Zirconate Pyrochlore Frustrated Magnets: Crystal Growth by the Floating Zone Technique
Abstract
:1. Introduction
2. Factors Affecting Crystal Growth
2.1. High Melting Point
2.2. Evaporation
2.3. Mixed Valence State
2.4. Crystal Structure
3. Methods
4. Floating Zone Growth of ZrO (with La → Gd)
4.1. Feed Rod Preparation
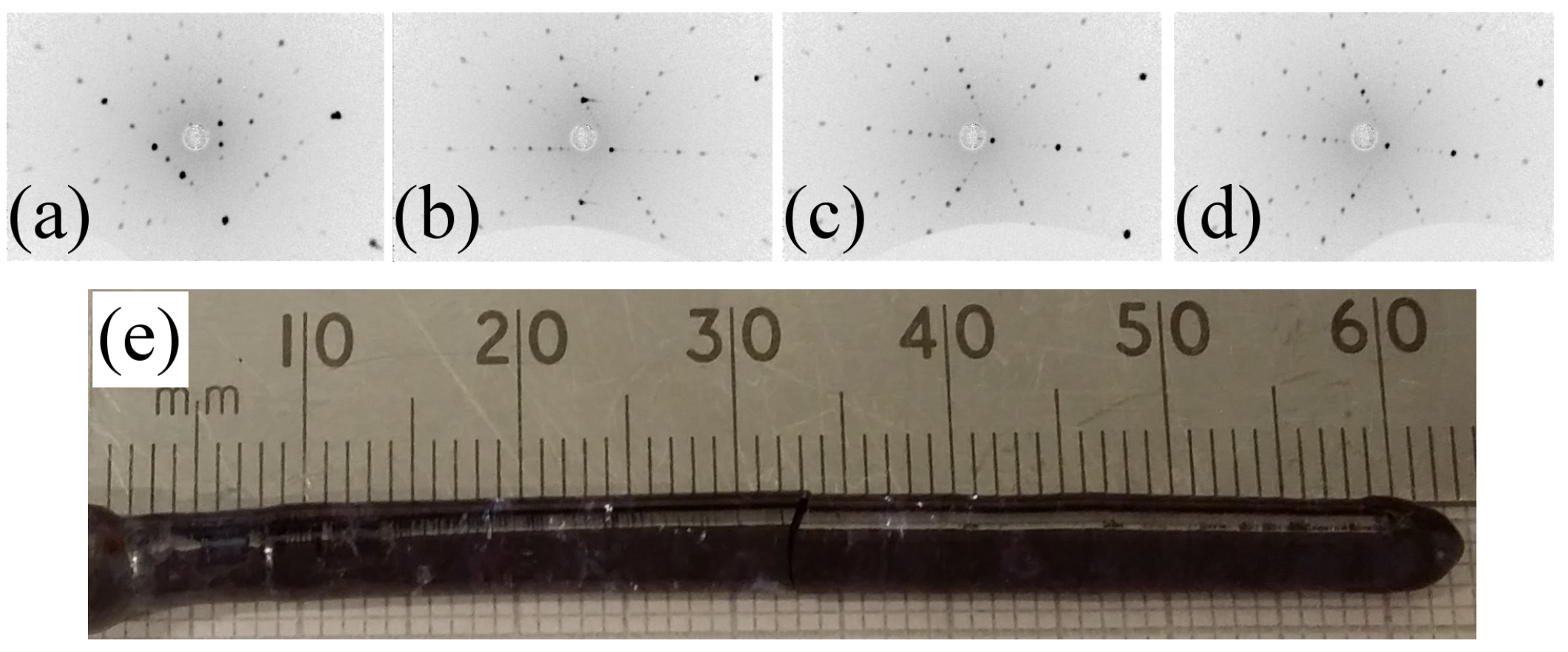
4.2. Crystal Growth Details
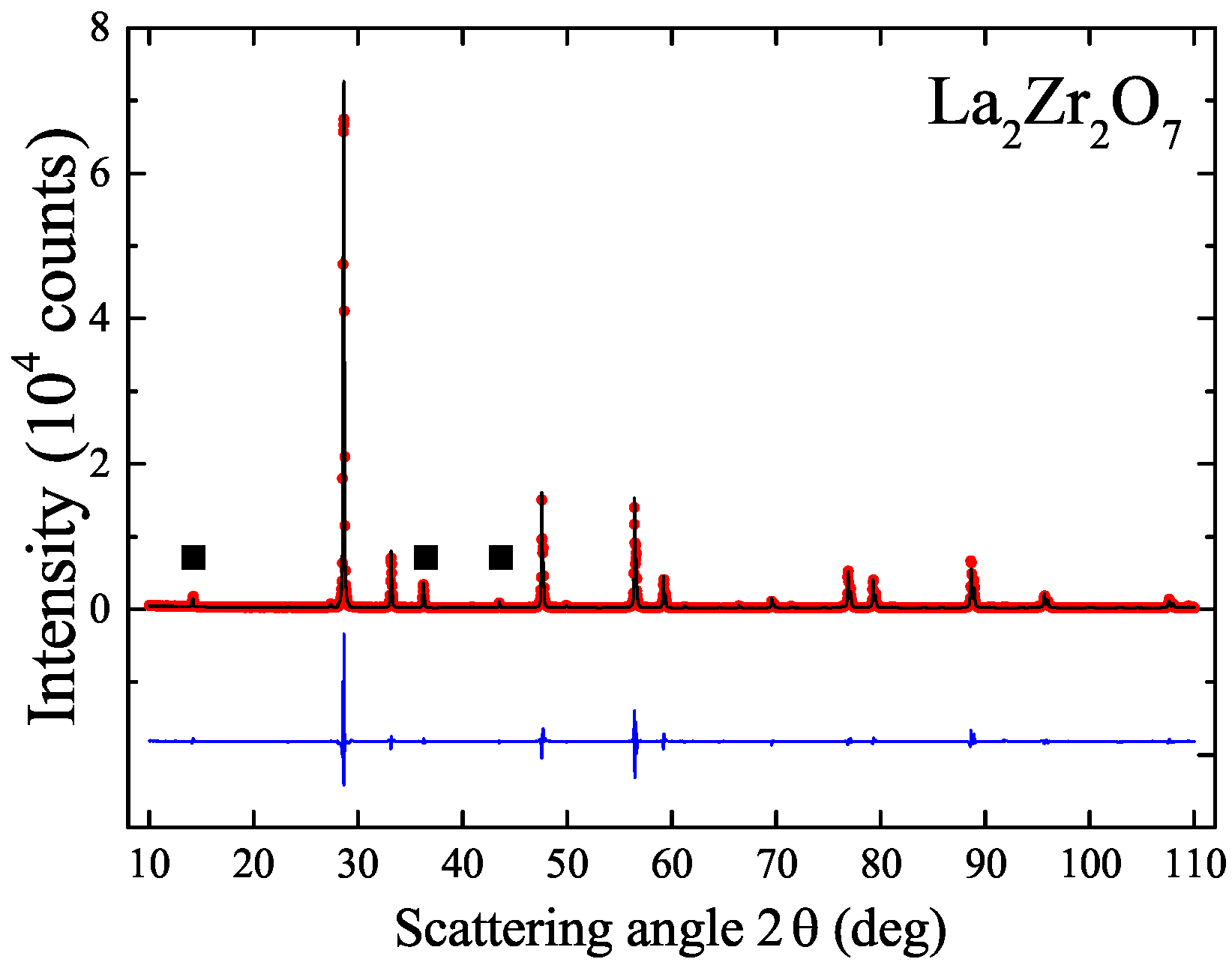
4.2.1. LaZrO
4.2.2. PrZrO
4.2.3. NdZrO
4.2.4. SmZrO
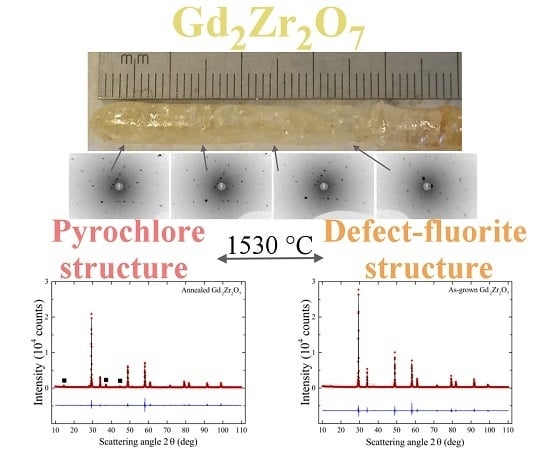
4.2.5. GdZrO
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Harris, M.J.; Bramwell, S.T.; McMorrow, D.F.; Zeiske, T.; Godfrey, K.W. Geometrical Frustration in the Ferromagnetic Pyrochlore Ho2Ti2O7. Phys. Rev. Lett. 1997, 79, 2554–2557. [Google Scholar] [CrossRef]
- Ramirez, A.P.; Hayashi, A.; Cava, R.J.; Siddharthan, R.; Shastry, B.S. Zero-Point entropy in ’spin ice’. Nature 1999, 399, 333–335. [Google Scholar] [CrossRef]
- Gaulin, B.D.; Reimers, J.N.; Mason, T.E.; Greedan, J.E.; Tun, Z. Spin freezing in the geometrically frustrated pyrochlore antiferromagnet Tb2Mo2O7. Phys. Rev. Lett. 1992, 69, 3244–3247. [Google Scholar] [CrossRef] [PubMed]
- Gardner, J.S.; Gaulin, B.D.; Lee, S.-H.; Broholm, C.; Raju, N.P.; Greedan, J.E. Glassy Statics and Dynamics in the Chemically Ordered Pyrochlore Antiferromagnet Y2Mo2O7. Phys. Rev. Lett. 1999, 83, 211–214. [Google Scholar] [CrossRef]
- Gardner, J.S.; Dunsiger, S.R.; Gaulin, B.D.; Gingras, M.J.P.; Greedan, J.E.; Kiefl, R.F.; Lumsden, M.D.; MacFarlane, W.A.; Raju, N.P.; Sonier, J.E.; et al. Cooperative Paramagnetism in the Geometrically Frustrated Pyrochlore Antiferromagnet Tb2Ti2O7. Phys. Rev. Lett. 1999, 82, 1012–1015. [Google Scholar] [CrossRef]
- Gardner, J.S.; Gingras, M.J.P.; Greedan, J.E. Magnetic pyrochlore oxides. Rev. Mod. Phys. 2010, 82, 53–107. [Google Scholar] [CrossRef]
- Ramirez, A.P. Strongly Geometrically Frustrated Magnets. Annu. Rev. Mater. Sci. 1994, 24, 453–480. [Google Scholar] [CrossRef]
- Greedan, J.E. Geometrically frustrated magnetic materials. J. Mater. Chem. 2001, 11, 37–53. [Google Scholar] [CrossRef]
- Subramanian, M.A.; Aravamudan, G.; Subba Rao, G.V. Oxide Pyrochlores —A Review. Prog. Solid St. Chem. 1983, 15, 55–143. [Google Scholar] [CrossRef]
- Gardner, J.S.; Gaulin, B.D.; Paul, D.M.K. Single crystal growth by the floating-zone method of a geometrically frustrated pyrochlore antiferromagnet, Tb2Ti2O7. J. Cryst. Growth 1998, 191, 740–745. [Google Scholar] [CrossRef]
- Balakrishnan, G.; Petrenko, O.A.; Lees, M.R.; Paul, D.M. Single crystal growth of rare earth titanate pyrochlores. J. Phys.: Condens. Matter. 1998, 10, L723–L725. [Google Scholar] [CrossRef]
- Prabhakaran, D.; Boothroyd, A.T. Crystal growth of spin-ice pyrochlores by the floating-zone method. J. Cryst. Growth 2011, 318, 1053–1056. [Google Scholar] [CrossRef]
- Chang, L.-J.; Onoda, S.; Su, Y.; Kao, Y.-J.; Tsuei, K.-D.; Yasui, Y.; Kakurai, K.; Lees, M.R. Higgs transition from a magnetic coulomb liquid to a ferromagnet in Yb2Ti2O7. Nat. Commun. 2012, 3, 992. [Google Scholar] [CrossRef] [PubMed]
- Ross, K.A.; Proffen, T.; Dabkowska, H.A.; Quilliam, J.A.; Yaraskavitch, L.R.; Kycia, J.B.; Gaulin, B.D. Lightly stuffed pyrochlore structure of single-crystalline Yb2Ti2O7 grown by the optical floating zone technique. Phys. Rev. B 2012, 86, 174424. [Google Scholar] [CrossRef]
- Li, Q.; Xu, L.; Fan, C.; Zhang, F.; Lv, Y.; Ni, B.; Zhao, Z.; Sun, X. Single crystal growth of the pyrochlores R2Ti2O7 (R=rare earth) by the optical floating-zone method. J. Cryst. Growth 2013, 377, 96–100. [Google Scholar] [CrossRef]
- Taguchi, Y.; Ohgushi, K.; Tokura, Y. Optical probe of the metal-insulator transition in pyrochlore-type molybdate. Phys. Rev. B 2002, 65, 115102. [Google Scholar] [CrossRef]
- Kézsmárki, I.; Hanasaki, N.; Hashimoto, D.; Iguchi, S.; Taguchi, Y.; Miyasaka, S.; Tokura, Y. Charge dynamics near the electron-correlation induced metal-insulator transition in pyrochlore-type molybdates. Phys. Rev. Lett. 2004, 93, 266401. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Onoda, S.; Balents, L. Generic quantum spin ice. Phys. Rev. B 2012, 86, 104412. [Google Scholar] [CrossRef]
- Gingras, M.J.P.; McClarty, P.A. Quantum spin ice: a search for gapless quantum spin liquids in pyrochlore magnets. Rep. Prog. Phys. 2014, 77, 056501. [Google Scholar] [CrossRef] [PubMed]
- Kimura, K.; Nakatsuji, S.; Wen, J.-J.; Broholm, C.; Stone, M.B.; Nishibori, E.; Sawa, H. Quantum fluctuations in spin-ice-like Pr2Zr2O7. Nat. Commun. 2013, 4, 1934. [Google Scholar] [CrossRef] [PubMed]
- Ciomaga Hatnean, M.; Decorse, C.; Lees, M.R.; Petrenko, O.A.; Keeble, D.S.; Balakrishnan, G. Structural and magnetic properties of single crystals of the geometrically frustrated zirconium pyrochlore, Pr2Zr2O7. Mater. Res. Express 2014, 1, 026109. [Google Scholar] [CrossRef]
- Ciomaga Hatnean, M.; Lees, M.R.; Balakrishnan, G. Growth of single-crystals of rare-earth zirconate pyrochlores, Ln2Zr2O7 (with Ln=La, Nd, Sm, and Gd) by the floating zone technique. J. Cryst. Growth 2015, 418, 1–6. [Google Scholar] [CrossRef]
- Matsuhira, K.; Sekine, C.; Paulsen, C.; Wakeshima, M.; Hinatsu, Y.; Kitazawa, T.; Kiuchi, Y.; Hiroi, Z.; Takagi, S. Spin freezing in the pyrochlore antiferromagnet Pr2Zr2O7. J. Phys.: Conf. Ser. 2009, 145, 012031. [Google Scholar] [CrossRef]
- Koohpayeh, S.M.; Wen, J.-J.; Trump, B.A.; Broholm, C.L.; McQueen, T.M. Synthesis, floating zone crystal growth and characterization of the quantum spin ice Pr2Zr2O7 pyrochlore. J. Cryst. Growth 2014, 402, 291–298. [Google Scholar] [CrossRef]
- Roth, R.S. Pyrochlore-Type compounds containing double oxides of trivalent and tetravalent ions. J. Res. Natl. Bur. Stand. 1956, 56, 2643. [Google Scholar] [CrossRef]
- Shannon, R.D. Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chaleogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Ewing, R.C.; Weber, W.J.; Lian, J. Nuclear waste disposal —pyrochlore (A2B2O7): Nuclear waste form for the immobilization of plutonium and "minor" actinides. J. Appl. Phys. 2004, 95, 5949–5971. [Google Scholar] [CrossRef]
- Michel, D.; Jorba, M.P.Y.; Collongues, R. Etude de la transformation ordre-desordre de la structure fluorite a la structure pyrochlore pour des phases (1 − x)ZrO2 − xLn2O3. Mat. Res. Bull. 1974, 9, 1457–1468. [Google Scholar] [CrossRef]
- Michel, D.; Jorba, M.P.Y.; Collongues, R. Study by raman spectroscopy of order-disorder phenomena occurring in some binary oxides with fluorite- related structures. J. Raman Spectrosc. 1976, 5, 163–180. [Google Scholar] [CrossRef]
- Rushton, M.J.D.; Grimes, R.W. Predicted pyrochlore to fluorite disorder temperature for A2Zr2O7 compositions. J. Mater. Res. 2004, 19, 1603–1604. [Google Scholar] [CrossRef]
- Ohtani, H.; Matsumoto, S.; Sundman, B.; Sakuma, T.; Hasebe, M. Equilibrium between fluorite and pyrochlore structures in the ZrO2 - Nd2O3 system. Mater. Trans. 2005, 46, 1167–1174. [Google Scholar] [CrossRef]
- Karaulov, A.G.; Zoz, E.I. Phase formation in the ZrO2 - HfO2 - Gd2O3 and ZrO2 - HfO2 - Yb2O3 systems. B. Acad. Sci. USSR Ch. + 1966, 15, 2011–2016. [Google Scholar] [CrossRef]
- Radha, A.V.; Ushakov, S.V.; Navrotsky, A. Thermochemistry of lanthanum zirconate pyrochlore. J. Mater. Res. 2009, 24, 3350–3357. [Google Scholar] [CrossRef]
- Karaulov, A.G.; Zoz, E.I. Phase formation in the ZrO2 - HfO2 - Gd2O3 and ZrO2 - HfO2 - Yb2O3 systems. Refract. Ind. Ceram. 1999, 40, 479–483. [Google Scholar] [CrossRef]
- Payne, J.L.; Tucker, M.G.; Evans, I.R. From fluorite to pyrochlore: Characterisation of local and average structure of neodymium zirconate, Nd2Zr2O7. J. Solid State Chem. 2013, 205, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.R.S.; Shekar, N.V.C.; Sahu, P.C. Pressure induced structural transformation of pyrochlore Gd2Zr2O7. Solid State Commun. 2008, 147, 357–359. [Google Scholar] [CrossRef]
- Surblé, S.; Heathman, S.; Raison, P.E.; Bouëxière, D.; Popa, K.; Caciuffo, R. Pressure-Induced structural transition in Ln2Zr2O7 (Ln = Ce, Nd, Gd) pyrochlores. Phys. Chem. Minerals 2010, 37, 761–767. [Google Scholar] [CrossRef]
- Xiao, H.Y.; Zhang, F.X.; Gao, F.; Lang, M.; Ewing, R.C.; Weber, W.J. Zirconate pyrochlores under high pressure. Phys. Chem. Chem. Phys. 2010, 12, 12472–12477. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Blanchard, P.E.R.; Clements, R.; Kennedy, B.J.; Ling, C.D.; Reynolds, E.; Avdeev, M.; Stampfl, A.P.J.; Zhang, Z.; Jang, L.-Y. Does local disorder occur in the pyrochlore zirconates? Inorg. Chem. 2012, 51, 13237–13244. [Google Scholar] [CrossRef] [PubMed]
- Moriga, T.; Yoshiasa, A.; Kanamaru, F.; Koto, K.; Yoshimura, M.; Somiya, S. Crystal structure analyses of the pyrochlore and fluorite-type Zr2Gd2O7 and anti-phase domain structure. Solid State Ionics 1989, 31, 319–328. [Google Scholar] [CrossRef]
- Reynolds, E.; Blanchard, P.E.R.; Kennedy, B.J.; Ling, C.D.; Liu, S.; Avdeev, M.; Zhang, Z.; Cuello, G.J.; Tadich, A.; Jang, L.-Y. Anion Disorder in Lanthanoid Zirconates Gd2-xTbxZr2O7. Inorg. Chem. 2013, 52, 8409–8415. [Google Scholar] [CrossRef] [PubMed]
- Ciomaga Hatnean, M.; Lees, M.R.; Petrenko, O.A.; Keeble, D.S.; Balakrishnan, G.; Gutmann, M.J.; Klekovkina, V.V.; Malkin, B.Z. Structural and magnetic investigations of single-crystalline neodymium zirconate pyrochlore Nd2Zr2O7. Phys. Rev. B 2015, 91, 174416. [Google Scholar] [CrossRef]
- Lhotel, E.; Petit, S.; Guitteny, S.; Florea, O.; Ciomaga Hatnean, M.; Colin, C.; Ressouche, E.; Lees, M.R.; Balakrishnan, G. Fluctuations and All-In—All-Out Ordering in Dipole-Octupole Nd2Zr2O7. Phys. Rev. Lett. 2015, 115, 197202. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Lhotel, E.; Canals, B.; Ciomaga Hatnean, M.; Ollivier, J.; Mutka, H.; Ressouche, E.; Wildes, A.R.; Lees, M.R.; Balakrishnan, G. Observation of magnetic fragmentation in spin ice. Nat. Phys. Advanced Online Publication 2016. [Google Scholar] [CrossRef]
- Mandal, B.P.; Tyagi, A.K. Pyrochlores: Potential multifunctional materials. BARC Newsletter 2010, 313, 6–13. [Google Scholar]












| Ion | Coordination Number | Effective Ionic Radius (Å) | |
|---|---|---|---|
| La | 8 | 1.16 | 1.61 |
| Pr | 8 | 1.126 | 1.56 |
| Pr | 8 | 0.96 | — |
| Nd | 8 | 1.109 | 1.54 |
| Sm | 8 | 1.079 | 1.50 |
| Eu | 8 | 1.066 | 1.48 |
| Gd | 8 | 1.053 | 1.46 |
| Tb | 8 | 1.04 | 1.44 |
| Tb | 8 | 0.88 | — |
| Dy | 8 | 1.027 | 1.43 |
| Ho | 8 | 1.015 | 1.41 |
| Er | 8 | 1.004 | 1.39 |
| Tm | 8 | 0.994 | 1.38 |
| Yb | 8 | 0.985 | 1.37 |
| Lu | 8 | 0.977 | 1.36 |
| Zr | 6 | 0.72 | — |
| Zr | 8 | 0.84 | — |
| Chemical Composition | Pyrochlore ↔ Defect-Fluorite | Data Acquisition | Melting Point (C) |
|---|---|---|---|
| Transition Temperature (C) | |||
| LaZrO | — | — | 2100–2350 [25,32,33] |
| PrZrO | — | — | 2300 [32] |
| NdZrO | 2300 | experiment [28,31] | 2300–2350 [25,32] |
| SmZrO | 2000 | experiment [28] | 2350 [32] |
| GdZrO | 1530 | experiment [28] | 2570 [34] |
| TbZrO | ∼1350 | prediction [30] | — |
| Chemical | Sample Label | Sintering | Space Group | Lattice Parameter (Å) | |
|---|---|---|---|---|---|
| Composition | Temperature (C) | Structure-Type | Present Work | Literature | |
| LaZrO | LZO | 1550 | (Pyrochlore) | 10.8115(1) | 10.805 [9] |
| PrZrO | PZO | 1500 | (Pyrochlore) | 10.7098(1) | 10.715 [9] |
| NdZrO | NZO | 1500 | (Pyrochlore) | 10.6751(1) | 10.678 [9] |
| SmZrO | SZO | 1500 | (Pyrochlore) | 10.5984(1) | 10.594 [9] |
| GdZrO | GZO_1 | 1450 | (Pyrochlore) | 10.4988(1) | 10.528 [9] |
| GdZrO | GZO_2 | 1600 | (Defect-fluorite) | 5.2737(1) | 5.2636(1) [41] |
| TbZrO | TZO | 1600 | (Defect-fluorite) | 5.2260(1) | 5.225 [42] |
| AZrO | Growth rate | Atmosphere | Pressure | Feed and Seed | Remarks |
|---|---|---|---|---|---|
| (mm/h) | Rotation Rate (rpm) | ||||
| LaZrO | 10–15 | air | ambient | 20–30 | colourless boules |
| PrZrO | 10–15 | air | ambient | 20–30 | dark-brown boules |
| 10–15 | O | 1–4 bars | 20–30 | dark-brown boules | |
| 10–15 | Ar | 1 bar | 20–30 | bright-green boules | |
| NdZrO | 10–15 | air | ambient | 20–30 | dark-purple boules |
| 10–15 | O | 2.5 bars | 20–30 | poor quality boules | |
| SmZrO | 5–15 | O | 4 bars | 20–30 | light-orange |
| 5–15 | air | ambient | 20–30 | poor quality boules | |
| GdZrO | 10–15 | air | ambient | 20–30 | light-yellow |
| 10–15 | O | 1–2 bars | 20–30 | poor quality boules |
| Chemical composition | Space Group Structure-Type | Lattice Parameter (Å) | |
|---|---|---|---|
| Present Work | |||
| LaZrO | (Pyrochlore) | 10.7992(1) | |
| PrZrO | (Pyrochlore) | 10.7010(1) | |
| NdZrO | (Pyrochlore) | 10.6134(1) | |
| SmZrO | (Pyrochlore) | 10.5907(1) | |
| GdZrO | As-grown | (Defect-fluorite) | 5.2614(1) |
| GdZrO | Annealed | (Pyrochlore) | 10.5341(1) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ciomaga Hatnean, M.; Decorse, C.; Lees, M.R.; Petrenko, O.A.; Balakrishnan, G. Zirconate Pyrochlore Frustrated Magnets: Crystal Growth by the Floating Zone Technique. Crystals 2016, 6, 79. https://doi.org/10.3390/cryst6070079
Ciomaga Hatnean M, Decorse C, Lees MR, Petrenko OA, Balakrishnan G. Zirconate Pyrochlore Frustrated Magnets: Crystal Growth by the Floating Zone Technique. Crystals. 2016; 6(7):79. https://doi.org/10.3390/cryst6070079
Chicago/Turabian StyleCiomaga Hatnean, Monica, Claudia Decorse, Martin R. Lees, Oleg A. Petrenko, and Geetha Balakrishnan. 2016. "Zirconate Pyrochlore Frustrated Magnets: Crystal Growth by the Floating Zone Technique" Crystals 6, no. 7: 79. https://doi.org/10.3390/cryst6070079