Technique for High-Quality Protein Crystal Growth by Control of Subgrain Formation under an External Electric Field
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
3.1. Improvement of Crystal Quality under an Applied Electric Field
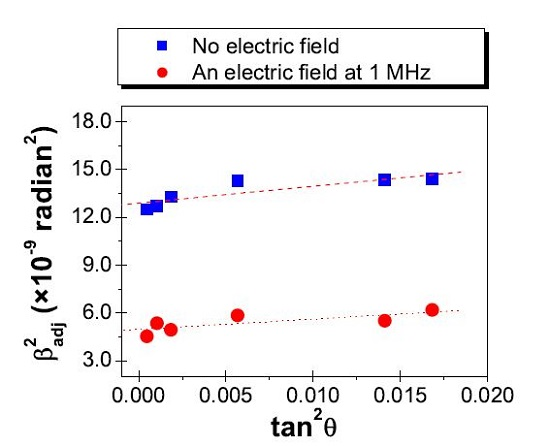
3.2. Control of Subgrain Formation under an Applied Electric Field
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sato, T.; Yamada, Y.; Saijo, S.; Hori, T.; Hirose, R.; Tanaka, N.; Sazaki, G.; Nakajima, K.; Igarashi, N.; Tanaka, M.; et al. Enhancement in the perfection of orthorhombic lysozyme crystals grown in a high magnetic field (10 T). Acta Crystallogr. 2000, D56, 1079–1083. [Google Scholar] [CrossRef]
- Lin, S.; Zhou, M.; Azzi, A.; Xu, G.; Wakayama, N.; Ataka, M. Magnet used for protein crystallization: Novel attempts to improve the crystal quality. Biochem. Biophys. Res. Commun. 2000, 275, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Ataka, M.; Wakayama, N. Effects of a magnetic field and magnetization force on protein crystal growth. Why does a magnet improve the quality of some crystals? Acta Crystallogr. 2002, D58, 1708–1710. [Google Scholar] [CrossRef]
- Wakayama, N. Effects of a strong magnetic field on protein crystal growth. Cryst. Growth Des. 2003, 3, 17–24. [Google Scholar] [CrossRef]
- Kinoshita, T.; Ataka, M.; Warizaya, M.; Neya, M.; Fujii, T. Improving quality and harvest period of protein crystals for structure-based drug design: Effects of a gel and a magnetic field on bovine adenosine deaminase crystals. Acta Crystallogr. 2003, D59, 1333–1335. [Google Scholar] [CrossRef]
- Lübbert, D.; Meents, A.; Weckert, E. Accurate rocking-curve measurements on protein crystals grown in a homogeneous magnetic field of 2.4 T. Acta Crystallogr. 2004, D60, 987–998. [Google Scholar]
- Moreno, A.; Quiroz-García, B.; Yokaichiya, F.; Stojanoff, V.; Rudolph, P. Protein crystal growth in gels and stationary magnetic fields. Cryst. Res. Technol. 2007, 42, 231–236. [Google Scholar] [CrossRef]
- Heijna, M.; Poodt, P.; Tsukamoto, K.; de Grip, W.; Christianen, P.; Maan, J.; Hendrix, J.; van Enckevort, W.; Vlieg, E. Magnetically controlled gravity for protein crystal growth. Appl. Phys. Lett. 2007, 90, 264105. [Google Scholar] [CrossRef] [Green Version]
- DeLucas, L.; Smith, C.; Smith, H.; Vijay-Kumar, S.; Senadhi, S.; Ealick, S.; Carter, D.; Snyder, R.; Weber, P.; Salemme, F. Protein crystal growth in microgravity. Science 1989, 246, 651–654. [Google Scholar] [CrossRef] [PubMed]
- McPherson, A. Virus and protein crystal growth on earth and in microgravity. J. Phys. D 1993, 26, B104–B112. [Google Scholar] [CrossRef]
- Snell, E.; Weisgerber, S.; Helliwell, J.; Weckert, E.; Holzer, K.; Schroer, K. Improvements in lysozyme protein crystal perfection through microgravity growth. Acta Crystallogr. 1995, D51, 1099–1102. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Tanaka, H.; Inaka, K.; Shinozaki, S.; Yamanaka, A.; Takahashi, S.; Yamanaka, M.; Hirota, E.; Sugiyama, S.; Kato, M.; et al. JAXA-GCF project-high-quality protein crystals grown under microgravity environment for better understanding of protein structure. Microgravity Sci. Technol. 2006, 18, 184–189. [Google Scholar] [CrossRef]
- Takahashi, S.; Tsurumura, T.; Aritake, K.; Furubayashi, N.; Sato, M.; Yamanaka, M.; Hirota, E.; Sano, S.; Kobayashi, T.; Tanaka, T.; et al. High-quality crystals of human haematopoietic prostaglandin D synthase with novel inhibitors. Acta Crystallogr. 2010, F66, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Inaka, K.; Takahashi, S.; Aritake, K.; Tsurumura, T.; Furubayashi, N.; Yan, B.; Hirota, E.; Sano, S.; Sato, M.; Kobayashi, T.; et al. High-quality protein crystal growth of mouse lipocalin-type prostaglandin D synthase in microgravity. Cryst. Growth Des. 2011, 11, 2107–2111. [Google Scholar] [CrossRef] [PubMed]
- Yoshikawa, S.; Kukimoto-Niino, M.; Parker, L.; Handa, N.; Terada, T.; Fujimoto, T.; Terazawa, Y.; Wakiyama, M.; Sato, M.; Sano, S.; et al. Structural basis for the altered drug sensitivities of non-small cell lung cancer-associated mutants of human epidermal growth factor receptor. Oncogene 2012, 32, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Adawy, A.; Rebuffet, E.; Törnroth-Horsefield, S.; de Grip, W.J.; van Enckevort, W.J.; Vlieg, E. High resolution protein crystals using an efficient convection-free geometry. Cryst. Growth Des. 2013, 13, 775–781. [Google Scholar] [CrossRef]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.; Capelle, B.; Aubry, A. Crystallization of proteins under an external electric field. J. Cryst. Growth 1999, 200, 575–582. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Improvement of Crystal Quality for Tetragonal Hen-Egg White Lysozyme Crystals under Application of an External AC Electric Field. J. Appl. Crystallogr. 2013, 46, 25–29. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Enhancement of crystal homogeneity of protein crystals under application of an external alternating current electric field. AIP Conf. Proc. 2014, 1618, 265–268. [Google Scholar]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Control of Subgrain Formation in Protein Crystals by the Application of an External Electric Field. Cryst. Growth Des. 2014, 14, 5662–5667. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Crystallization of high-quality protein crystals using an external electric field. J. Appl. Crystallogr. 2015, 48, 1507–1513. [Google Scholar] [CrossRef]
- Vekilov, P.G.; Thomas, B.R.; Rosenberger, F. Effects of convective solute and impurity transport in protein crystal growth. J. Phys. Chem. B 1998, 102, 5208–5216. [Google Scholar] [CrossRef]
- Kadowaki, A.; Yoshizaki, I.; Adachi, S.; Komatsu, H.; Odawara, O.; Yoda, S. Effects of forced solution flow on protein-crystal quality and growth process. Cryst. Growth Des. 2006, 6, 2398–2403. [Google Scholar] [CrossRef]
- Otálora, F.; Gavira, J.A.; Ng, J.D.; García-Ruiz, J.M. Counterdiffusion methods applied to protein crystallization. Prog. Biophys. Mol. Biol. 2009, 101, 26–37. [Google Scholar] [CrossRef] [PubMed]
- Maruyama, M.; Kawahara, H.; Sazaki, G.; Maki, S.; Takahashi, Y.; Yoshikawa, H.Y.; Sugiyama, S.; Adachi, H.; Takano, K.; Matsumura, H.; et al. Effects of a forced solution flow on the step advancement on {110} faces of tetragonal lysozyme crystals: Direct visualization of individual steps under a forced solution flow. Cryst. Growth Des. 2012, 12, 2856–2863. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.; Moreno, A. Investigations on protein crystal growth by the gel acupuncture method. Acta Crystallogr. 1994, D50, 484–490. [Google Scholar] [CrossRef] [PubMed]
- Vidal, O.; Robert, M.; Arnoux, B.; Capelle, B. Crystalline quality of lysozyme crystals grown in agarose and silica gels studied by X-ray diffraction techniques. J. Cryst. Growth 1999, 196, 559–571. [Google Scholar] [CrossRef]
- Lorber, B.; Sauter, C.; Ng, J.; Zhu, D.; Giegé, R.; Vidal, O.; Robert, M.; Capelle, B. Characterization of protein and virus crystals by quasi-planar wave X-ray topography: A comparison between crystals grown in solution and in agarose gel. J. Cryst. Growth 1999, 204, 357–368. [Google Scholar] [CrossRef]
- Dong, J.; Boggon, T.J.; Chayen, N.E.; Raftery, J.; Bi, R.C.; Helliwell, J.R. Bound-solvent structures for microgravity-, ground control-, gel-and microbatch-grown hen egg-white lysozyme crystals at 1.8 A resolution. Acta Crystallogr. 1999, D55, 745–752. [Google Scholar] [CrossRef]
- Garcıa-Ruiz, J.; Novella, M.; Moreno, R.; Gavira, J. Agarose as crystallization media for proteins: I: Transport processes. J. Cryst. Growth 2001, 232, 165–172. [Google Scholar] [CrossRef]
- Gavira, J.A.; García-Ruiz, J.M. Agarose as crystallisation media for proteins II: Trapping of gel fibres into the crystals. Acta Crystallogr. 2002, D58, 1653–1656. [Google Scholar] [CrossRef]
- Sugiyama, S.; Maruyama, M.; Sazaki, G.; Hirose, M.; Adachi, H.; Takano, K.; Murakami, S.; Inoue, T.; Mori, Y.; Matsumura, H. Growth of protein crystals in hydrogels prevents osmotic shock. JACS 2012, 134, 5786–5789. [Google Scholar] [CrossRef] [PubMed]
- Taleb, M.; Didierjean, C.; Jelsch, C.; Mangeot, J.; Aubry, A. Equilibrium kinetics of lysozyme crystallization under an external electric field. J. Cryst. Growth 2001, 232, 250–255. [Google Scholar] [CrossRef]
- Nanev, C.; Penkova, A. Nucleation of lysozyme crystals under external electric and ultrasonic fields. J. Cryst. Growth 2001, 232, 285–293. [Google Scholar] [CrossRef]
- Charron, C.; Didierjean, C.; Mangeot, J.; Aubry, A. TheOctopus’ plate for protein crystallization under an electric field. J. Appl. Crystallogr. 2003, 36, 1482–1483. [Google Scholar] [CrossRef]
- Mirkin, N.; Frontana-Uribe, B.; Rodríguez-Romero, A.; Hernández-Santoyo, A.; Moreno, A. The influence of an internal electric field upon protein crystallization using the gel-acupuncture method. Acta Crystallogr. 2003, D59, 1533–1538. [Google Scholar] [CrossRef]
- Moreno, A.; Sazaki, G. The use of a new ad hoc growth cell with parallel electrodes for the nucleation control of lysozyme. J. Cryst. Growth 2004, 264, 438–444. [Google Scholar] [CrossRef]
- Penkova, A.; Gliko, O.; Dimitrov, I.; Hodjaoglu, F.; Nanev, C.; Vekilov, P. Enhancement and suppression of protein crystal nucleation due to electrically driven convection. J. Cryst. Growth 2005, 275, e1527–e1532. [Google Scholar] [CrossRef]
- Penkova, A.; Pan, W.; Hodjaoglu, F.; Vekilov, P. Nucleation of protein crystals under the influence of solution shear flow. Ann. N. Y. Acad. Sci. 2006, 1077, 214–231. [Google Scholar] [CrossRef] [PubMed]
- Al-Haq, M.; Lebrasseur, E.; Choi, W.; Tsuchiya, H.; Torii, T.; Yamazaki, H.; Shinohara, E. An apparatus for electric-field-induced protein crystallization. J. Appl. Crystallogr. 2007, 40, 199–201. [Google Scholar] [CrossRef]
- Al-Haq, M.; Lebrasseur, E.; Tsuchiya, H.; Torii, T. Protein crystallization under an electric field. Crystallogr. Rev. 2007, 13, 29–64. [Google Scholar] [CrossRef]
- Hammadi, Z.; Astier, J.; Morin, R.; Veesler, S. Protein Crystallization Induced by a Localized Voltage. Cryst. Growth Des. 2007, 7, 1472–1475. [Google Scholar] [CrossRef]
- Pérez, Y.; Eid, D.; Acosta, F.; Marín-García, L.; Jakoncic, J.; Stojanoff, V.; Frontana-Uribe, B.; Moreno, A. Electrochemically Assisted Protein Crystallization of Commercial Cytochrome c without Previous Purification. Cryst. Growth Des. 2008, 8, 2493–2496. [Google Scholar]
- Mirkin, N.; Jaconcic, J.; Stojanoff, V.; Moreno, A. High resolution X-ray crystallographic structure of bovine heart cytochrome c and its application to the design of an electron transfer biosensor. Proteins Struct. Funct. Bioinform. 2008, 70, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Hou, D.; Chang, H. ac field enhanced protein crystallization. Appl. Phys. Lett. 2008, 92, 223902. [Google Scholar] [CrossRef]
- Revalor, E.; Hammadi, Z.; Astier, J.; Grossier, R.; Garcia, E.; Hoff, C.; Furuta, K.; Okustu, T.; Morin, R.; Veesler, S. Usual and unusual crystallization from solution. J. Cryst. Growth 2010, 312, 939–946. [Google Scholar] [CrossRef]
- Wakamatsu, T. Transparent Cell for Protein Crystallization under Low Applied Voltage. Jpn. J. Appl. Phys. 2011, 50, 048003. [Google Scholar] [CrossRef]
- Wakamatsu, T.; Toyoshima, S.; Shimizu, H. Observation of electric-field induced aggregation in crystallizing protein solutions by forward light scattering. Appl. Phys. Lett. 2011, 99, 153701. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujiwara, K.; Uda, S. Control of Nucleation Rate for Tetragonal Hen-Egg White Lysozyme Crystals by Application of an Electric Field with Variable Frequencies. Cryst. Growth Des. 2009, 9, 2420–2424. [Google Scholar] [CrossRef]
- Koizumi, H.; Tomita, Y.; Uda, S.; Fujiwara, K.; Nozawa, J. Nucleation Rate Enhancement of Porcine Insulin by Application of an External AC Electric Field. J. Cryst. Growth 2012, 352, 155–157. [Google Scholar] [CrossRef]
- Koizumi, H.; Fujiwara, K.; Uda, S. Role of the Electric Double Layer in Controlling the Nucleation Rate for Tetragonal Hen Egg White Lysozyme Crystals by Application of an External Electric Field. Cryst. Growth Des. 2010, 10, 2591–2595. [Google Scholar] [CrossRef]
- Uda, S.; Koh, S.; Huang, X. The electric field-induced transformation of the melting state of langasite from incongruent into congruent. J. Cryst. Growth 2006, 292, 1–4. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Nozawa, J. Effect of various precipitants on the nucleation rate of tetragonal hen egg-white lysozyme crystals in an AC external electric field. J. Cryst. Growth 2010, 312, 3503–3508. [Google Scholar] [CrossRef]
- Koizumi, H.; Uda, S.; Fujiwara, K.; Nozawa, J. Control of Effect on the Nucleation Rate for Hen Egg White Lysozyme Crystals under Application of an External ac Electric Field. Langmuir 2011, 27, 8333–8338. [Google Scholar] [PubMed]
- Dobrianov, I.; Finkelstein, K.; Lemay, S.; Thorne, R. X-ray topographic studies of protein crystal perfection and growth. Acta Crystallogr. 1998, D54, 922–937. [Google Scholar] [CrossRef]
- Izumi, K.; Taguchi, K.; Kobayashi, Y.; Tachibana, M.; Kojima, K.; Ataka, M. Screw dislocation lines in lysozyme crystals observed by Laue topography using synchrotron radiation. J. Cryst. Growth 1999, 206, 155–158. [Google Scholar] [CrossRef]
- Fourme, R.; Ducruix, A.; Riès-Kautt, M.; Capelle, B. Probing the quality of crystals of biological macromolecules using maximum resolution of diffraction data, Bragg reflection profiles and X-ray topographs. J. Cryst. Growth 1999, 196, 535–545. [Google Scholar] [CrossRef]
- Otálora, F.; García-Ruiz, J.M.; Antonio Gavira, J.; Capelle, B. Topography and high resolution diffraction studies in tetragonal lysozyme. J. Cryst. Growth 1999, 196, 546–558. [Google Scholar] [CrossRef]
- Caylor, C.; Dobrianov, I.; Lemay, S.; Kimmer, C.; Kriminski, S.; Finkelstein, K.; Zipfel, W.; Webb, W.; Thomas, B.; Chernov, A.; et al. Macromolecular impurities and disorder in protein crystals. Proteins Struct. Funct. Bioinform. 1999, 36, 270–281. [Google Scholar] [CrossRef]
- Boggon, T.; Helliwell, J.; Judge, R.; Olczak, A.; Siddons, D.; Snell, E.; Stojanoff, V. Synchrotron X-ray reciprocal-space mapping, topography and diffraction resolution studies of macromolecular crystal quality. Acta Crystallogr. 2000, D56, 868–880. [Google Scholar] [CrossRef]
- Capelle, B.; Epelboin, Y.; Hartwig, J.; Moraleda, A.; Otálora, F.; Stojanoff, V. Characterization of dislocations in protein crystals by means of synchrotron double-crystal topography. J. Appl. Crystallogr. 2004, 37, 67–71. [Google Scholar] [CrossRef]
- Hu, Z.; Chu, Y.; Lai, B.; Thomas, B.; Chernov, A. Diffraction and imaging study of imperfections of crystallized lysozyme with coherent X-rays. Acta Crystallogr. 2004, D60, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, I.; Fukuyama, S.; Koizumi, H.; Tachibana, M.; Kojima, K.; Matsuura, Y.; Tanaka, M.; Igarashi, N.; Kadowaki, A.; Rong, L.; et al. Impurity-induced defect and its effect on protein crystal perfection. J. Cryst. Growth 2006, 290, 185–191. [Google Scholar] [CrossRef]
- Koishi, M.; Ohya, N.; Mukobayashi, Y.; Koizumi, H.; Kojima, K.; Tachibana, M. Observation of Clear Images of Dislocations in Protein Crystals by Synchrotron Monochromatic-Beam X-ray Topography. Cryst. Growth Des. 2007, 7, 2182–2186. [Google Scholar] [CrossRef]
- Mukobayashi, Y.; Kitajima, N.; Yamamoto, Y.; Kajiwara, K.; Sugiyama, H.; Hirano, K.; Kojima, K.; Tachibana, M. Observation of dislocations in hen egg-white lysozyme crystals by synchrotron monochromatic-beam X-ray topography. Phys. Status Solidi A 2009, 206, 1825–1828. [Google Scholar] [CrossRef]
- Sawaura, T.; Fujii, D.; Shen, M.; Yamamoto, Y.; Wako, K.; Kojima, K.; Tachibana, M. Characterization of dislocations in monoclinic hen egg-white lysozyme crystals by synchrotron monochromatic-beam X-ray topography. J Cryst. Growth 2011, 318, 1071–1074. [Google Scholar] [CrossRef]
- Colapietro, M.; Cappuccio, G.; Marciante, C.; Pifferi, A.; Spagna, R.; Helliwell, J. The X-ray diffraction station at the ADONE wiggler facility: Preliminary results (including crystal perfection). J. Appl. Crystallogr. 1992, 25, 192–194. [Google Scholar] [CrossRef]
- Hordon, M.; Averbach, B. X-ray measurements of dislocation density in deformed copper and aluminum single crystals. Acta Metall. 1961, 9, 237–246. [Google Scholar] [CrossRef]
- Ayers, J. The measurement of threading dislocation densities in semiconductor crystals by X-ray diffraction. J. Cryst. Growth 1994, 135, 71–77. [Google Scholar] [CrossRef]
- Klapper, H. Crystals; Freyhardt, H.C., Ed.; Springer-Verlag: Berlin, Germany, 1991; Volume 13. [Google Scholar]
- Bowen, D.; Tanner, B. High Resolution X-Ray Diffraction and Topography; Teylor & Francis: London, UK, 1998; Chapter 8; p. 172. [Google Scholar]
- Authier, A. Dynamical Theory of X-Ray Diffraction; Springer: Berlin, Germany, 2001. [Google Scholar]
- Koizumi, H.; Tachibana, M.; Yoshizaki, I.; Fukuyama, S.; Tsukamoto, K.; Suzuki, Y.; Uda, S.; Kojima, K. Dislocations in High-Quality Glucose Isomerase Crystals Grown from Seed Crystals. Cryst. Growth Des. 2014, 14, 5111–5116. [Google Scholar] [CrossRef]
- Tachibana, M.; Koizumi, H.; Izumi, K.; Kajiwara, K.; Kojima, K. Identification of dislocations in large tetragonal hen egg-white lysozyme crystals by synchrotron white-beam topography. J. Synchrotron Radiat. 2003, 10, 416–420. [Google Scholar] [CrossRef] [PubMed]
- Matsuzuki, Y.; Kubota, T.; Liu, X.; Ataka, M.; Takano, K. AFM observation of the surface morphology and impurity effects on orthorhombic hen egg-white lysozyme crystals. J. Cryst. Growth 2002, 242, 199–208. [Google Scholar] [CrossRef]
- Yoshizaki, I.; Kadowaki, A.; Iimura, Y.; Igarashi, N.; Yoda, S.; Komatsu, H. Impurity effects on lysozyme crystal growth. J. Synchrotron Radiat. 2003, 11, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Vekilov, P.G.; Monaco, L.A.; Thomas, B.R.; Stojanoff, V.; Rosenberger, F. Repartitioning of NaCl and protein impurities in lysozyme crystallization. Acta Crystallogr. 1996, D52, 785–798. [Google Scholar] [CrossRef]
- Kudryavtsev, A.; Christopher, G.; Smith, C.; Mirov, S.; Rosenblum, W.; DeLucas, L. The effect of ordering of internal water in thaumatin and lysozyme crystals as revealed by Raman method. J. Cryst. Growth 2000, 219, 102–114. [Google Scholar] [CrossRef]
- Vekilov, P.G.; Feeling-Taylor, A.; Yau, S.T.; Petsev, D. Solvent entropy contribution to the free energy of protein crystallization. Acta Crystallogr. 2002, D58, 1611–1616. [Google Scholar] [CrossRef]







| Reflection | Average FWHMs (Standard Deviation) | |
|---|---|---|
| No Electric Field | Applied Field at 1 MHz | |
| 440 reflection | 0.0054° (27) | 0.0032° (18) |
| 12 12 0 reflection | 0.0060° (29) | 0.0034° (19) |
| Condition | FWHM () | Entropy Change |
|---|---|---|
| No electric field | 407.3 ± 3.9 | |
| Applied field at 1 MHz | 394.8 ± 3.2 | Decrease |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koizumi, H.; Uda, S.; Fujiwara, K.; Tachibana, M.; Kojima, K.; Nozawa, J. Technique for High-Quality Protein Crystal Growth by Control of Subgrain Formation under an External Electric Field. Crystals 2016, 6, 95. https://doi.org/10.3390/cryst6080095
Koizumi H, Uda S, Fujiwara K, Tachibana M, Kojima K, Nozawa J. Technique for High-Quality Protein Crystal Growth by Control of Subgrain Formation under an External Electric Field. Crystals. 2016; 6(8):95. https://doi.org/10.3390/cryst6080095
Chicago/Turabian StyleKoizumi, Haruhiko, Satoshi Uda, Kozo Fujiwara, Masaru Tachibana, Kenichi Kojima, and Jun Nozawa. 2016. "Technique for High-Quality Protein Crystal Growth by Control of Subgrain Formation under an External Electric Field" Crystals 6, no. 8: 95. https://doi.org/10.3390/cryst6080095