The Synthesis and Molecular Structure of 1-(3,4-Dihydroxyphenethyl)-3-hydroxy-2-methylpyridin-4(1H)-one Hydrochloride Methanol Solvate
Abstract
:1. Introduction
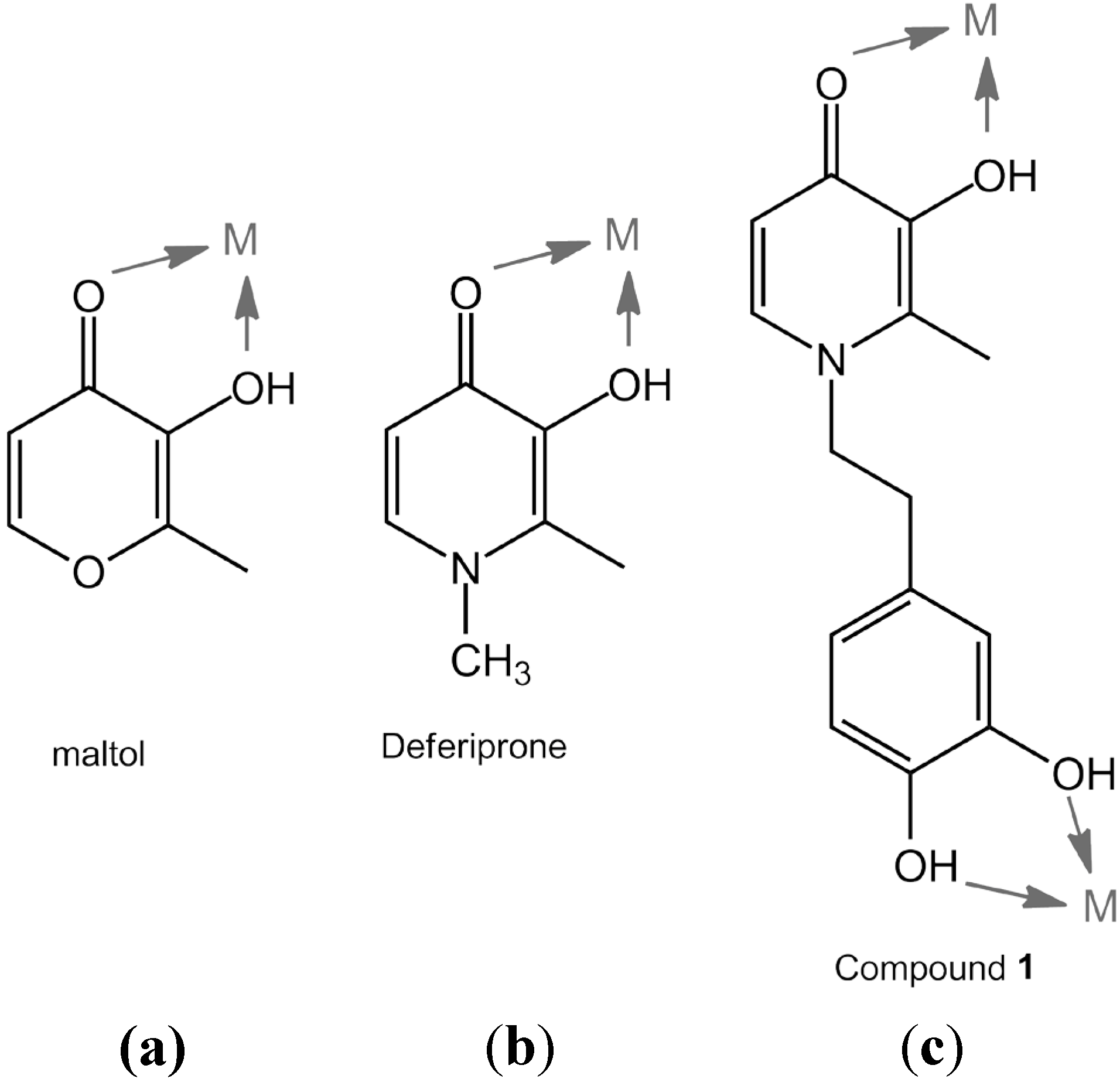
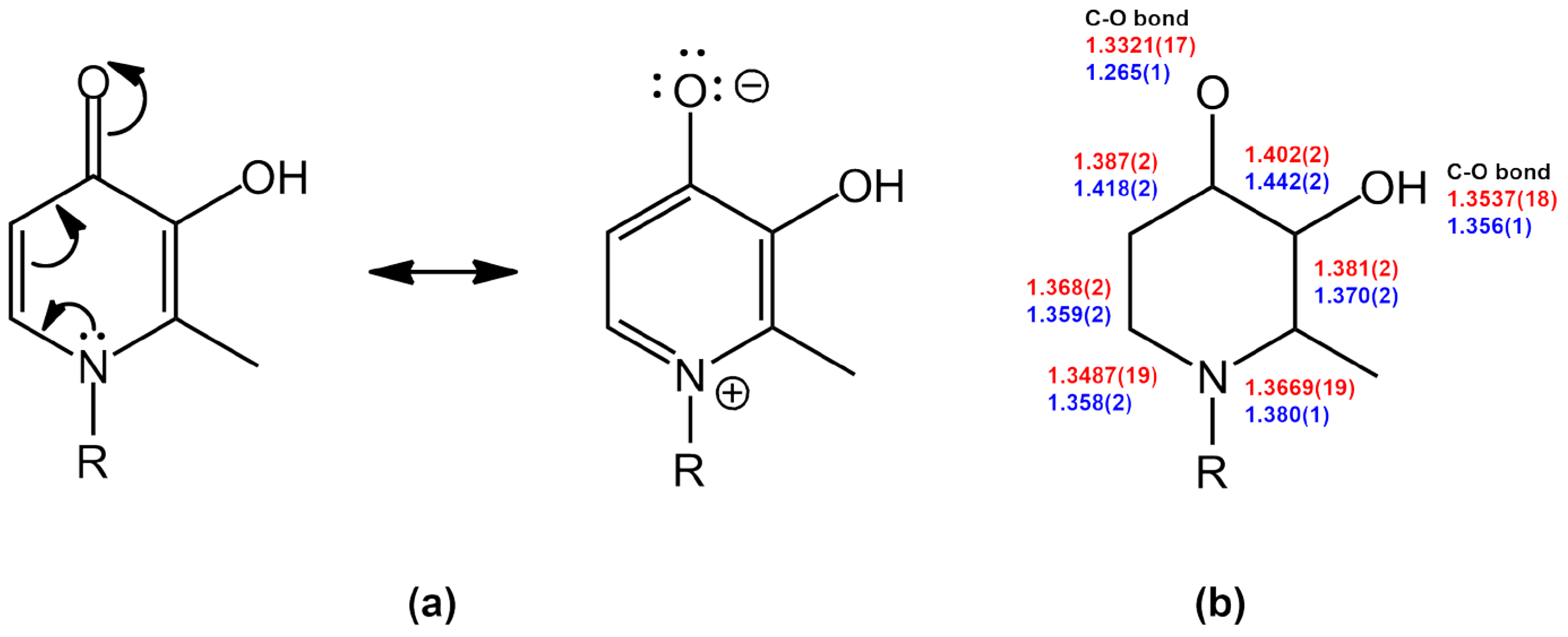
2. Results and Discussion
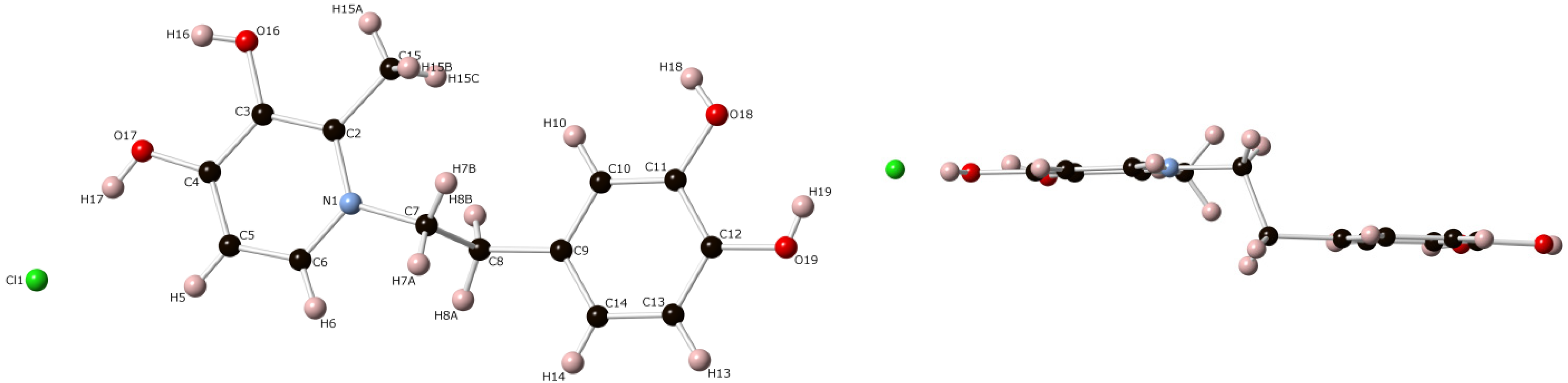
3. Experimental Section
3.1. General
3.2. Synthesis of 1-(3,4-Dihydroxyphenethyl)-3-hydroxy-2-methylpyridin-4(1H)-one (1)
3.3. Crystal Structure Determination
4. Conclusions
Acknowledgments
Conflict of Interest
References and Notes
- Santos, M.A.; Marques, S.M.; Chaves, S. Hydroxypyridinones as “privileged” chelating structures for the design of medicinal drugs. Coord. Chem. Rev. 2012, 256, 240–259. [Google Scholar] [CrossRef]
- Thompson, K.H.; Barta, C.A.; Orvig, C. Metal complexes of maltol and close analogues in medicinal inorganic chemistry. Chem. Soc. Rev. 2006, 35, 545–556. [Google Scholar]
- Datta, A.; Raymond, K.N. Gd-hydroxypyridinone (HOPO)-high relaxivity magnetic resonance imagining (MRI) contrast agents. Acc. Chem. Res. 2009, 42, 938–947. [Google Scholar] [CrossRef]
- Philpot, C. Bioinorganic chemistry: getting a grip on iron. Nat. Chem. Biol. 2010, 6, 568–570. [Google Scholar] [CrossRef]
- Xiao, G.; van der Helm, D.; Hider, R.C.; Dobbin, P.S. Structure-stability relationships of 3-hydroxypyridin-4-one complexes. J. Chem. Soc. Dalton Trans. 1992, 22, 3265–3271. [Google Scholar]
- Nelson, W.O.; Rettig, S.J.; Orvig, C. Aluminum and gallium complexes of 1-ethyl-3-hydroxy-2-methyl-4-pyridinone: a new exoclathrate matrix. Inorg. Chem. 1989, 28, 3153–3157. [Google Scholar] [CrossRef]
- Giesecke, J. The structure of catecholamines. V. The crystal and molecular structure of epinine hydrobromide. Acta Cryst. 1976, B32, 2337–2340. [Google Scholar]
- SAINT 7.23A; Bruker AXS, Inc.: Madison, WI, USA, 2006.
- SADABS 2008; Bruker AXS, Inc.: Madison, WI, USA, 2008.
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, A64, 112–122. [Google Scholar]
- CCDC (Cambridge Crystallographic Data Centre), SW101014.cif; No. CCDC 919059; CCDC: Cambridge, UK, 2013.
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hall, S.R.; Roy, R.; McLaughlin, D.T.; Sullivan, K.J.; Barclay, L.R.C.; Vogels, C.M.; Decken, A.; Westcott, S.A. The Synthesis and Molecular Structure of 1-(3,4-Dihydroxyphenethyl)-3-hydroxy-2-methylpyridin-4(1H)-one Hydrochloride Methanol Solvate. Crystals 2013, 3, 333-338. https://doi.org/10.3390/cryst3020333
Hall SR, Roy R, McLaughlin DT, Sullivan KJ, Barclay LRC, Vogels CM, Decken A, Westcott SA. The Synthesis and Molecular Structure of 1-(3,4-Dihydroxyphenethyl)-3-hydroxy-2-methylpyridin-4(1H)-one Hydrochloride Methanol Solvate. Crystals. 2013; 3(2):333-338. https://doi.org/10.3390/cryst3020333
Chicago/Turabian StyleHall, Steven R., Raymond Roy, Dylan T. McLaughlin, Kate J. Sullivan, L. Ross C. Barclay, Christopher M. Vogels, Andreas Decken, and Stephen A. Westcott. 2013. "The Synthesis and Molecular Structure of 1-(3,4-Dihydroxyphenethyl)-3-hydroxy-2-methylpyridin-4(1H)-one Hydrochloride Methanol Solvate" Crystals 3, no. 2: 333-338. https://doi.org/10.3390/cryst3020333




