Influence of Mesogenic Properties of Cruciform-Shaped Liquid Crystals by Incorporating Side-Arms with a Laterally-Substituted-Fluorine
Abstract
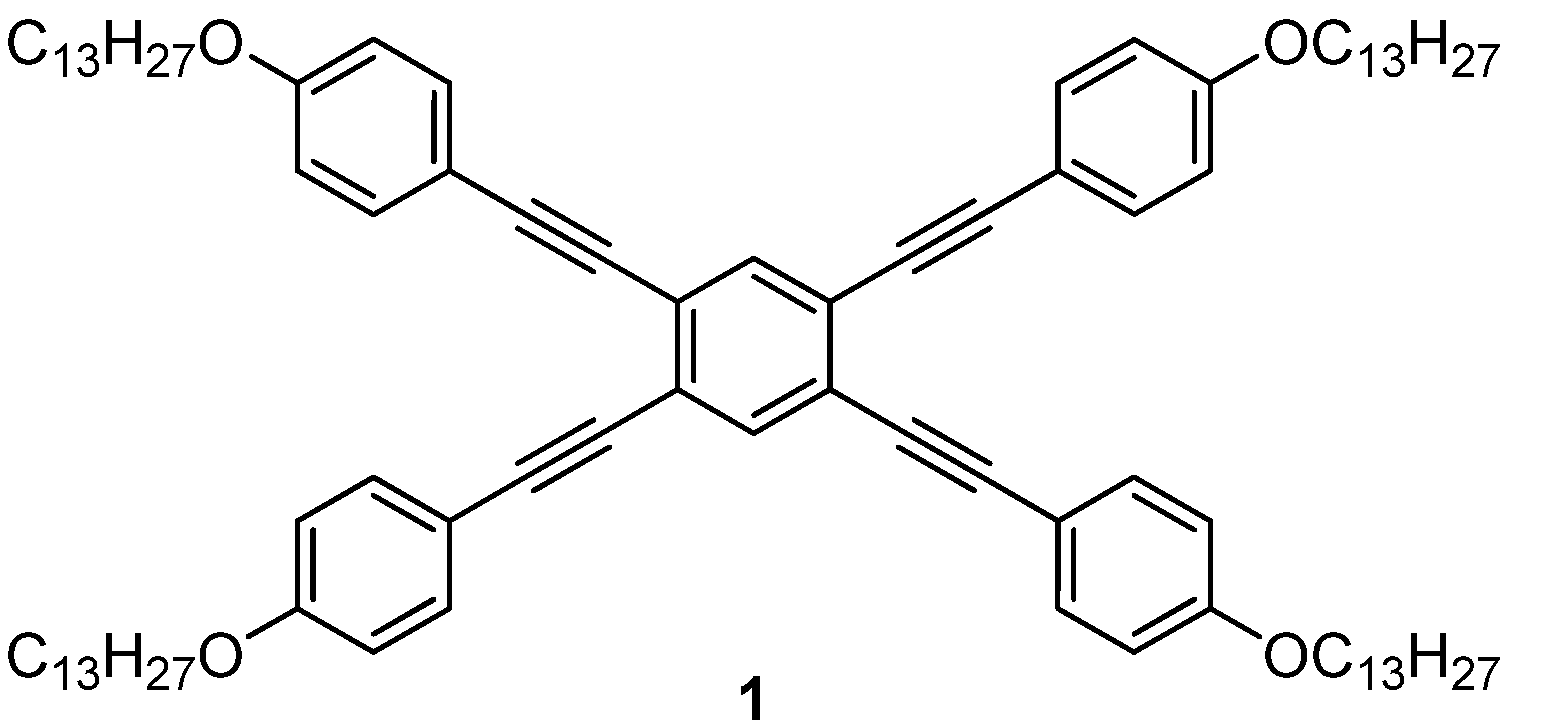
:1. Introduction
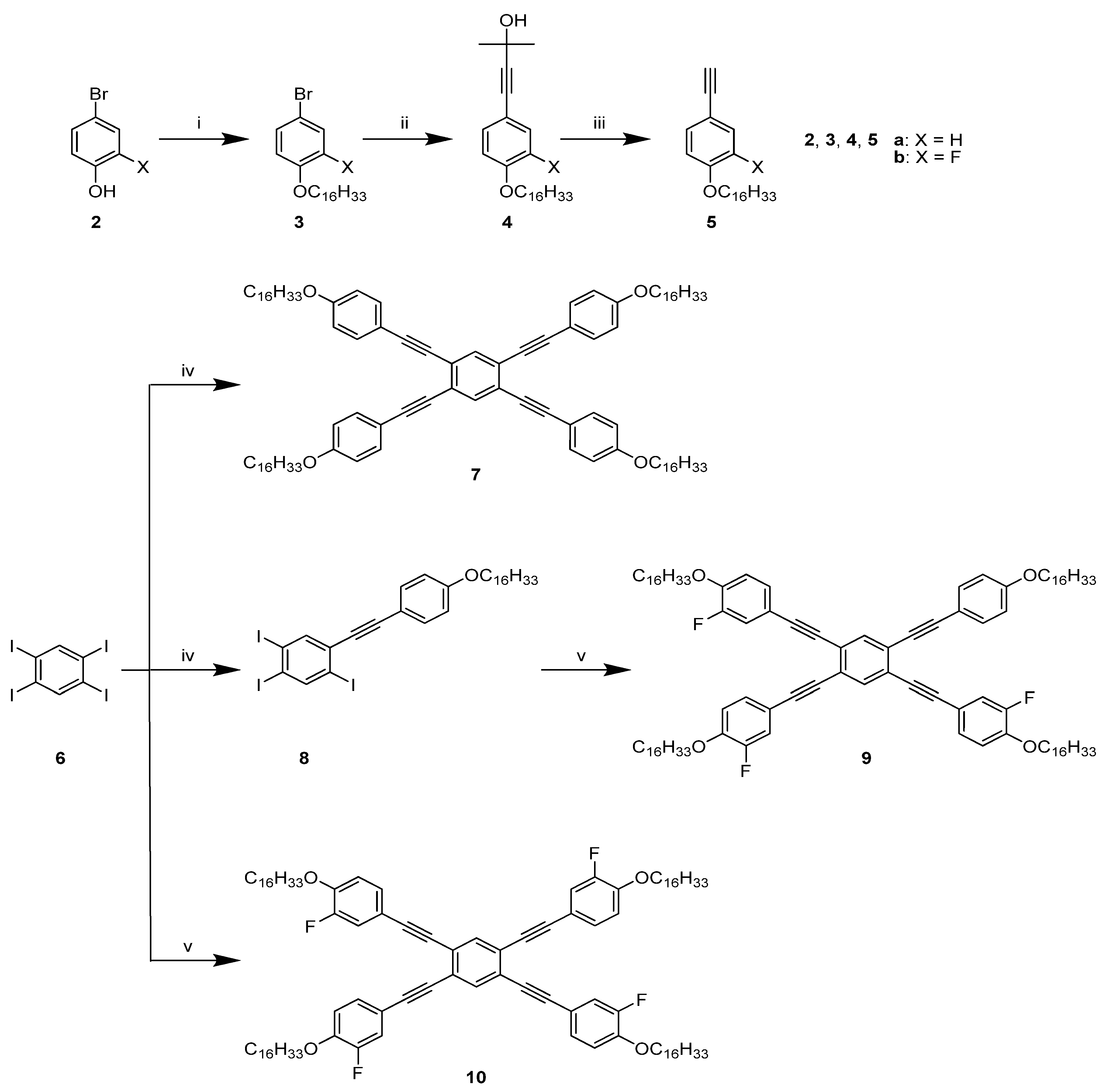
2. Results and Discussion
2.1. Synthesis
2.2. Liquid Crystalline Properties
| Compound | Thermal treatments b | Phase behavior | T [°C] (∆H [kJ mol−1]) |
|---|---|---|---|
| 7 | heating | Cryst- iso | 84.1 (30.58) |
| cooling | iso-N-Cryst | 74.0 (−0.69), 60.7 (−78.75) | |
| 9 | heating | Cryst-iso | 81.3 (93.72) |
| cooling | iso-N-SmC-Cryst a | 66.1 (−1.05), 64.9 (−3.43), 41.7 (−67.32) | |
| 10 | heating | Cryst- iso | 78.3 (38.00) |
| cooling | iso-SmC-Cryst | 62.1 (−4.85), 40.5 (−25.92) |
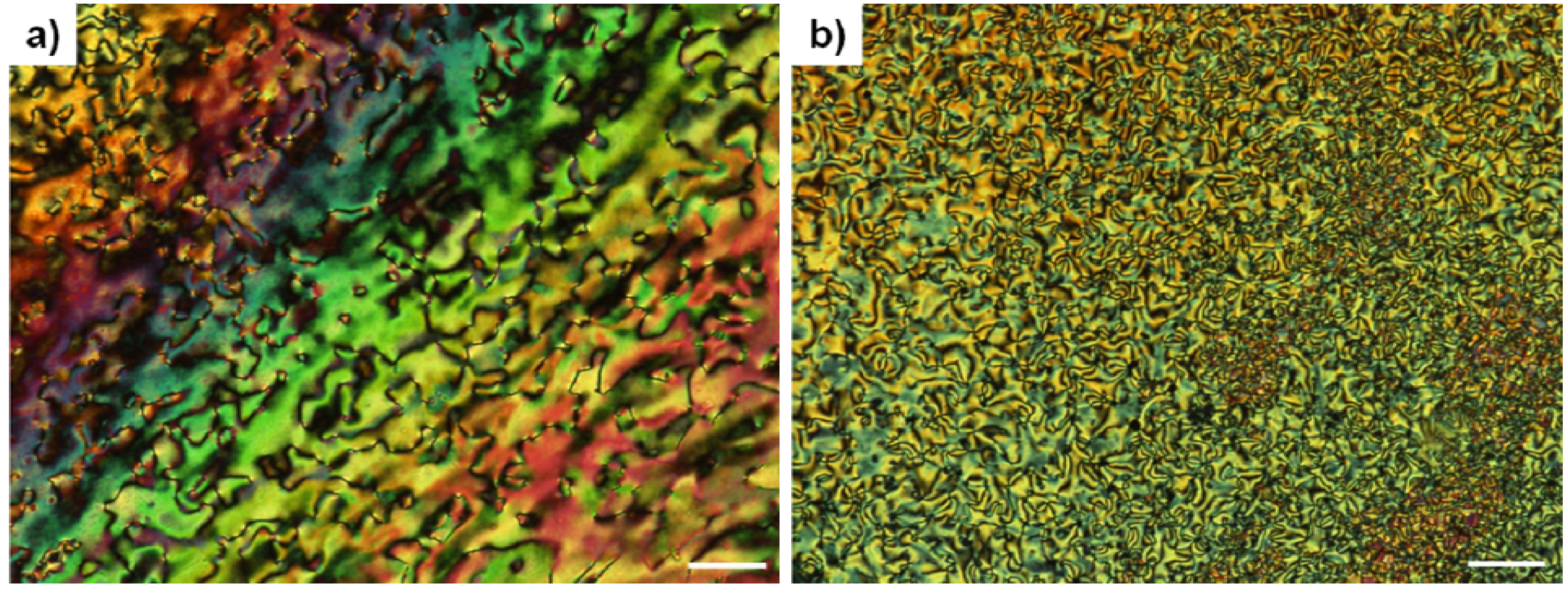
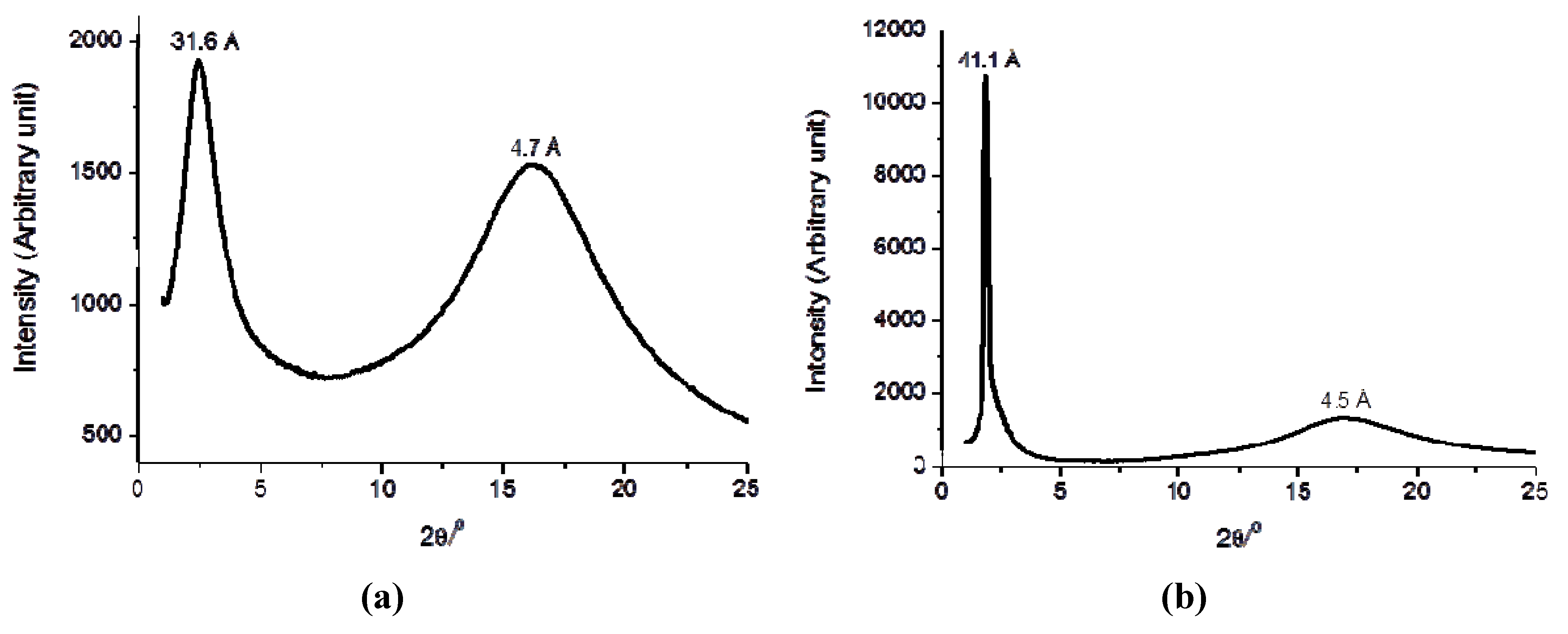
3. X-ray Diffraction Studies
4. Experimental Section
4.1. 4-Bromo-2-fluoro-1-(hexadecyloxy)benzene (3b)
4.2. 4-(3-Fluoro-4-(hexadecyloxy)phenyl)-2-methylbut-3-yn-2-ol (4b)
4.3. 4-Ethynyl-2-fluoro-1-(hexadecyloxy)benzene (5b)
4.4. 1,2,4,5-Tetrakis((4-(hexadecyloxy)phenyl)ethynyl)benzene (7)
4.5. 1-(4-Hexadecyloxyphenylethynyl)-2,4,5-triiodobenzene (8)
4.6. 1,2,4-Tris(3-fluoro-4-hexadecyloxyphenylethynyl)-5-(4-hexadecyloxy-phenylethynyl)benzene (9)
4.7. 1,2,4,5-Tetrakis((3-fluoro-4-(hexadecyloxy)phenyl)ethynyl)benzene (10)
5. Conclusions
Acknowledgments
References
- Chandrasekhar, S. Biaxial nematic liquid crystals in low molecular weight thermotropic systems. Mol. Cryst. Liq. Cryst. 1994, 243, 1–9. [Google Scholar] [CrossRef]
- Norbert, W.D.J.A.; Goodby, J.W.; Hird, M.; Kenneth, J.T. The synthesis and mesomorphic behaviour of the 1,2,4,5-tetrasubstituted benzenes with (4-tridecyloxyphenyl) ethynyl and (4-tridecyloxyphenyl) carbonyloxy substituents. Liq. Cryst. 1997, 22, 631–642. [Google Scholar] [CrossRef]
- Ohta, K.; Yamaguchi, N.; Yamamoto, I. Discotic liquid crystals of transition metal complexes. Part 24 synthesis and mesomorphism of porphyrin derivatives substituted with two or four bulky groups. J. Mater. Chem. 1998, 8, 2637–2650. [Google Scholar] [CrossRef]
- Chandrasekhar, S.; Nair, G.G.; Shankar Rao, D.; Krishna Prasad, S.; Prafecke, K.; Blunk, D. Schlieren textures in free-standing nematic films: Evidence of biaxiality. Liq. Cryst. 1998, 24, 67–70. [Google Scholar] [CrossRef]
- Nguyen, H.-T.; Destrade, C.; Malthécte, J. Phasmids and polycatenar mesogens. Adv. Mater. 1997, 9, 375–388. [Google Scholar] [CrossRef]
- Fletcher, I.; Luckhurst, G. The synthesis and characterization of novel non-symmetric dimers with rod-like and disc-like mesogenic units. Liq. Cryst. 1995, 18, 175–183. [Google Scholar] [CrossRef]
- Praefcke, K.; Kohne, B.; Singer, D.; Demus, D.; Pelzl, G.; Diele, S. Thermotropic biaxial nematic phases with negative optical character. Liq. Cryst. 1990, 7, 589–594. [Google Scholar] [CrossRef]
- Kreuder, W.; Ringsdorf, H.; Herrmann-Schönherr, O.; Wendorff, J.H. The “wheel of mainz” as a liquid crystal?—Structural variation and mesophade properties of trimeric descotic compounds. Angew. Chem. Int. Ed. Engl. 1987, 26, 1249–1252. [Google Scholar] [CrossRef]
- Fasina, T.M.; Collings, J.C.; Lydon, D.P.; Albesa-Jove, D.; Batsanov, A.S.; Howard, J.A.K.; Nguyen, P.; Bruce, M.; Scott, A.J.; Clegg, W.; Watt, S.W.; Viney, C.; Marder, T.B. Synthesis, optical properties, crystal structures and phase behaviour of selectively fluorinated 1,4-bis(4[prime or minute]-pyridylethynyl)benzenes, 4-(phenylethynyl)pyridines and 9,10-bis(4[prime or minute]-pyridylethynyl)anthracene, and a Zn(NO3)2 coordination polymer. J. Mater. Chem. 2004, 14, 2395–2404. [Google Scholar] [CrossRef]
- Kang, H.; Evmenenko, G.; Dutta, P.; Clays, K.; Song, K.; Marks, T.J. X-shaped electro-optic chromophore with remarkably blue-shifted optical absorption. Synthesis, characterization, linear/nonlinear optical properties, self-assembly, and thin film microstructural characteristics. J. Am. Chem. Soc 2006, 128, 6194–6205. [Google Scholar]
- Knox, J.E.; Halls, M.D.; Hratchian, H.P.; Schlegel, H.B. Chemical failure modes of AlQ3-based OLEDs: AlQ3 hydrolysis. Phys. Chem. Chem. Phys. 2006, 8, 1371–1377. [Google Scholar] [CrossRef]
- Müllen, K.; Scherf, U. Organic Light Emitting Devices: Synthesis Properties and Applications; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Schwab, P.F.H.; Levin, M.D.; Michl, J. Molecular rods. 1. Simple axial rods. Chem. Rev. 1999, 99, 1863–1934. [Google Scholar] [CrossRef]
- Shirota, Y. Organic materials for electronic and optoelectronic devices. J. Mater. Chem. 2000, 10, 1–25. [Google Scholar] [CrossRef]
- Shukla, V.K.; Kumar, S.; Deva, D. Light induced effects on the morphology and optical properties of tris-(8-hydroxyquinoline) aluminium small molecular thin film. Synth. Met. 2006, 156, 387–391. [Google Scholar] [CrossRef]
- Zhao, Y.; Slepkov, A.D.; Akoto, C.O.; McDonald, R.; Hegmann, F.A.; Tykwinski, R.R. Synthesis, structure, and nonlinear optical properties of cross-conjugated perphenylated iso-polydiacetylenes. Chem. Eur. J. 2005, 11, 321–329. [Google Scholar] [CrossRef]
- Kang, H.; Zhu, P.; Yang, Y.; Facchetti, A.; Marks, T.J. Self-assembled electrooptic thin films with remarkably blue-shifted optical absorption based on an X-shaped chromophore. J. Am. Chem. Soc. 2004, 126, 15974–15975. [Google Scholar]
- Zhang, X.-B.; Feng, J.-K.; Ren, A.-M.; Sun, C.-C. Theoretical study of two-photon absorption properties for donor/acceptor-functionalized tetrakis(phenylethynyl)benzenes and bis(dehydrobenzoannuleno)benzenes. Opt. Mater. 2007, 29, 955–962. [Google Scholar] [CrossRef]
- Brombosz, S.M.; Zucchero, A.J.; Phillips, R.L.; Vazquez, D.; Wilson, A.; Bunz, U.H.F. Terpyridine-based cruciform-Zn2+ complexes as anion-responsive fluorophores. Org. Lett. 2007, 9, 4519–4522. [Google Scholar] [CrossRef]
- Hauck, M.; Schönhaber, J.; Zucchero, A.J.; Hardcastle, K.I.; Müller, T.J.J.; Bunz, U.H.F. Phenothiazine cruciforms: Synthesis and metallochromic properties. J. Org. Chem. 2007, 72, 6714–6725. [Google Scholar]
- Wilson, J.N.; Bunz, U.H.F. Switching of intramolecular charge transfer in cruciforms: Metal ion sensing. J. Am. Chem. Soc. 2005, 127, 4124–4125. [Google Scholar] [CrossRef]
- Gray, G.W.; Hird, M.; Toyne, K.J. The synthesis of several lateral difluoro-substituted 4,4′′-dialkyl- and 4,4′′-alkoxyalkyl-terphenyls and a rationalisation of the effect of such substitution on mesophase type and transition temperatures. Mol. Cryst. Liq. Cryst. 1991, 204, 43–64. [Google Scholar] [CrossRef]
- Chen, H.-H.; Lin, H.-A.; Lai, Y.-H.; Lin, S.-Y.; Chiang, C.-H.; Hsu, H.-F.; Shih, T.-L.; Lee, J.-J.; Lai, C.-C.; Kuo, T.-S. Enantiotropic nematics from cross-like 1,2,4,5-tetrakis(4′-alkyl-4-ethynylbiphenyl)benzenes and their biaxiality studies. Chem. Eur. J. 2012, 18, 9543–9551. [Google Scholar] [CrossRef]
- Hird, M. Fluorinated liquid crystals—Properties and applications. Chem. Soc. Rev. 2007, 36, 2070–2095. [Google Scholar] [CrossRef]
- Artal, M.C.; Toyne, K.J.; Goodby, J.W.; Barbera, J.; Photinos, D.J. Synthesis and mesogenic properties of novel board-like liquid crystals. J. Mater. Chem. 2001, 11, 2801–2807. [Google Scholar] [CrossRef]
- Hsu, H.-F.; Chen, H.-C.; Kuo, C.-H.; Wang, B.-C.; Chiu, H.-T. Design and investigation of calamitic liquid crystals with low aspect-ratios: Rigid y-shaped 1,2,4-tris(4-alkoxyphenylethynyl)benzenes. J. Mater. Chem. 2005, 15, 4854–4861. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lin, Y.-H.; Ezhumalai, Y.; Yang, Y.-L.; Liao, C.-T.; Hsu, H.-F.; Wu, C. Influence of Mesogenic Properties of Cruciform-Shaped Liquid Crystals by Incorporating Side-Arms with a Laterally-Substituted-Fluorine. Crystals 2013, 3, 339-349. https://doi.org/10.3390/cryst3020339
Lin Y-H, Ezhumalai Y, Yang Y-L, Liao C-T, Hsu H-F, Wu C. Influence of Mesogenic Properties of Cruciform-Shaped Liquid Crystals by Incorporating Side-Arms with a Laterally-Substituted-Fluorine. Crystals. 2013; 3(2):339-349. https://doi.org/10.3390/cryst3020339
Chicago/Turabian StyleLin, Yi-Hui, Yamuna Ezhumalai, Yu-Ling Yang, Ching-Ting Liao, Hsiu-Fu Hsu, and Chunhung Wu. 2013. "Influence of Mesogenic Properties of Cruciform-Shaped Liquid Crystals by Incorporating Side-Arms with a Laterally-Substituted-Fluorine" Crystals 3, no. 2: 339-349. https://doi.org/10.3390/cryst3020339
APA StyleLin, Y.-H., Ezhumalai, Y., Yang, Y.-L., Liao, C.-T., Hsu, H.-F., & Wu, C. (2013). Influence of Mesogenic Properties of Cruciform-Shaped Liquid Crystals by Incorporating Side-Arms with a Laterally-Substituted-Fluorine. Crystals, 3(2), 339-349. https://doi.org/10.3390/cryst3020339




