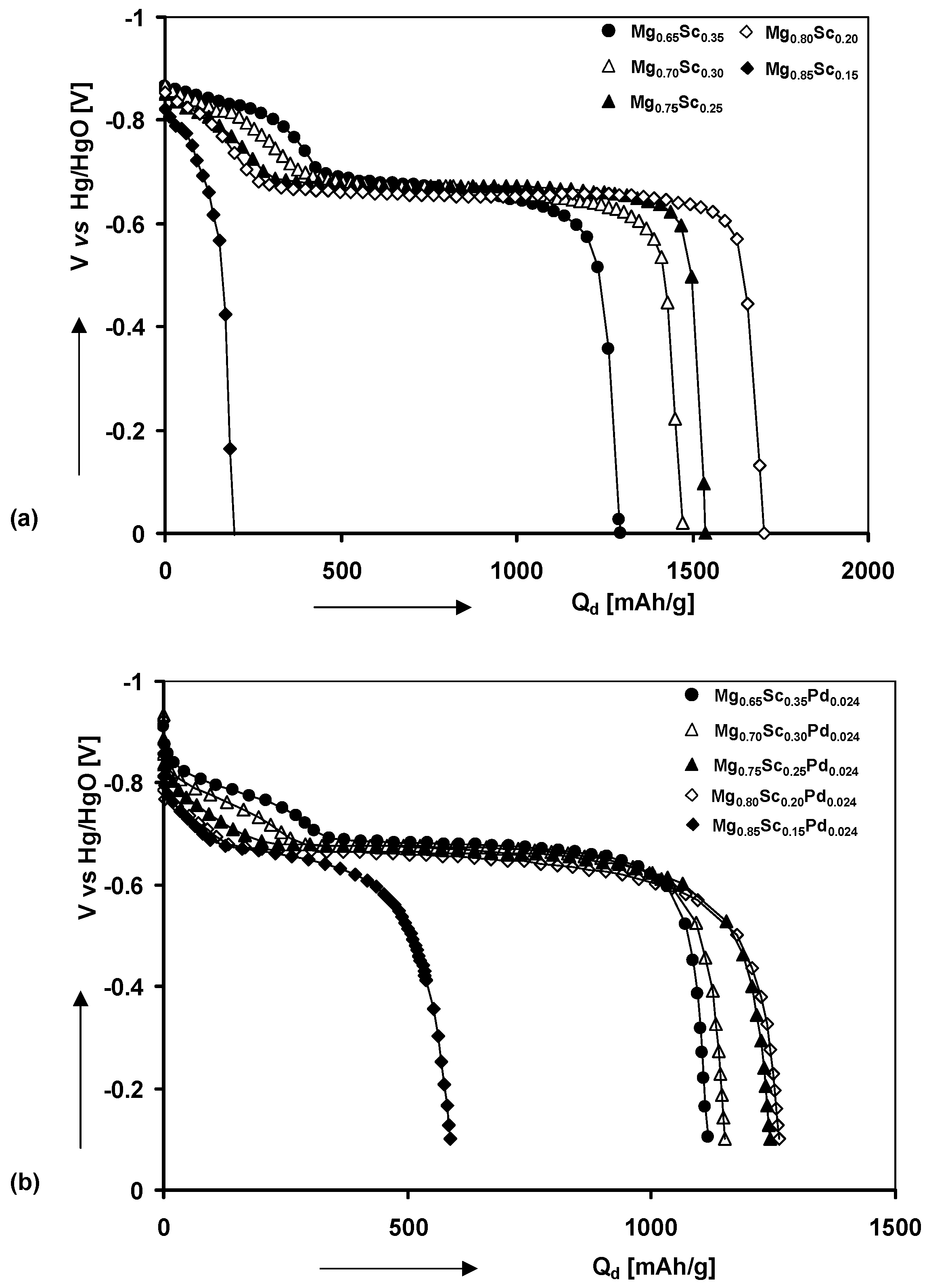
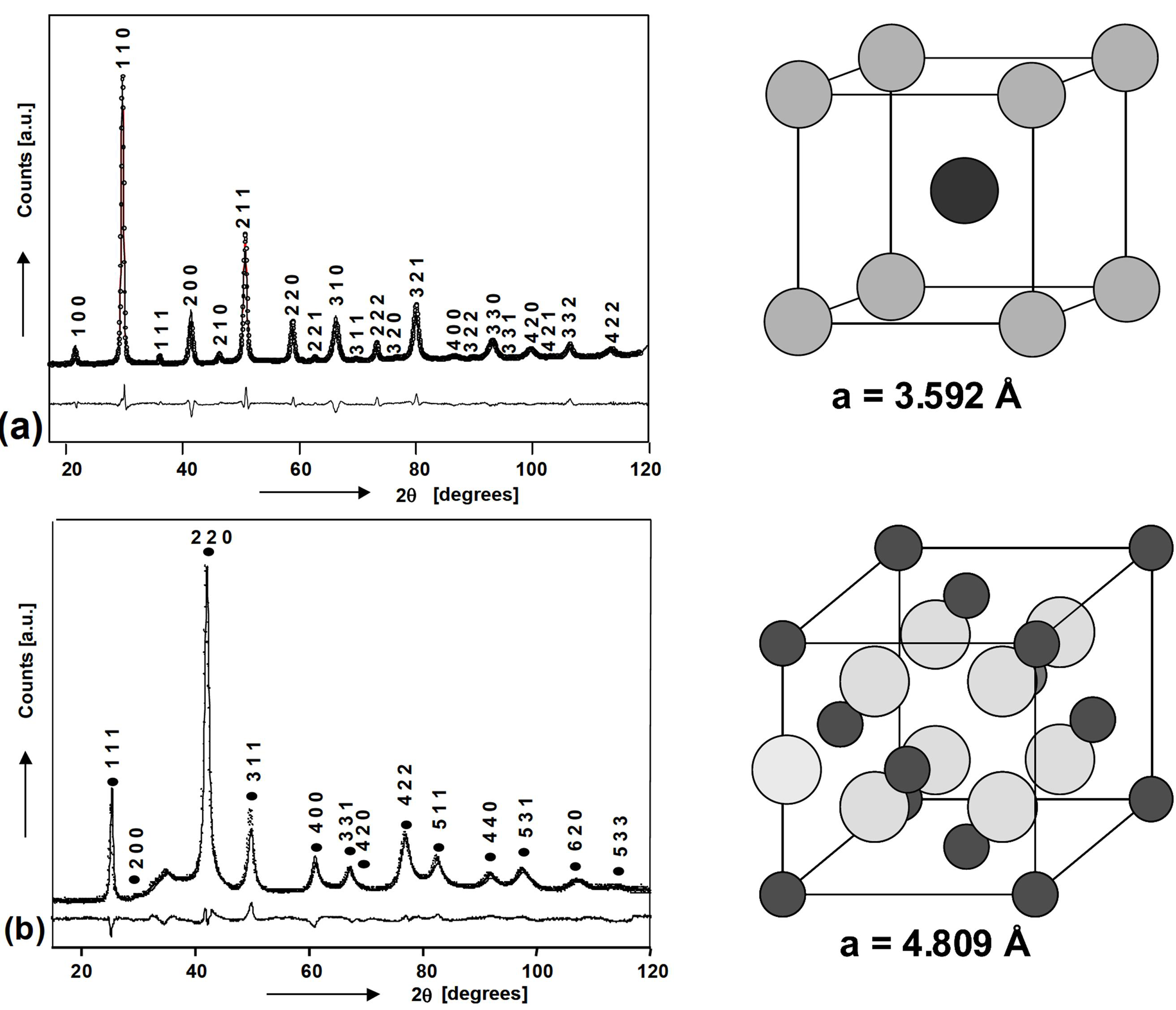
4.1. Magnesium Scandium Alloys
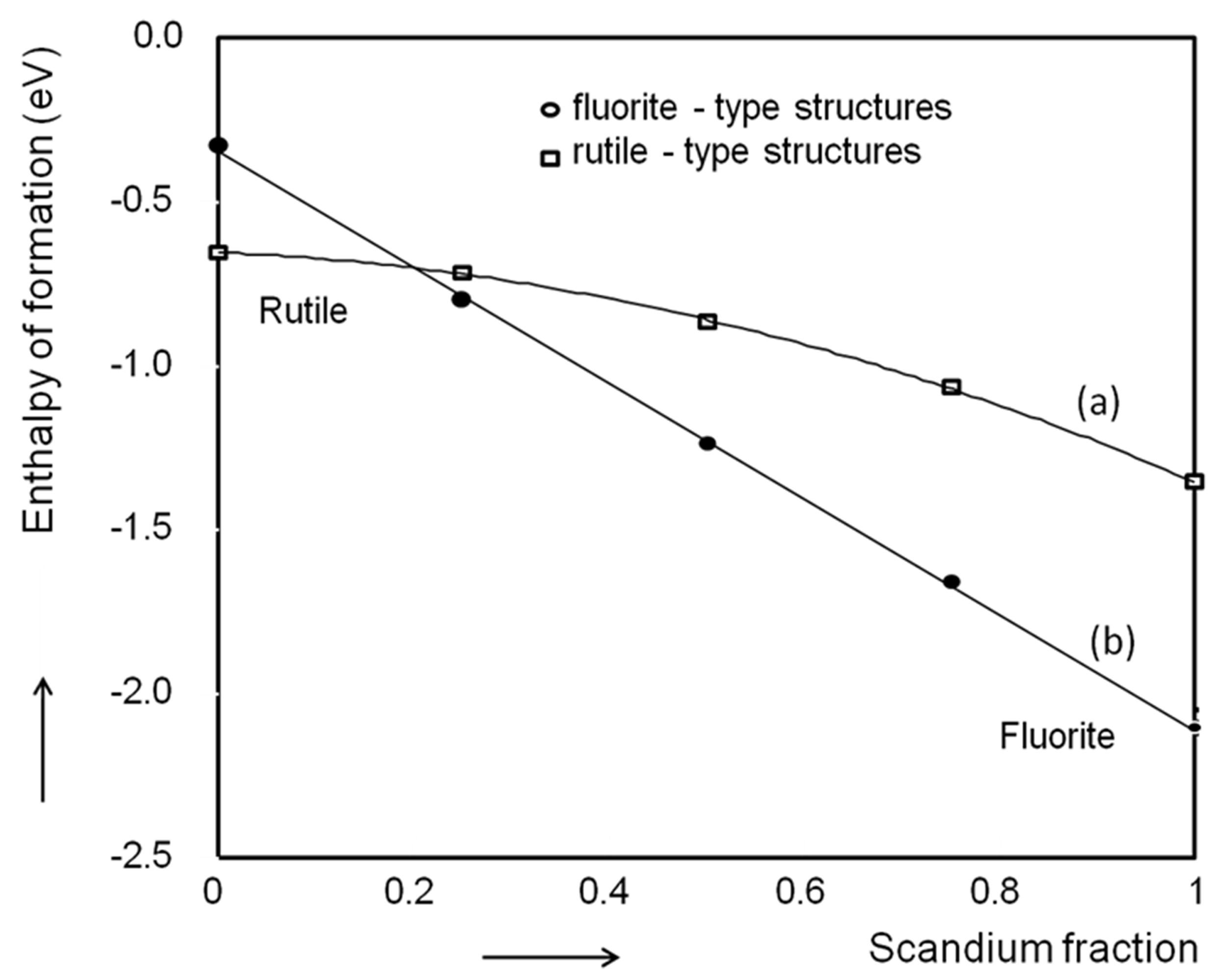
4.2. Magnesium Titanium Alloys
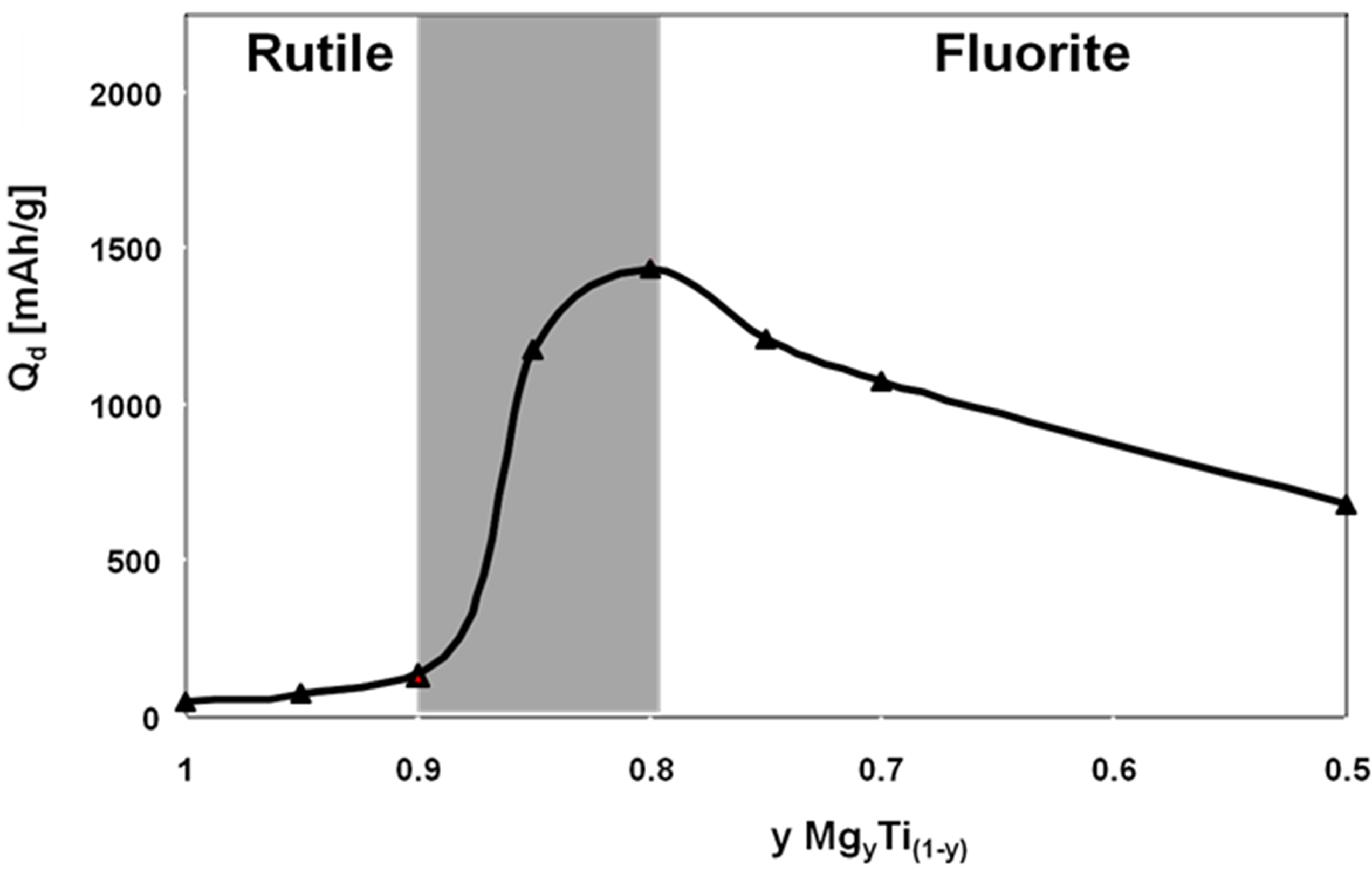
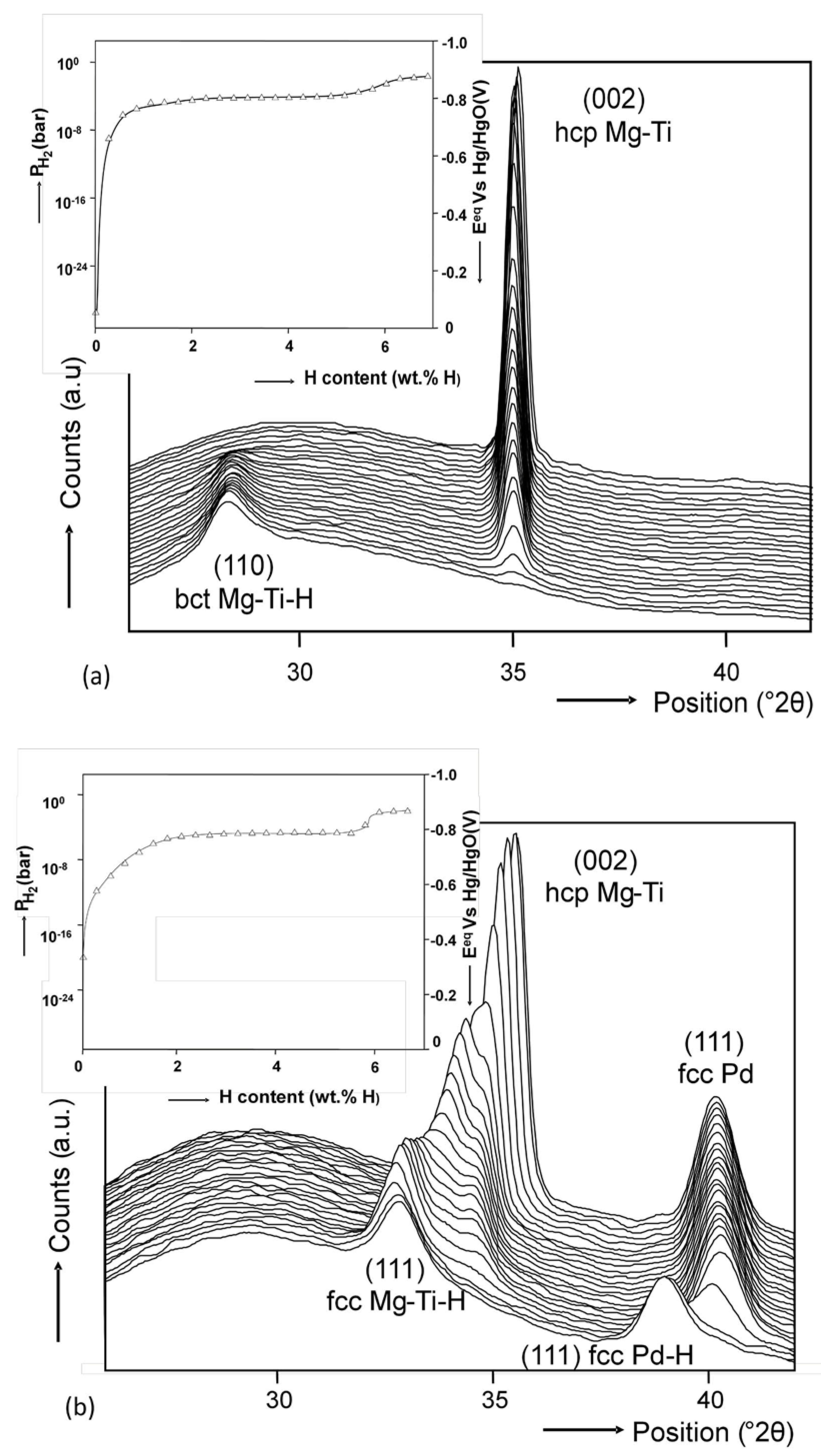
4.3. Mg-Based Ternary Alloys
 increases steeply and soon reaches a sloping plateau. Since Si is heavier than Al, the storage capacity is lower than that of the Al-containing ternary alloys. The occurrence of the second plateau was also observed in other systems, such as Mg2CuAl0.375, LaCo6, Ti-Fe and NdCo5. Interestingly, the Mg-Ti-Al-H system shows a plateau near atmospheric pressure and temperature.
increases steeply and soon reaches a sloping plateau. Since Si is heavier than Al, the storage capacity is lower than that of the Al-containing ternary alloys. The occurrence of the second plateau was also observed in other systems, such as Mg2CuAl0.375, LaCo6, Ti-Fe and NdCo5. Interestingly, the Mg-Ti-Al-H system shows a plateau near atmospheric pressure and temperature. 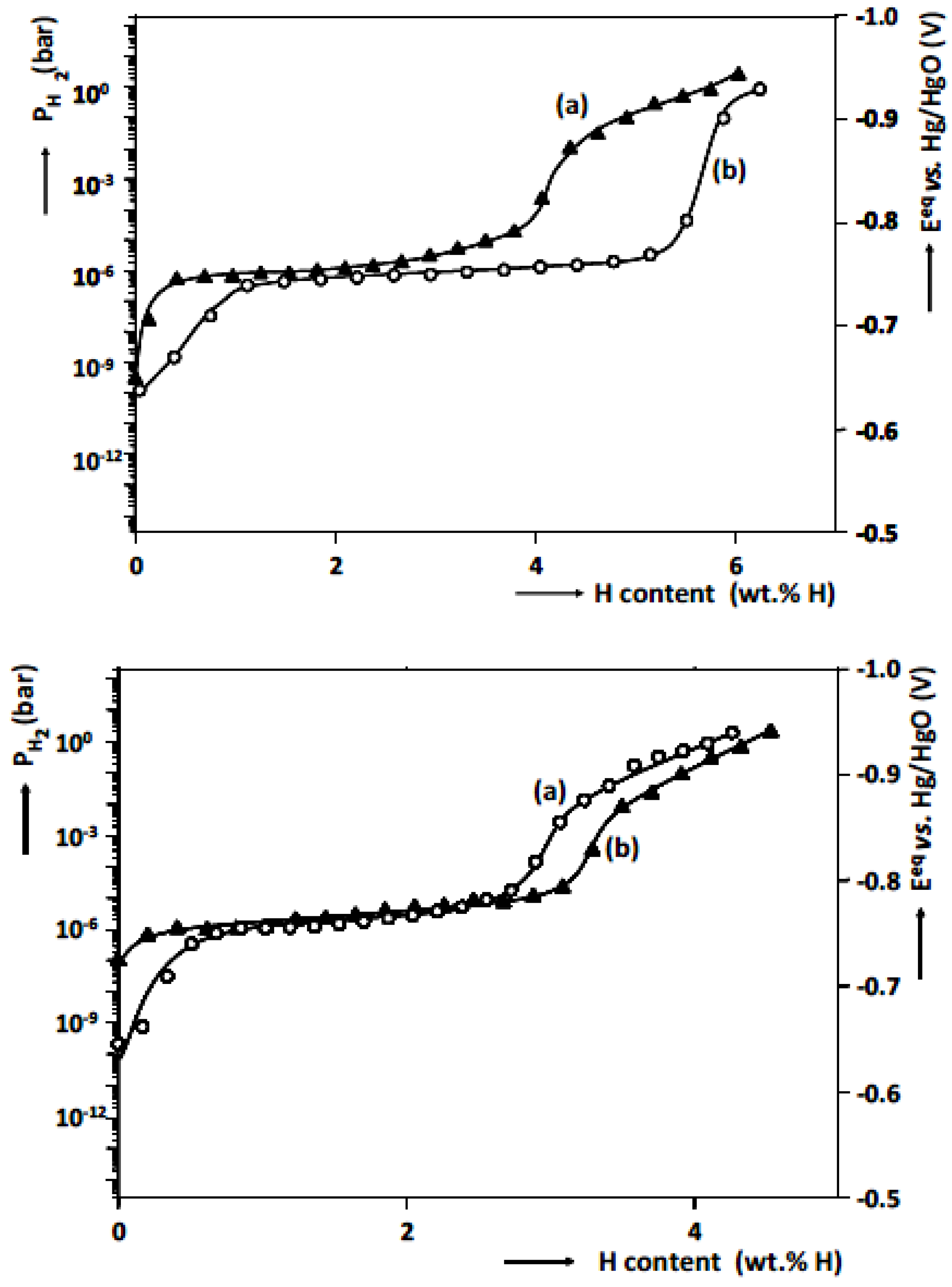
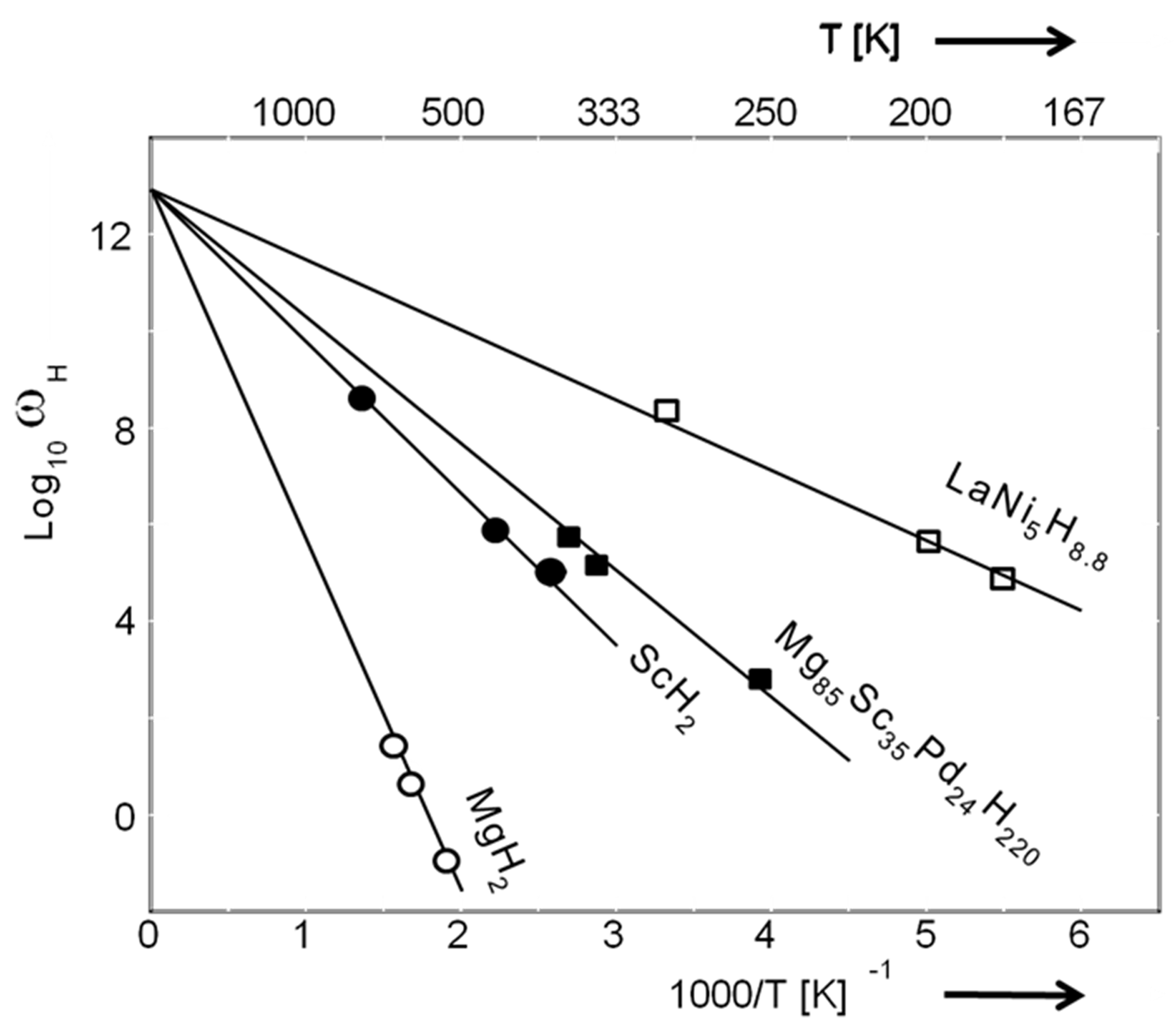
5. Hydrogenography

6. Conclusions
References
- Guo, Z.X.; Shang, C.; Aguey-Zinsou, K.F. Materials challenges for hydrogen storage. J. Eur. Ceram. Soc. 2008, 28, 1467–1473. [Google Scholar] [CrossRef]
- Momirlan, M.; Veziroglu, T.N. The properties of hydrogen as fuel tomorrow in sustainable energy system for a cleaner planet. Int. J. Hydrogen Energy 2005, 30, 795–802. [Google Scholar] [CrossRef]
- Züttel, A.; Borgschulte, A.; Schlapbach, L. Hydrogen as A Future Energy Carrier; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008; p. 5. [Google Scholar]
- Schlapbach, L.; Züttel, A. Hydrogen-storage materials for mobile applications. Nature 2001, 414, 353–358. [Google Scholar] [CrossRef]
- Ross, D.K. Hydrogen storage: The major technological barrier to the development of hydrogen fuel cell cars. Vacuum 2006, 80, 1084–1089. [Google Scholar] [CrossRef]
- Fukai, Y. The Metal-Hydrogen System, Basic Bulk Properties. In Springer Series in Materials Science; Springer: Berlin, Germany, 1993; pp. 55–88. [Google Scholar]
- Van Vucht, J.H.N.; Kujipers, F.A.; Bruning, H.C.A.M. Reversible room-temperature absorption of large quantites of hydrogen by intermetallic compounds. Philips Res. Rep. 1970, 25, 133–140. [Google Scholar]
- Selvam, P.; Viswanathan, B.; Swamy, C.S.; Srinivasan, V. Magnesium and magnesium alloy hydrides. Int. J. Hydrogen Energy 1986, 11, 169–192. [Google Scholar] [CrossRef]
- Luz, Z.; Genossar, J.; Rudman, P.S. Identification of the diffusing atom in MgH2. J. Less Common Metals 1980, 73, 113–118. [Google Scholar] [CrossRef]
- Züttel, A. Materials for hydrogen storage. Mater. Today 2003, 6, 24–33. [Google Scholar] [CrossRef]
- Willems, J.J.G. Metal Hydride Electrodes Stability of LaNi5-Related Compounds; Philips Research Laboratories: Eindhoven, The Netherlands, 1984; Volume 39, pp. 1–94. [Google Scholar]
- Notten, P.H.L. Rechargeable Nickel-Metal hydride Batteries: A Successful New Concept. In Interstitial Intermetallic Alloys; Grandjean, F., Long, G.L., Buschow, K.H.J., Eds.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1995; Volume 281, p. 151. [Google Scholar]
- Platt, J.R. Electrochromism, a Possible Change of Color Producible in Dyes by an Electric Field. J. Chem. Phys. 1961, 34, 862–863. [Google Scholar] [CrossRef]
- Deb, S.K. A Novel electrophotographic system. Appl. Opt. 1969, 8, 192–195. [Google Scholar]
- Huiberts, J.N.; Griessen, R.; Rector, J.H.; Wijngaarden, R.J.; Dekker, J.P.; de Groot, D.G.; Koeman, N.J. Yttrium and lanthanum hydride films with switchable optical properties. Nature 1996, 380, 231–234. [Google Scholar] [CrossRef]
- Griessen, R.; Giebels, I.A.M.E.; Dam, B. Optical Properties of Metal-Hydrides: Switchable Mirrors. Available online: http://www.nat.vu.nl/en/Images/ReviewSwitchableMirrors10AUG04_tcm69-85550.pdf (accessed on 28 November 2011).
- Notten, P.H.L.; Kremers, M.; Griessen, R. Optical switching of Y-hydride thin film electrodes. J. Electrochem. Soc. 1996, 143, 3348–3353. [Google Scholar] [CrossRef]
- Van der Sluis, P.M.; Ouwerkerk, M.; Duine, P.A. Optical switching based on magnesium lanthanide alloy hydrides. Appl. Phys. Lett. 1997, 70, 3356–3358. [Google Scholar] [CrossRef]
- Griessen, R.; van der Sluis, P. Schaltbare Spiegel-Elektronenkorrelationen in der Anwendung. Physik Unserer Zeit 2001, 32, 76–83. [Google Scholar] [CrossRef]
- Niessen, R.A.H.; Notten, P.H.L. Electrochemical hydrogen storage characteristics of thin film MgX (X=Sc, Ti, V, Cr) compounds. Electrochem. Solid StateLett. 2005, 8, A534–A538. [Google Scholar] [CrossRef]
- Richardson, T.J.; Slack, J.L.; Armitage, R.D.; Kostecki, R.; Farangis, B.; Rubin, M.D. Switchable mirrors based on nickel-magnesium films. Appl. Phys. Lett. 2001, 78, 3047–3049. [Google Scholar] [CrossRef]
- Karazhanov, S.Zh.; Ulyashin, A.G.; Vajeeston, P.; Ravindran, P. Hydrides as materials for semiconductor electronics. Philos. Mag. 2008, 88, 2461–2476. [Google Scholar] [CrossRef]
- Reilly, J.J.; Wiswall, R.H. Reaction of hydrogen with alloys of magnesium and nickel and the formation of Mg2NiH4. Inorg. Chem. 1968, 7, 2254–2256. [Google Scholar] [CrossRef]
- Janot, R.; Aymard, L.; Rougier, A.; Nazri, G.; Tarascon, J. Enhanced hydrogen sorption capacities and kinetics of Mg2Ni alloys by ball-milling with carbon and Pd coating. J. Mater. Res. 2003, 18, 1749–1752. [Google Scholar] [CrossRef]
- Zhang, S.G.; Hara, Y.; Suda, S.; Morikawa, T.; Inoue, H.; Iwakura, C. Physicochemical and electrochemical hydriding-dehydriding characteristics of amorphous MgNix (x = 1.0, 1.5, 2.0) alloys prepared by mechanical alloying. J. Solid State Electrochem. 2001, 5, 23–28. [Google Scholar] [CrossRef]
- Süleyman, Er.; Tiwari, D.; de Wijs, G.A.; Brocks, G. Tunable Hydrogen Storage in Magnesium-Transition Metal Compounds: First-Principles Calculations. Phys. Rev. B 2009, 79, 1–8. [Google Scholar]
- Niessen, R.A.H.; Notten, P.H.L. Hydrogen storage in thin film magnesium-scandium alloys. J Alloy Compd. 2005, 404-406, 457–460. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Niessen, R.A.H.; Notten, P.H.L. Electrochemical hydrogen storage in MgSc alloys: A comparative study between thin films and bulk materials. J. Alloy Compd. 2006, 417, 280–291. [Google Scholar] [CrossRef]
- Latroche, M.; Kalisvaart, P.; Notten, P.H.L. Crystal structure of Mg0.65Sc0.35Dx deuterides studied by X-ray and neutron powder diffraction. J. Solid State Chem. 2006, 179, 3024–3032. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Latroche, M.; Cuevas, F.; Notten, P.H.L. In situ neutron diffraction study on Pd-doped Mg0.65Sc0.35 electrode material. J. Solid State Chem. 2008, 181, 1141–1148. [Google Scholar] [CrossRef]
- Conradi, M.S.; Mendenhall, M.P.; Ivancic, T.M.; Carl, E.A.; Browning, C.D.; Notten, P.H.L.; Kalisvaart, W.P.; Magusin, P.C.M.M.; Bowman, R.C., Jr.; Hwang, S.; Adolphi, N.L. NMR to determine rates of motion and structures in metal-hydrides. J. Alloy Compd. 2007, 446-447, 499–503. [Google Scholar] [CrossRef]
- Pauw, B.R.; Kalisvaart, W.P.; Tao, S.X.; Koper, M.T.M.; Jansen, A.P.J.; Notten, P.H.L. Cubic MgH2 stabilized by alloying with transition metals: A density functional theory study. Acta Mater. 2008, 56, 2948–2954. [Google Scholar] [CrossRef]
- Vermeulen, P.; Niessen, R.A.H.; Notten, P.H.L. Hydrogen storage in metastable MgyTi(1−y) thin films. Electrochem. Commun. 2006, 8, 27–32. [Google Scholar] [CrossRef]
- Vermeulen, P.; Wondergem, H.J.; Graat, P.C.J.; Borsa, D.M.; Schreuders, H.; Dam, B.; Griessen, R.; Notten, P.H.L. In situ electrochemical XRD study of (de)hydrogenation of MgyTi(100−y) thin films. J. Mater. Chem. 2008, 18, 3680–3687. [Google Scholar] [CrossRef]
- Rousselot, S.; Bichat, M.P.; Guay, D.; Roué, L. Structure and electrochemical behaviour of metastable Mg50Ti50 alloy prepared by ball milling. J. Power Sources 2008, 175, 621–624. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Notten, P.H.L. Mechanical alloying and electrochemical hydrogen storage of Mg-based systems. J. Mater. Res. 2008, 23, 2179–2187. [Google Scholar] [CrossRef]
- Kalisvaart, W.P.; Wondergem, H.J.; Bakker, F.; Notten, P.H.L. Mg-Ti based materials for electrochemical hydrogen storage. J. Mater. Res. 2007, 22, 1640–1649. [Google Scholar] [CrossRef]
- Kyoi, D.; Sato, T.; Rönnebro, E.; Kitamura, N.; Ueda, A.; Ito, M.; Katsuyama, S.; Hara, S.; Noréus, D.; Sakai, T. A new ternary magnesium-titanium hydride Mg7TiHx with hydrogen desorption properties better than both binary magnesium and titanium hydrides. J. Alloy Compd. 2004, 372, 213–217. [Google Scholar] [CrossRef]
- De Boer, F.R.; Boom, R.; Mattens, W.C.M.; Miedema, A.R.; Niessen, A.K. Cohesion in Metals; North-Holland: Amsterdam, The Netherlands, 1988; p. 127. [Google Scholar]
- Liang, G.; Schulz, R. Synthesis of Mg-Ti alloy by mechanical alloying. J. Mater. Sci. 2003, 38, 1179–1184. [Google Scholar] [CrossRef]
- Srinivasan, S.; Magusin, P.C.M.M.; Kalisvaart, W.P.; Notten, P.H.L.; Cuevas, F.; Latroche, M.; van Santen, R.A. Nanostructures of Mg0.65Ti0.35Dx studied with x-ray diffraction, neutron diffraction, and magic-angle-spinning 2H NMR spectroscopy. Phys. Rev. B 2010, 81, 1–10. [Google Scholar]
- Miedema, A.R.; de Châtel, P.F.; de Boer, F.R. Cohesion in Alloys-fundamentals of a Semi-empirical model. Physica B 1980, 100, 1–28. [Google Scholar]
- Miedema, A.R. The electronegativity parameter for transition metals: Heat of formation and charge transfer of alloys. J. Less Common Metals 1973, 32, 117–136. [Google Scholar] [CrossRef]
- Vermeulen, P.; van Thiel, E.F.M.J.; Notten, P.H.L. Ternary MgTiX-alloys: A promising route towards low-temperature, high-capacity, hydrogen-storage materials. Chem. Eur. J. 2007, 13, 9892–9898. [Google Scholar] [CrossRef]
- Gremaud, R.; Broedersz, C.P.; Borsa, D.M.; Borgschulte, A.; Mauron, P.; Schreuders, H.; Rector, J.H.; Dam, B.; Griessen, R. Hydrogenography: An optical combinatorial method to find new light-weight hydrogen-storage materials. Adv. Mater. 2007, 19, 2813–2817. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Manivasagam, T.G.; Kiraz, K.; Notten, P.H.L. Electrochemical and Optical Properties of Magnesium-Alloy Hydrides Reviewed. Crystals 2012, 2, 1410-1433. https://doi.org/10.3390/cryst2041410
Manivasagam TG, Kiraz K, Notten PHL. Electrochemical and Optical Properties of Magnesium-Alloy Hydrides Reviewed. Crystals. 2012; 2(4):1410-1433. https://doi.org/10.3390/cryst2041410
Chicago/Turabian StyleManivasagam, Thirugnasambandam G., Kamil Kiraz, and Peter H. L. Notten. 2012. "Electrochemical and Optical Properties of Magnesium-Alloy Hydrides Reviewed" Crystals 2, no. 4: 1410-1433. https://doi.org/10.3390/cryst2041410




