Synthesis, Crystal Structure, and Nonlinear Optical Properties of a New Alkali and Alkaline Earth Metal Carbonate RbNa5Ca5(CO3)8
Abstract
:1. Introduction
2. Results and Discussion
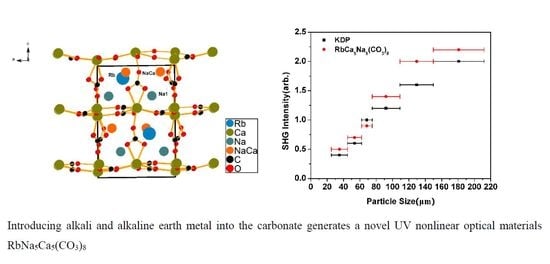
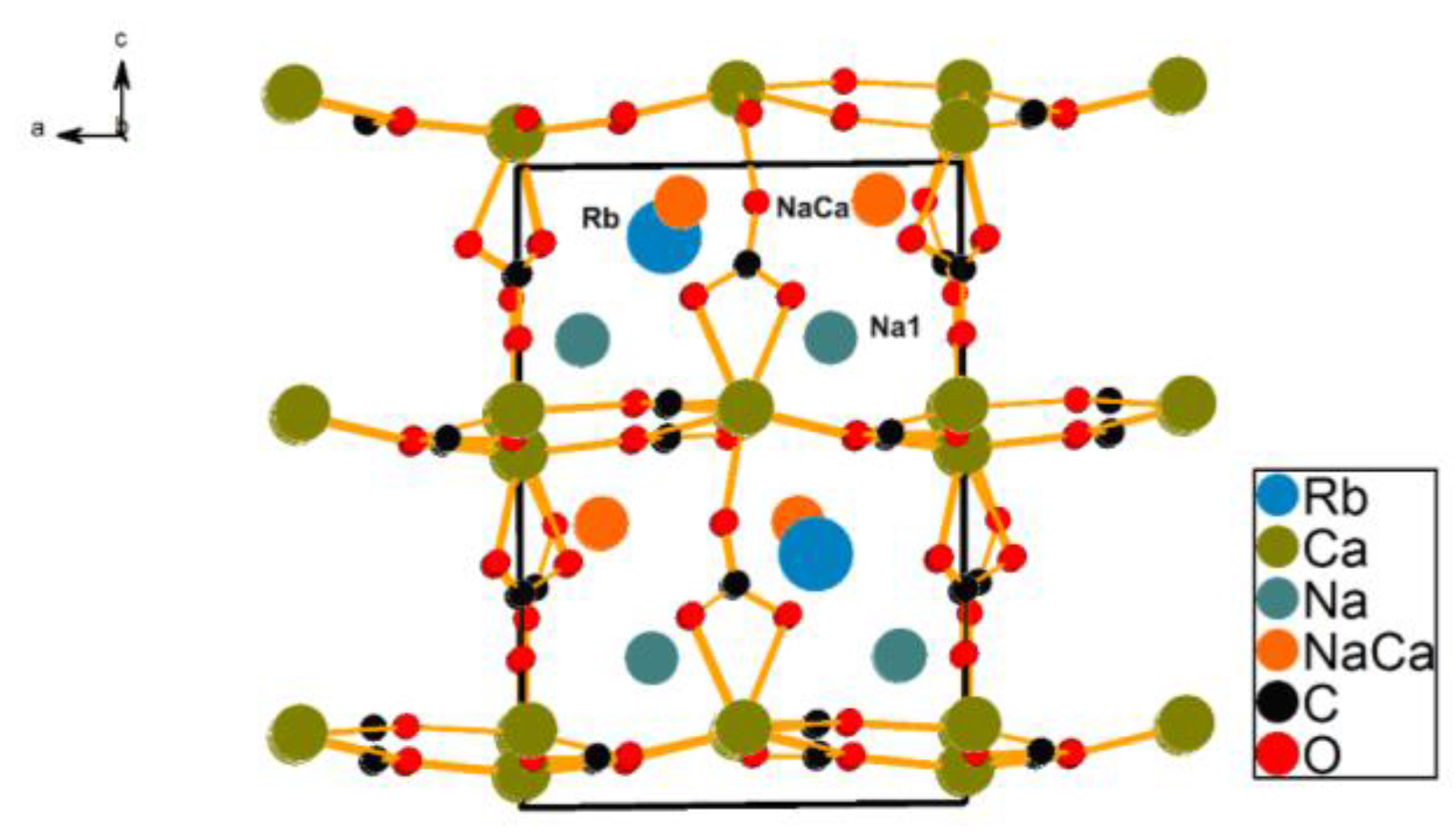
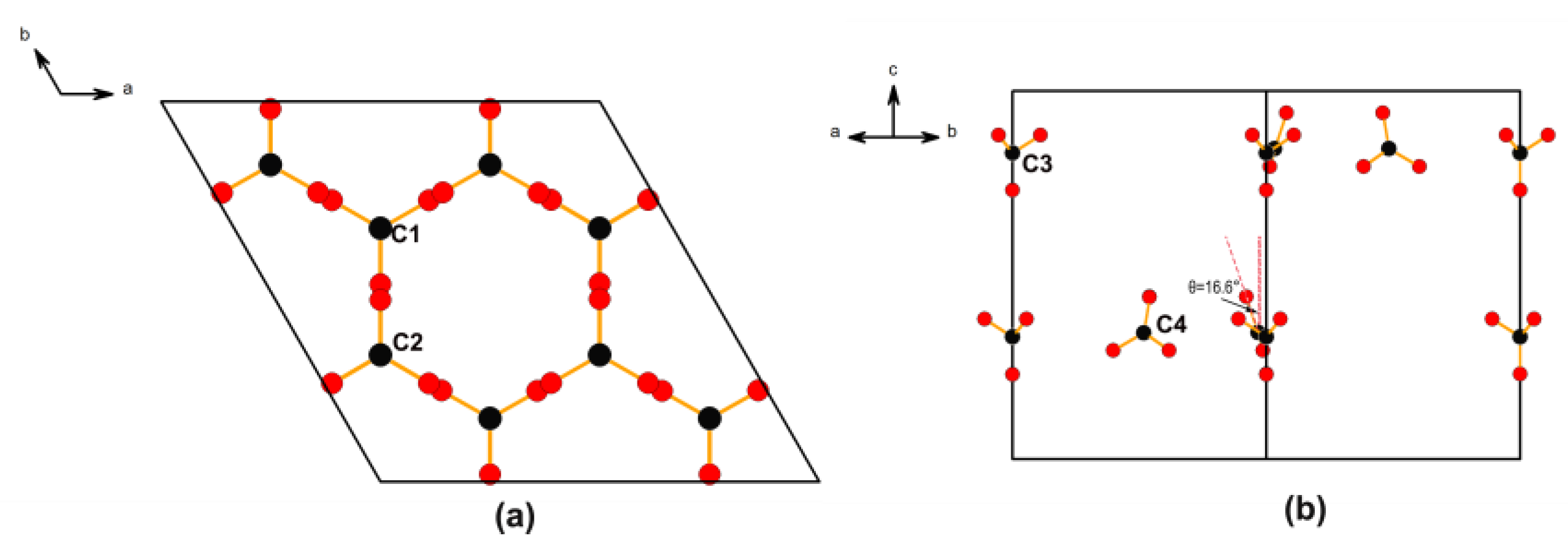
2.1. Crystal Structure Description
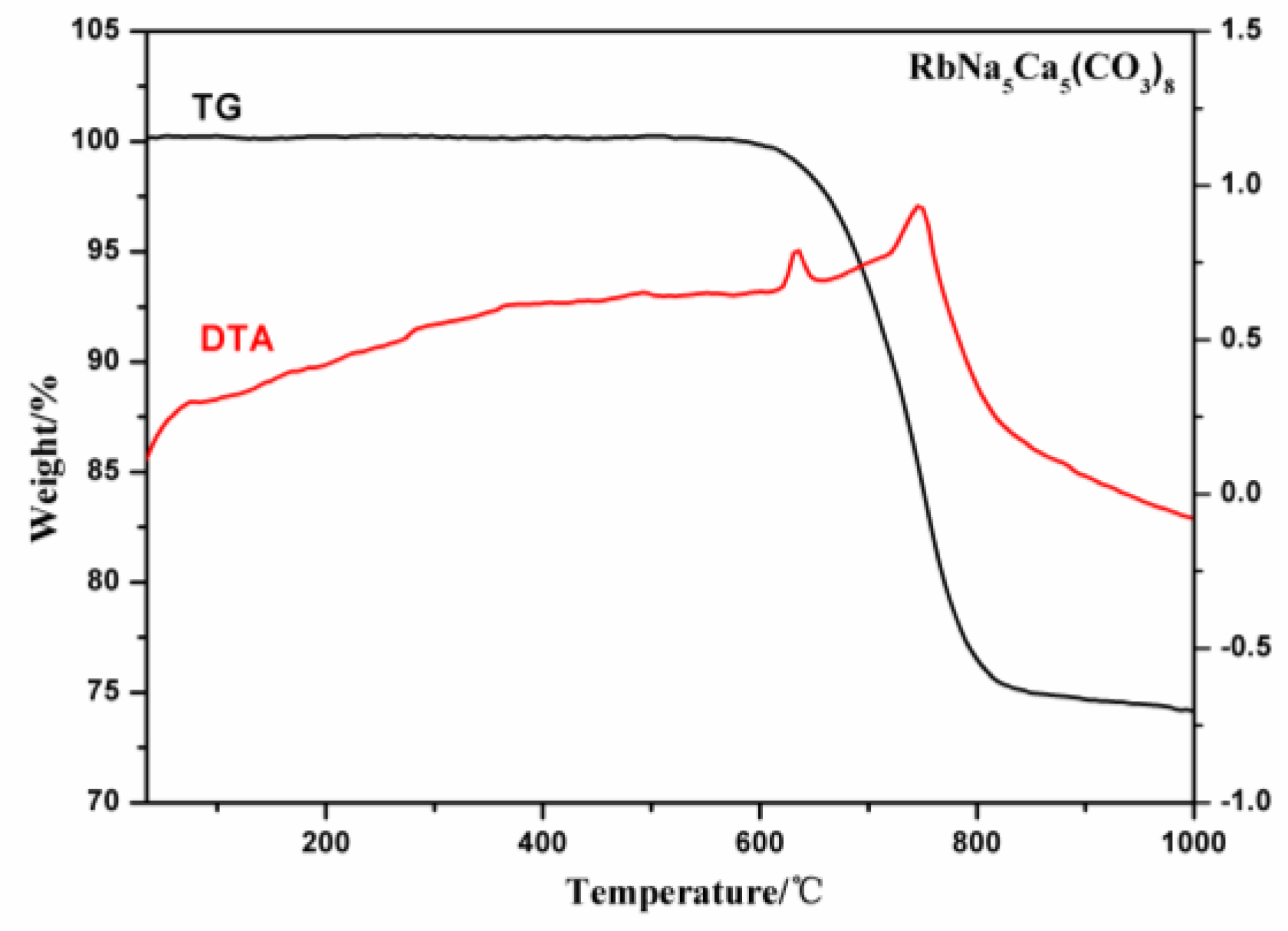
2.2. TG Analysis
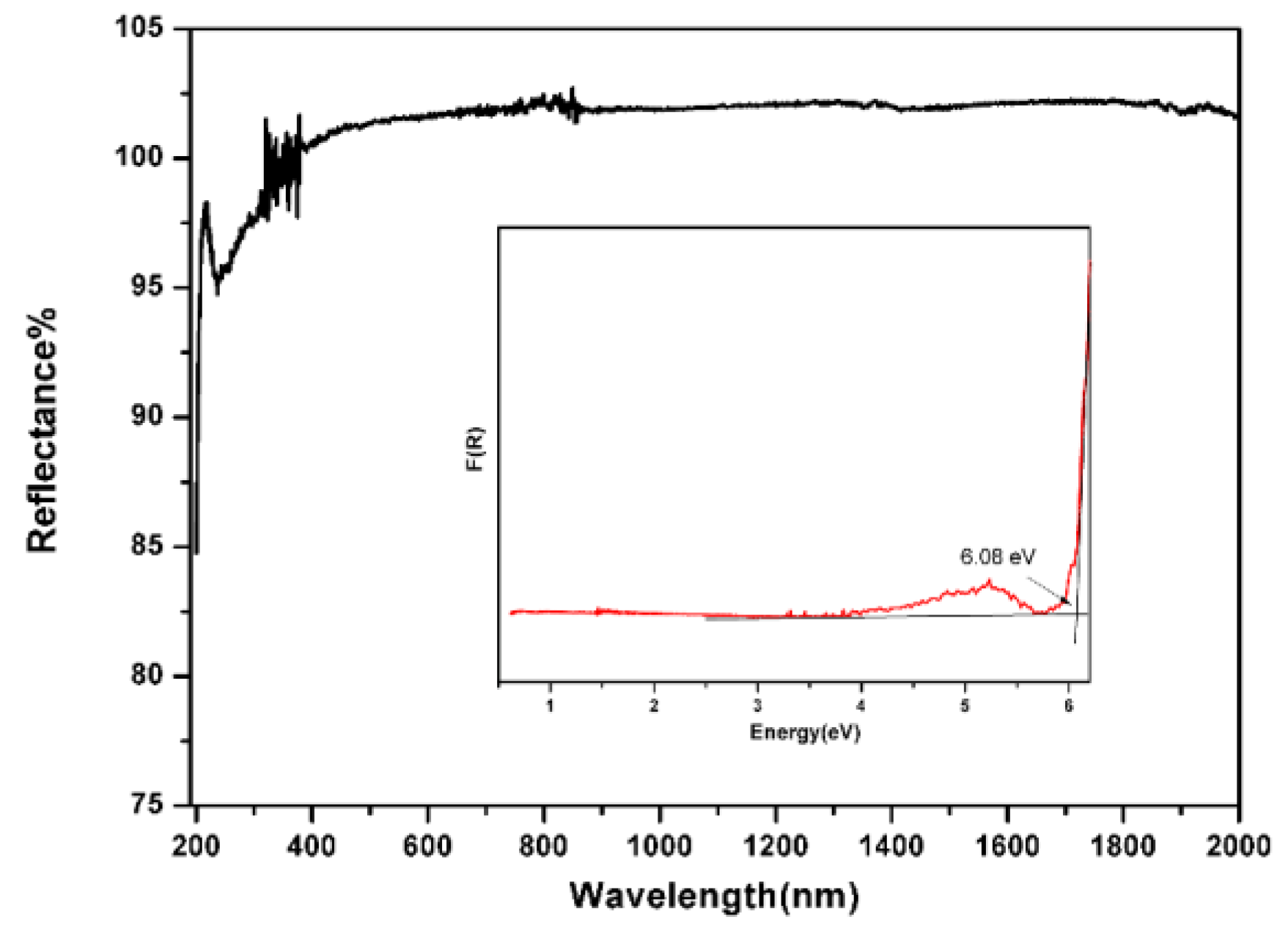
2.3. Diffuse-Reflectance Spectroscopy
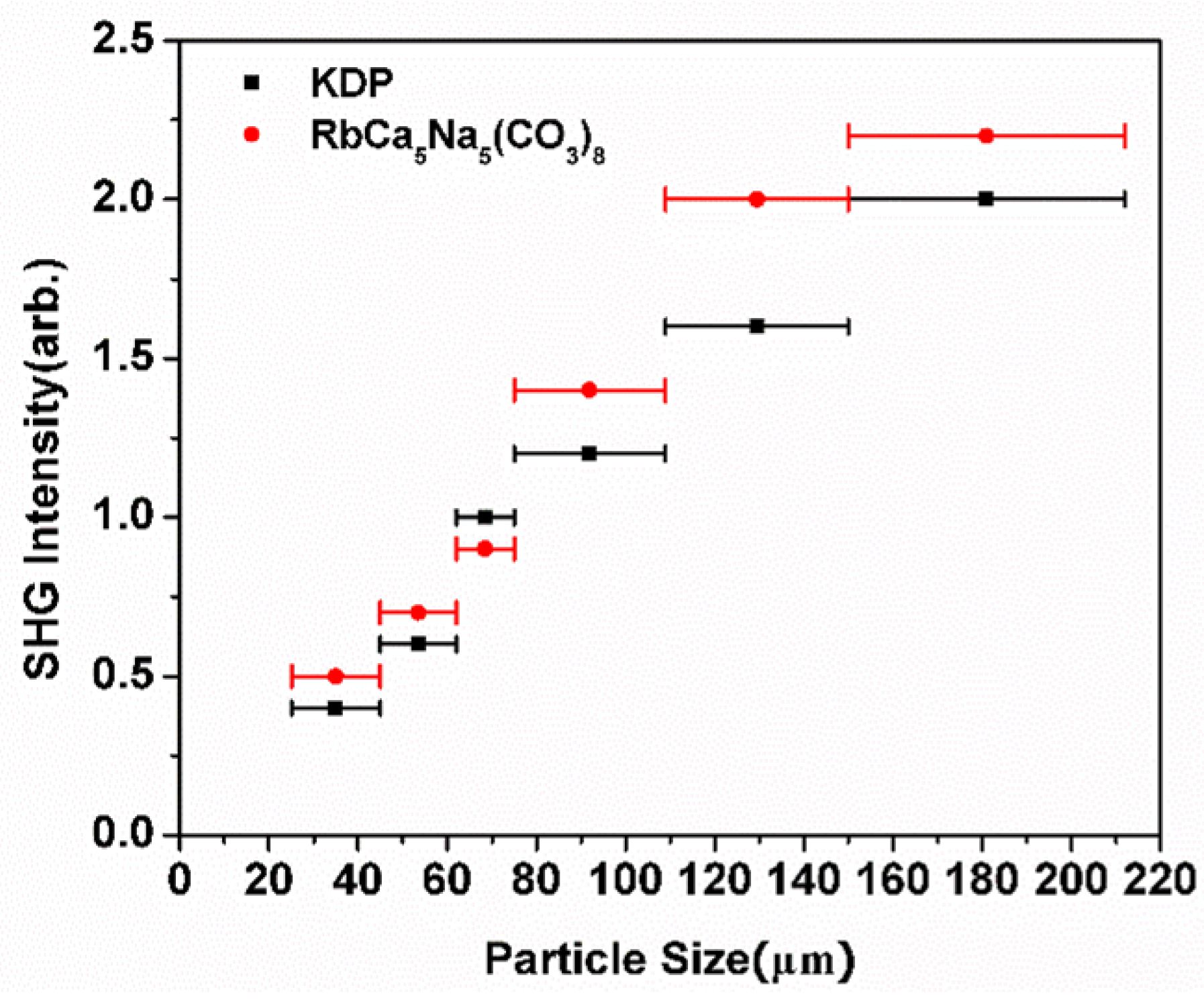
2.4. NLO Properties
2.5. Relationship between Structure and NLO Properties
3. Materials and Methods
3.1. Reagents
3.2. Synthesis and Crystal Growth
3.3. Single-Crystal X-Ray Diffraction
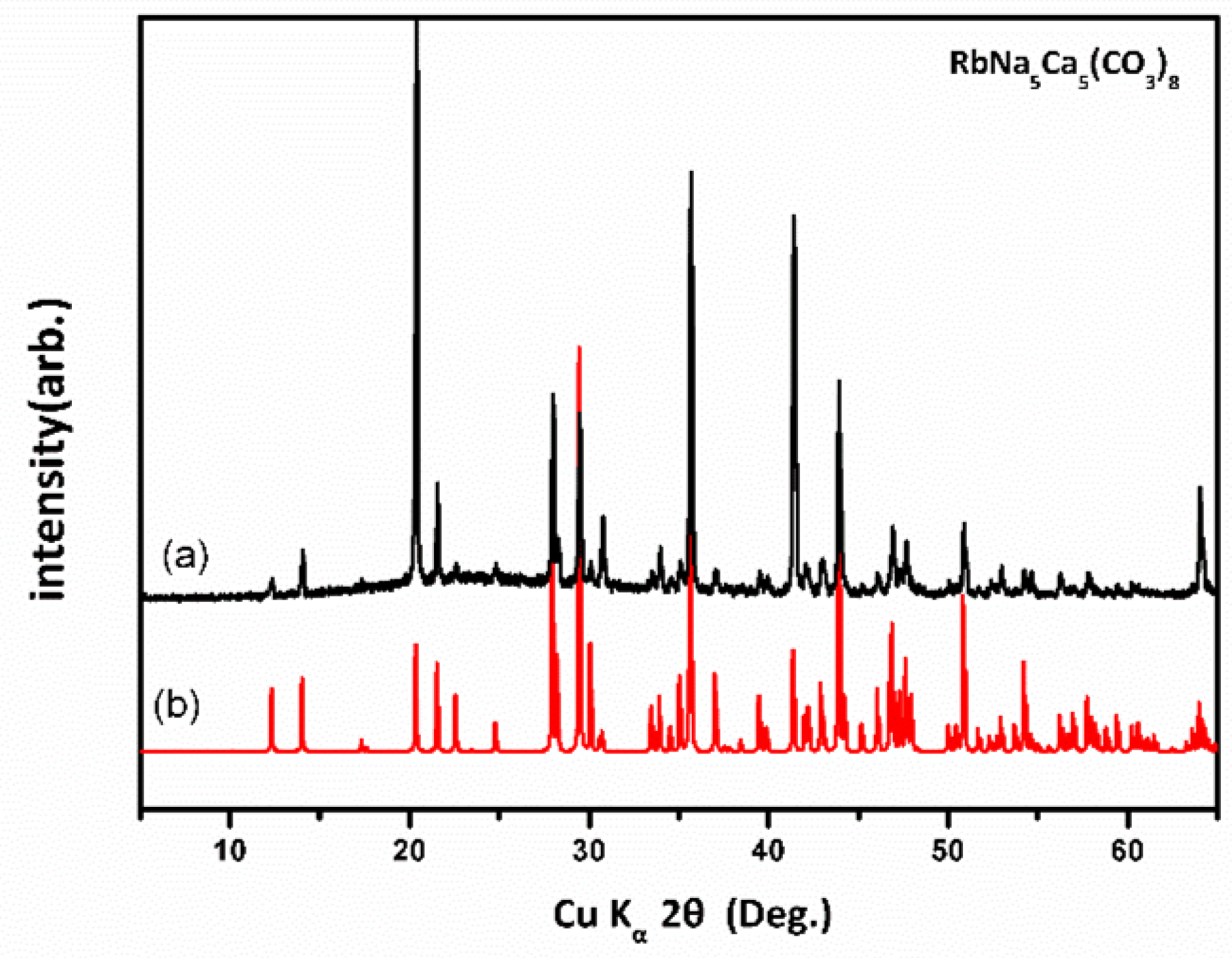
3.4. Powder X-Ray Diffraction
3.5. Thermal Analysis
3.6. UV-VIS Diffuse Reflectance Spectroscopy
3.7. Second-Harmonic Generation
3.8. Scanning Electron Microscope (SEM)/Energy-Dispersive Analysis by X-Ray (EDX)
4. Conclusions
Supplementary Materials
Author Contributions
Conflicts of Interest
References
- Tran, T.T.; Yu, H.; Rondinelli, J.R.; Poeppelmeier, K.R.; Halasyamani, P.S. Deep Ultraviolet Nonlinear Optical Materials. Chem. Mater. 2016, 28, 5238–5258. [Google Scholar] [CrossRef]
- Tran, T.T.; He, J.G.; Rondinelli, J.M.; Halasyamani, P.S. RbMgCO3F: A New Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material. J. Am. Chem. Soc. 2015, 137, 10504–10507. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Hu, C.L.; Kong, F.; Zhang, J.H.; Mao, J.G.; Sun, J. Cs2GeB4O9: A new second-order nonlinear-optical crystal. Inorg. Chem. 2013, 52, 5831–5837. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, N. Nonlinear optical crystal BiAlGa2(BO3)4. Solid State Sci. 2007, 9, 713–717. [Google Scholar] [CrossRef]
- Chen, J.; Luo, M.; Ye, N. Syntheses, characterization and nonlinear optical properties of sodium–scandium carbonate Na5Sc(CO3)4·2H2O. Solid State Sci. 2014, 36, 24–28. [Google Scholar] [CrossRef]
- Huang, L.; Zou, G.; Cai, H.; Wang, S.; Lin, C.; Ye, N. Sr2(OH)3NO3: the first nitrate as a deep UV nonlinear optical material with large SHG responses. J. Mater. Chem. C 2015, 3, 5268–5274. [Google Scholar] [CrossRef]
- Ye, N.; Zeng, W.R.; Jiang, J.; Wu, B.C.; Chen, C.T.; Feng, B.H.; Zhang, X.L. New nonlinear optical crystal K2Al2B2O7. J. Opt. Soc. Am. B 2000, 17, 764–768. [Google Scholar] [CrossRef]
- Sasaki, T.; Mori, Y.; Yoshimura, M.; Yap, Y.K.; Kamimura, T. Recent development of nonlinear optical borate crystals: key materials for generation of visible and UV light. Mater. Sci. Eng. R Rep. 2000, 30, 1–54. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, G.M. A new-type ultraviolet SHG crystal-Beta-BaB2O4. Sci. Sin. Ser. B 1985, 28, 235–243. [Google Scholar]
- Chen, C.; Wu, Y.; Jiang, A.; Wu, B.; You, G.; Li, R.; Lin, S. New nonlinear-optical crystal: LiB3O5. J. Opt. Soc. Am. B 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Wu, Y.; Sasaki, T.; Nakai, S.; Yokotani, A.; Tang, H.; Chen, C. CsB3O5: A new nonlinear optical crystal. Appl. Phys. Lett. 1993, 62, 2614. [Google Scholar] [CrossRef]
- Chen, C.T.; Liu, G.Z. Recent Advances in Nonlinear Optical and Electro-Optical Materials. Annu. Rev. Mater. Sci. 1986, 16, 203–243. [Google Scholar] [CrossRef]
- Chen, C.T.; Li, R.K. The Anionic Group Theory of The Non-Linear Optical Effect and Its Applications in the Development of New High-Quality NLO Crystals in the Borate Series. Int. Rev. Phys. Chem. 1989, 8, 65–91. [Google Scholar] [CrossRef]
- Chen, C.T. Localized Quantal Theoretical Treatment, Based on an Anionic Coordination Polyhedron Model, for the Eo and Shg Effects in Crystals of the Mixed-Oxide Types. Scientia Sinica 1979, 22, 756–776. [Google Scholar]
- Zou, G.; Zhang, L.; Ye, N. Synthesis, structure, and characterization of a new promising nonlinear optical crystal: Cd5(BO3)3F. CrystEngComm 2013, 15, 2422. [Google Scholar] [CrossRef]
- Yu, H.; Wu, H.; Pan, S.; Wang, Y.; Yang, Z.; Su, X. New salt-inclusion borate, Li3Ca9(BO3)7·2[LiF]: A promising UV NLO material with the coplanar and high density BO3 triangles. Inorg. Chem. 2013, 52, 5359–5365. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gong, P.; Bai, L.; Xu, X.; Zhang, S.; Sun, Z.; Lin, Z.; Hong, M.; Chen, C.; Luo, J. Beryllium-free Li4Sr(BO3)2 for deep-ultraviolet nonlinear optical applications. Nat. Commun. 2014, 5, 4019. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.J.; Li, R.K. Structure and optical properties of a noncentrosymmetric borate RbSr4(BO3)3. J. Solid State Chem. 2013, 197, 366–369. [Google Scholar] [CrossRef]
- Zou, G.; Ye, N.; Huang, L.; Lin, X. Alkaline-alkaline earth fluoride carbonate crystals ABCO3F (A = K, Rb, Cs; B = Ca, Sr, Ba) as nonlinear optical materials. J. Am. Chem. Soc. 2011, 133, 20001–20007. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Halasyamani, P.S.; Rondinelli, J.M. Role of Acentric Displacements on the Crystal Structure and Second-Harmonic Generating Properties of RbPbCO3F and CsPbCO3F. Inorg. Chem. 2014, 53, 6241–6251. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Huang, L.; Ye, N.; Lin, C.; Cheng, W.; Huang, H. CsPbCO3F: a strong second-harmonic generation material derived from enhancement via p-pi interaction. J. Am. Chem. Soc. 2013, 135, 18560–18566. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Halasyamani, P.S. New fluoride carbonates: centrosymmetric KPb2(CO3)2F and noncentrosymmetric K2.70Pb5.15(CO3)5F3. Inorg. Chem. 2013, 52, 2466–2473. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Ye, N.; Zou, G.; Lin, C.; Cheng, W. Na8Lu2(CO3)6F2and Na3Lu(CO3)2F2: Rare Earth Fluoride Carbonates as Deep-UV Nonlinear Optical Materials. Chem. Mater. 2013, 25, 3147–3153. [Google Scholar] [CrossRef]
- Luo, M.; Wang, G.; Lin, C.; Ye, N.; Zhou, Y.; Cheng, W. Na4La2(CO3)5 and CsNa5Ca5(CO3)8: Two new carbonates as UV nonlinear optical materials. Inorg. Chem. 2014, 53, 8098–8104. [Google Scholar] [CrossRef] [PubMed]
- Luo, M.; Lin, C.; Zou, G.; Ye, N.; Cheng, W. Sodium–rare earth carbonates with shorite structure and large second harmonic generation response. CrystEngComm 2014, 16, 4414–4421. [Google Scholar] [CrossRef]
- Kang, L.; Luo, S.Y.; Huang, H.W.; Ye, N.; Lin, Z.S.; Qin, J.G.; Chen, C.T. Prospects for Fluoride Carbonate Nonlinear Optical Crystals in the UV and Deep-UV Regions. J. Phys. Chem. C 2013, 117, 25684–25692. [Google Scholar] [CrossRef]
- Yang, Y.; Pan, S.L.; Hou, X.L.; Wang, C.Y.; Poeppelmeier, K.R.; Chen, Z.H.; Wu, H.P.; Zhou, Z.X. A congruently melting and deep UV nonlinear optical material: Li3Cs2B5O10. J. Mater. Chem. 2011, 21, 2890–2894. [Google Scholar] [CrossRef]
- Wang, S.; Ye, N.; Li, W.; Zhao, D. Alkaline beryllium borate NaBeB3O6 and ABe2B3O7 (A = K, Rb) as UV nonlinear optical crystals. J. Am. Chem. Soc. 2010, 132, 8779–8786. [Google Scholar] [CrossRef] [PubMed]
- Ye, N.; Chen, Q.X.; Wu, B.C.; Chen, C.T. Searching for new nonlinear optical materials on the basis of the anionic group theory. J. Appl. Phys. 1998, 84, 555–558. [Google Scholar] [CrossRef]
- Grice, J.D.; Maisonneuve, V.; Leblanc, M. Natural and Synthetic Fluoride Carbonates. Chem. Rev. 2006, 107, 114–132. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Tauc, J. UV-VIS. Mater. Res. Bull. 1970, 5, 721–730. [Google Scholar] [CrossRef]
- Kubelka, P.; Munk, F.Z. Ein Beitrag zur Optik der Farbanstriche. Tech. Phys. 1931, 12, 593–601. (In German) [Google Scholar]
| Crystals | SHG coefficient (×KDP)a | Structural criterion C | densities of the [CO3] (n/V) (Å-3) | (n/V) × C(Å−3) |
|---|---|---|---|---|
| KSrCO3F RbNa5Ca5(CO3)8 | 3.33 1 | 1 0.197 | 0.0089 0.0144 | 0.0089 0.0028 |
| Formula | RbNa5Ca5(CO3)8 |
|---|---|
| Formula Mass(amu) | 880.90 |
| Crystal System | Hexagonal |
| Space Group | P63mc |
| a (Å) | 10.075(3) |
| c (Å) | 12.639(5) |
| Α (°) | 90 |
| γ (°) | 120 |
| V (Å3) | 1111.2(7) |
| Z | 2 |
| ρ(calcd) (g/cm3) | 2.633 |
| Temperature (K) | 293(2) |
| Λ (Å) | 0.71073 |
| F(000) | 864 |
| Μ (mm−1) | 3.600 |
| θ (deg) | 2.33–27.50 |
| Rint | 0.0442 |
| R/wR (I > 2σ (I)) | 0.0409/0.1015 |
| R/wR (all data) | 0.0413/0.1018 |
| GOF on F2 | 1.117 |
| Absolute Structure Parameter | 0.00 |
| Largest Diff. Peak and Hole (e/Å-3) | 1.069 and –0.835 |
© 2016 by the author; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Q.; Luo, M. Synthesis, Crystal Structure, and Nonlinear Optical Properties of a New Alkali and Alkaline Earth Metal Carbonate RbNa5Ca5(CO3)8. Crystals 2017, 7, 10. https://doi.org/10.3390/cryst7010010
Chen Q, Luo M. Synthesis, Crystal Structure, and Nonlinear Optical Properties of a New Alkali and Alkaline Earth Metal Carbonate RbNa5Ca5(CO3)8. Crystals. 2017; 7(1):10. https://doi.org/10.3390/cryst7010010
Chicago/Turabian StyleChen, Qiaoling, and Min Luo. 2017. "Synthesis, Crystal Structure, and Nonlinear Optical Properties of a New Alkali and Alkaline Earth Metal Carbonate RbNa5Ca5(CO3)8" Crystals 7, no. 1: 10. https://doi.org/10.3390/cryst7010010