2-Azidoimidazolium Ions Captured by N-Heterocyclic Carbenes: Azole-Substituted Triazatrimethine Cyanines
Abstract
:1. Introduction
2. Results and Discussion
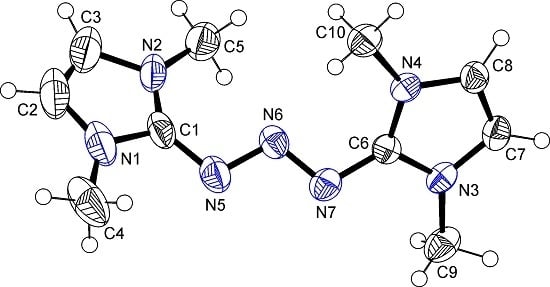
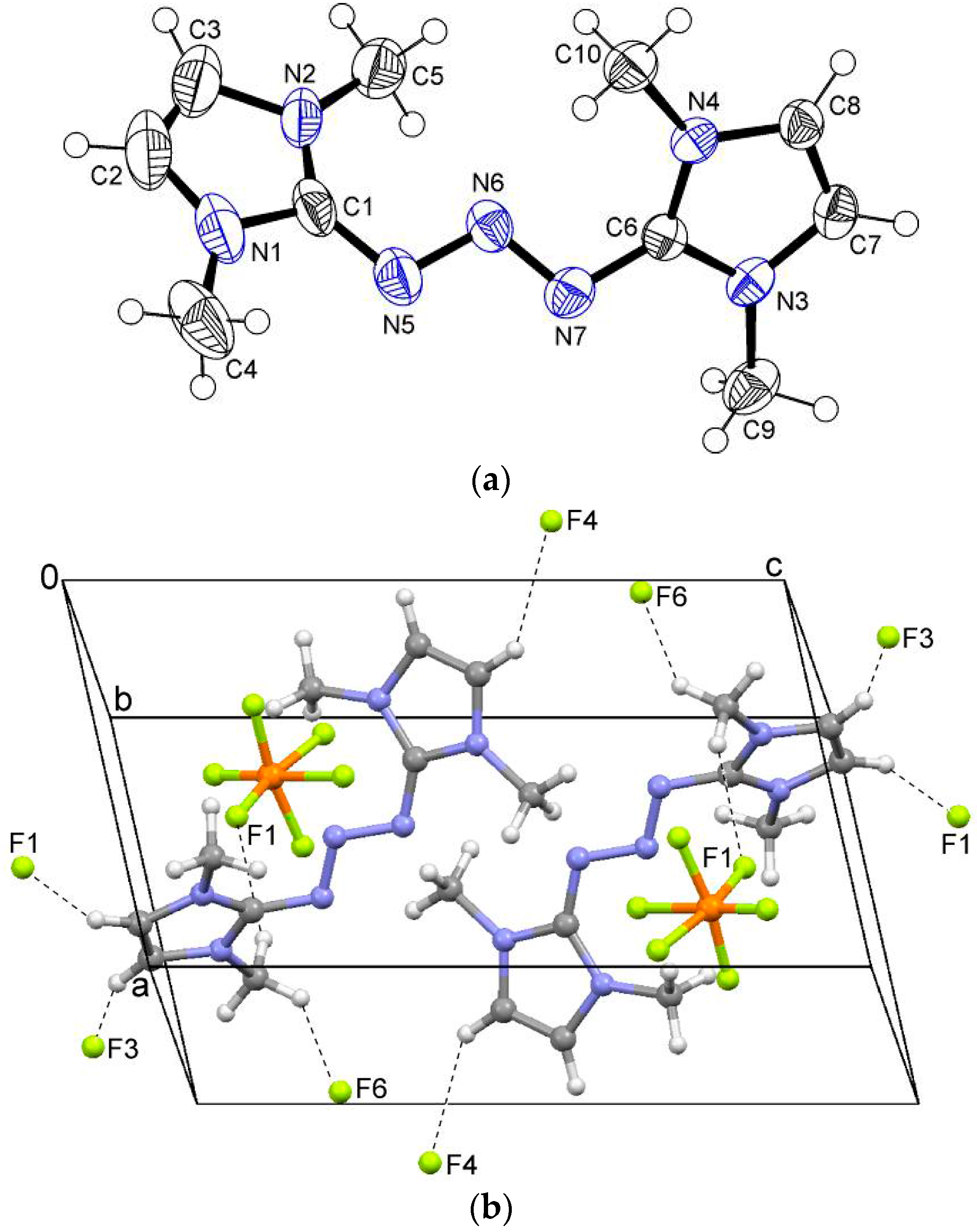
2.1. Crystal Structures
2.2. UV-Vis Spectroscopy
2.3. Differential Scanning Calorimetry (DSC) and Thermogravimetric Analysis (TGA)
2.4. Cyclic Voltammetry (CV)
3. Experimental Section
3.1. 1,3-Dimethyl-2-(1-(1,3-dimethylimidazolin-2-ylidene)triazen-3-yl)imidazolium Hexafluoridophosphate (1)
3.2. 1,3-Dimethoxy-2-(1-(1,3-dimethoxyimidazolin-2-ylidene)triazen-3-yl)imidazolium Hexafluoridophosphate (2)
3.3. 1,3-Dimethoxy-2-(1-(1-methyl-4-dimethylamino-1,2,4-triazolin-5-ylidene)triazen-3-yl)imidazolium Hexafluoridophosphate (3)
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Laus, G.; Schwärzler, A.; Schuster, P.; Bentivoglio, G.; Hummel, M.; Wurst, K.; Kahlenberg, V.; Lörting, T.; Schütz, J.; Peringer, P.; et al. N,N′-Di(alkyloxy)imidazolium Salts: New Patent-free Ionic Liquids and NHC Precatalysts. Z. Naturforsch. 2007, 62b, 295–308. [Google Scholar] [CrossRef]
- Kitamura, M.; Kato, S.; Yano, M.; Tashiro, N.; Shiratake, Y.; Sando, M.; Okauchi, T. A reagent for safe and efficient diazo–transfer to primary amines: 2–azido–1,3–dimethylimidazolinium hexafluorophosphate. Org. Biomol. Chem. 2014, 12, 4397–4406. [Google Scholar] [CrossRef] [PubMed]
- Zanirato, P.; Cerini, S. On the utility of the azido transfer protocol: Synthesis of 2- and 5-azido N-methylimidazoles, 1,3-thiazoles and N-methylpyrazole and their conversion to triazole–azole bisheteroaryls. Org. Biomol. Chem. 2005, 3, 1508–1513. [Google Scholar] [CrossRef] [PubMed]
- Khramov, D.M.; Bielawski, C.W. Triazene formation via reaction of imidazol-2-ylidenes with azides. Chem. Commun. 2005, 39, 4958–4960. [Google Scholar] [CrossRef] [PubMed]
- Khramov, D.M.; Bielawski, C.W. Donor–Acceptor Triazenes: Synthesis, Characterization, and Study of Their Electronic and Thermal Properties. J. Org. Chem. 2007, 72, 9407–9417. [Google Scholar] [CrossRef] [PubMed]
- Kunetskiy, R.A.; Cisarova, I.; Saman, D.; Lyapkalo, I.M. New Lipophilic 2-Amino-N,N′-dialkyl-4,5-dimethylimidazolium Cations: Synthesis, Structure, Properties, and Outstanding Thermal Stability in Alkaline Media. Chem. Eur. J. 2009, 15, 9477–9485. [Google Scholar] [CrossRef] [PubMed]
- Tennyson, A.G.; Ono, R.J.; Hudnall, T.W.; Khramov, D.M.; Er, J.A.V.; Kamplain, J.W.; Lynch, V.M.; Sessler, J.L.; Bielawski, C.W. Quinobis(imidazolylidene): Synthesis and Study of an Electron-Configurable Bis(N-Heterocyclic Carbene) and Its Bimetallic Complexes. Chem. Eur. J. 2010, 16, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Ghadwal, R.S.; Roesky, H.W.; Granitzka, M.; Stalke, D. A Facile Route to Functionalized N–Heterocyclic Carbenes (NHCs) with NHC Base-Stabilized Dichlorosilylene. J. Am. Chem. Soc. 2010, 132, 10018–10020. [Google Scholar] [CrossRef] [PubMed]
- Tennyson, A.G.; Moorhead, E.J.; Madison, B.L.; Er, J.A.V.; Lynch, V.M.; Bielawski, C.W. Methylation of Ylidene-Triazenes: Insight and Guidance for 1,3-Dipolar Cycloaddition Reactions. Eur. J. Org. Chem. 2010, 2010, 6277–6282. [Google Scholar] [CrossRef]
- Patil, S.; Bugarin, A. Crystal structure of (E)-1,3-dimethyl-2-[3-(3-nitrophenyl)triaz-2-en-1-ylidene]-2,3-dihydro-1H-imidazole. Acta Crystallogr. 2014, E70, 224–227. [Google Scholar] [CrossRef] [PubMed]
- Lysenko, S.; Daniliuc, C.G.; Jones, P.G.; Tamm, M. Tungsten alkylidyne complexes with ancillary imidazolin-2-iminato and imidazolidin-2-iminato ligands and their use in catalytic alkyne metathesis. J. Organomet. Chem. 2013, 744, 7–14. [Google Scholar] [CrossRef]
- Naef, R.; Balli, H. Synthesis, Structure and Photochemical Properties of 4,4′,7,7′-Tetra-substituted1,1′,3,3′-Tetraethylbenzimidazolotriazatrimethine Cyanines. Helv. Chim. Acta 1978, 61, 2958–2973. [Google Scholar] [CrossRef]
- Hanot, V.P.; Robert, T.D.; Kolnaar, J.J.A.; Haasnoot, J.G.; Kooijman, H.; Spek, A.L. Crystal structure and magnetic properties of a µ-halogeno-bridged copper(II) chain with neutral planar [Cu(batt)Cl] units (Hbatt = 1,3-bis[3-(5-amino-1,2,4-triazolyl)]triazene). Inorg. Chim. Acta 1997, 256, 327–329. [Google Scholar] [CrossRef]
- Hanot, V.P.; Robert, T.D.; Haasnoot, J.G.; Kooijman, H.; Spek, A.L. Crystal structure and resonance Raman spectroscopic study of {1,3-bis[3-(5-amino-1,2,4-triazolyl)]triazenido-N′4,N2,N″4}chloro palladium(II)-methanol (1/1). J. Chem. Cryst. 1998, 28, 343–351. [Google Scholar] [CrossRef]
- Hanot, V.P.; Robert, T.D.; Haasnoot, J.G.; Kooijman, H.; Spek, A.L. Crystal structure and spectroscopic study of bis{1,3-bis[3-(5-amino-1,2,4-triazolyl)]triazenido-N′4,N2,N″4}nickel(II) tetrahydrate. J. Chem. Cryst. 1999, 29, 299–308. [Google Scholar] [CrossRef]
- Serebryanskaya, T.V.; Ivashkevich, L.S.; Lyakhov, A.S.; Gaponik, P.N.; Ivashkevich, O.A. 1,3-Bis(2-alkyltetrazol-5-yl)triazenes and their Fe(II), Co(II) and Ni(II) complexes: Synthesis, spectroscopy, and thermal properties. Crystal structure of Fe(II) and Co(II) 1,3-bis(2-methyltetrazol-5-yl)triazenide complexes. Polyhedron 2010, 29, 2844–2850. [Google Scholar] [CrossRef]
- Eulgem, P.J.; Klein, A.; Maggiarosa, N.; Naumann, D.; Pohl, R.W.H. New Rare Earth Metal Complexes with Nitrogen-Rich Ligands: 5,5′-Bitetrazolate and 1,3-Bis(tetrazol-5-yl)triazenate–On the Borderline between Coordination and the Formation of Salt-Like Compounds. Chem. Eur. J. 2008, 14, 3727–3736. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.; Guru Row, T.N. Role of organic fluorine in crystal engineering. CrystEngComm 2011, 13, 2175–2186. [Google Scholar] [CrossRef]
- D’Oria, E.; Novoa, J.J. On the hydrogen bond nature of the C–H···F interactions in molecular crystals. An exhaustive investigation combining a crystallographic database search and ab initio theoretical calculations. CrystEngComm 2008, 10, 423–436. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of Bond Lengths determined by X-Ray and Neutron Diffraction. Part I. Bond Lengths in Organic Compounds. J. Chem. Soc. Perkin Trans. 1987, 2, S1–S19. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Abboud, J.-L.M.; Taft, R.W. The Solvatochromic Comparison Method. The π* Scale of Solvent Polarities. J. Am. Chem. Soc. 1977, 99, 6027–6038. [Google Scholar] [CrossRef]
- Taft, R.W.; Kamlet, M.J. The Solvatochromic Comparison Method. The α Scale of Solvent Hydrogen-Bond Donor (HBD) Acidities. J. Am. Chem. Soc. 1976, 98, 2886–2894. [Google Scholar] [CrossRef]
- Kamlet, M.J.; Taft, R.W. The Solvatochromic Comparison Method. The β Scale of Solvent Hydrogen-Bond Acceptor (HBA) Basicities. J. Am. Chem. Soc. 1976, 98, 377–383. [Google Scholar] [CrossRef]
- Marcus, Y. The Properties of Organic Liquids that are Relevant to their Use as Solvating Solvents. Chem. Soc. Rev. 1993, 22, 409–416. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Reichert, W.M.; Swatloski, R.P.; Broker, G.A.; Pitner, W.R.; Seddon, K.R.; Rogers, R.D. Efficient, halide free synthesis of new, low cost ionic liquids: 1,3-dialkylimidazolium salts containing methyl- and ethyl-sulfate anions. Green Chem. 2002, 4, 407–413. [Google Scholar] [CrossRef]
- Schwärzler, A.; Laus, G.; Kahlenberg, V.; Wurst, K.; Gelbrich, T.; Kreutz, C.; Kopacka, H.; Bonn, G.; Schottenberger, H. Quaternary 4-amino-1,2,4-triazolium Salts: Crystal structures of Ionic Liquids and N-heterocyclic carbene (NHC) complexes. Z. Naturforsch. 2009, 64b, 603–616. [Google Scholar] [CrossRef]
- Naumann, S.; Buchmeiser, M.R. Liberation of N-heterocyclic carbenes (NHCs) from thermally labile progenitors: Protected NHCs as versatile tools in organo- and polymerization catalysis. Catal. Sci. Technol. 2014, 4, 2466–2479. [Google Scholar] [CrossRef]







| Compound | 1 | 2 | 3 |
|---|---|---|---|
| CCDC No. | 1428586 | 1428587 | 1428588 |
| Empirical formula | C10H16N7·F6P | C10H16N7O4·F6P | C10H18N9O2·F6P |
| Formula weight | 379.27 | 443.27 | 441.3 |
| Crystal system | Triclinic | Monoclinic | Monoclinic |
| Space group | P | P21/c | P21 |
| a/Å | 7.608 (1) | 10.354 (1) | 10.567 (1) |
| b/Å | 8.386 (1) | 13.153 (1) | 6.573 (1) |
| c/Å | 13.806 (1) | 13.863 (1) | 14.267 (2) |
| α/° | 80.893 (2) | – | – |
| β/° | 77.396 (2) | 109.841 (8) | 111.390 (11) |
| γ/° | 70.006 (2) | – | – |
| Volume/Å3 | 804.5 (1) | 1775.8 (2) | 922.6 (2) |
| Z | 2 | 4 | 2 |
| Dx/g·cm−3 | 1.57 | 1.66 | 1.59 |
| μ/mm−1 | 0.24 | 0.25 | 0.24 |
| F (000) | 388 | 904 | 452 |
| T/K | 170 (2) | 173 (2) | 173 (2) |
| Crystal size/mm3 | 0.18 × 0.15 × 0.03 | 0.40 × 0.28 × 0.08 | 0.38 × 0.32 × 0.28 |
| θmax/° | 25.0 | 25.4 | 25.3 |
| Index ranges | –8 ≤ h ≤ 9, –9 ≤ k ≤ 9, 0 ≤ l ≤ 16 | –12 ≤ h ≤ 12, –15 ≤ k ≤ 15, –12 ≤ l ≤ 16 | –12 ≤ h ≤ 10, –7 ≤ k ≤ 7, –15 ≤ l ≤ 17 |
| Reflections collected | 3311 | 11235 | 5556 |
| Independent reflections (Rint) | 3311 | 3251 (0.026) | 3303 (0.023) |
| Observed reflections (I > 2σ (I)) | 2651 | 2701 | 3215 |
| Absorption correction | multi-scan | multi-scan | multi-scan |
| Restraints/parameters | 0/278 | 162/312 | 157/313 |
| Goodness-of-fit on F2 | 1.04 | 1.04 | 1.10 |
| R1/wR2 (I > 2σ (I)) | 0.057/0.124 | 0.037/0.088 | 0.031/0.078 |
| R1/wR2 (all data) | 0.080/0.134 | 0.048/0.094 | 0.032/0.079 |
| Δρmax/Δρmin/e·Å−3 | 0.277/–0.426 | 0.44/–0.26 | 0.24/–0.19 |
| Compound | Interaction | H···A | C···A | C–H···A | Symmetry Operation A |
|---|---|---|---|---|---|
| 1 | C8–H···F1 | 2.417 | 3.28 (1) | 151.0 | x, y, 1 + z |
| C9–HC···F1 | 2.417 | 3.18 (1) | 134.5 | 1 − x, 1 − y, 1 − z | |
| C7–H···F3 | 2.422 | 3.26 (2) | 146.4 | x, −1 + y, 1 + z | |
| C9–HA···F6 | 2.456 | 2.99 (1) | 114.1 | −x, 1 − y, 1 − z | |
| C10–HA···F5 | 2.463 | 3.19 (1) | 130.7 | 1 − x, 2 − y, 1 − z | |
| C2–H···F4 | 2.570 | 3.17 (1) | 131.2 | 1 + x, y, z | |
| 2 | C2–H···O3 | 2.295 | 3.128 (3) | 146.0 | 1 − x, 1/2 + y, 3/2 − z |
| C7–H···F6A | 2.281 | 3.21 (1) | 167.3 | 2 − x, −1/2 + y, 3/2 − z | |
| C8–H···F3A | 2.384 | 3.24 (1) | 149.8 | 2 − x, −y,1 − z | |
| C9–HB···F1A | 2.414 | 3.02 (1) | 119.5 | x, 1/2 − y, 1/2 + z | |
| C3–H···F5A | 2.460 | 3.30 (1) | 147.6 | 1 − x, 1 − y, 1 − z | |
| C4–HC···O4 | 2.575 | 3.355 (3) | 136.6 | 1 − x, −y, 1 − z | |
| 3 | C3–H···F6A | 2.512 | 3.34 (1) | 145.3 | x, 1 + y, z |
| C3–H···F3A | 2.542 | 3.27 (1) | 133.1 | x, 1 + y, z | |
| C7–H···F5A | 2.339 | 3.18 (1) | 147.2 | 1 + x, −1 + y, z | |
| C9–HB···F5A | 2.446 | 3.42 (1) | 173.7 | 1 − x, −1/2 + y, − z | |
| C4–HB···F1A | 2.421 | 3.19 (1) | 134.6 | 1 − x, −1/2 + y, 1 − z | |
| C4–HA···F3A | 2.464 | 3.10 (1) | 121.8 | 1 − x, 1/2 + y, 1 − z | |
| C10–HC···N5 | 2.584 | 3.378 (4) | 138.1 | x, −1 + y, z | |
| C5–HB···N7 | 2.555 | 3.480 (3) | 157.5 | 1 − x, 1/2 + y, −z | |
| C8–HB···O1 | 2.385 | 3.349 (2) | 168.2 | 2 − x, −1/2 + y, 1 − z |
| Compound | 1 | 2 | 3 |
|---|---|---|---|
| C1–N5 | 1.359 (7) | 1.353 (3) | 1.353 (3) |
| N5–N6 | 1.319 (4) | 1.302 (2) | 1.304 (3) |
| N6–N7 | 1.296 (5) | 1.309 (3) | 1.303 (3) |
| N7–C6 | 1.374 (4) | 1.351 (2) | 1.356 (3) |
| C1–N5–N6 | 110.3 (3) | 113.3 (2) | 114.5 (2) |
| N5–N6–N7 | 111.3 (3) | 109.8 (2) | 110.0 (2) |
| N6–N7–C6 | 110.0 (3) | 112.9 (2) | 113.0 (2) |
| C1–N5–N6–N7 | 173.9 (3) | 177.7 (2) | 177.1 (2) |
| N5–N6–N7–C6 | 175.0 (3) | 179.9 (2) | 178.2 (2) |
| Solvent | π* | α | 1 | 2 | 3 |
|---|---|---|---|---|---|
| λmax/nm | λmax/nm | λmax/nm | |||
| H2O | 1.09 | 1.17 | 391.0 | 403.0 | 361.5 |
| AcOH | 0.64 | 1.12 | 393.0 | 396.0 | 362.8 |
| MeOH | 0.60 | 0.98 | 395.5 | 394.5 | 364.3 |
| EtOH | 0.54 | 0.86 | 397.5 | 393.7 | 367.5 |
| 2-PrOH | 0.48 | 0.76 | 399.0 | 393.7 | 367.0 |
| CH2Cl2 | 0.82 | 0.13 | 403.5 | 392.0 | 371.7 |
| DMSO | 1.00 | 0 | 405.0 | – | – |
| Compound | Scan rate/mV·s−1 | (Ep)c/mV | (Ip)c/µA |
|---|---|---|---|
| 1 | 20 | n.d. | 0.6 |
| 50 | n.d. | 0.9 | |
| 100 | −1470 | 2.4 | |
| 2 | 20 | −1410 | 1.8 |
| 50 | −1445 | 3.0 | |
| 100 | −1475 | 3.8 | |
| 3 | 20 | −1390 | 1.2 |
| 50 | −1420 | 2.0 | |
| 100 | −1430 | 2.7 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haslinger, S.; Laus, G.; Kahlenberg, V.; Wurst, K.; Bechtold, T.; Vergeiner, S.; Schottenberger, H. 2-Azidoimidazolium Ions Captured by N-Heterocyclic Carbenes: Azole-Substituted Triazatrimethine Cyanines. Crystals 2016, 6, 40. https://doi.org/10.3390/cryst6040040
Haslinger S, Laus G, Kahlenberg V, Wurst K, Bechtold T, Vergeiner S, Schottenberger H. 2-Azidoimidazolium Ions Captured by N-Heterocyclic Carbenes: Azole-Substituted Triazatrimethine Cyanines. Crystals. 2016; 6(4):40. https://doi.org/10.3390/cryst6040040
Chicago/Turabian StyleHaslinger, Simone, Gerhard Laus, Volker Kahlenberg, Klaus Wurst, Thomas Bechtold, Stefan Vergeiner, and Herwig Schottenberger. 2016. "2-Azidoimidazolium Ions Captured by N-Heterocyclic Carbenes: Azole-Substituted Triazatrimethine Cyanines" Crystals 6, no. 4: 40. https://doi.org/10.3390/cryst6040040
APA StyleHaslinger, S., Laus, G., Kahlenberg, V., Wurst, K., Bechtold, T., Vergeiner, S., & Schottenberger, H. (2016). 2-Azidoimidazolium Ions Captured by N-Heterocyclic Carbenes: Azole-Substituted Triazatrimethine Cyanines. Crystals, 6(4), 40. https://doi.org/10.3390/cryst6040040