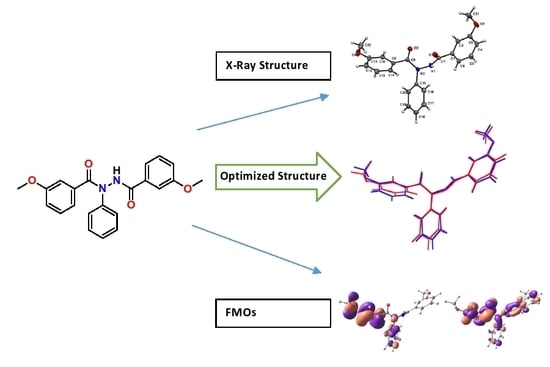
Synthesis, Crystal Structure, DFT Study of m-Methoxy-N′-(3-Methoxybenzoyl)-N-Phenylbenzohydrazide
Abstract
:1. Introduction
2. Results and Discussion
2.1. Crystal Structure
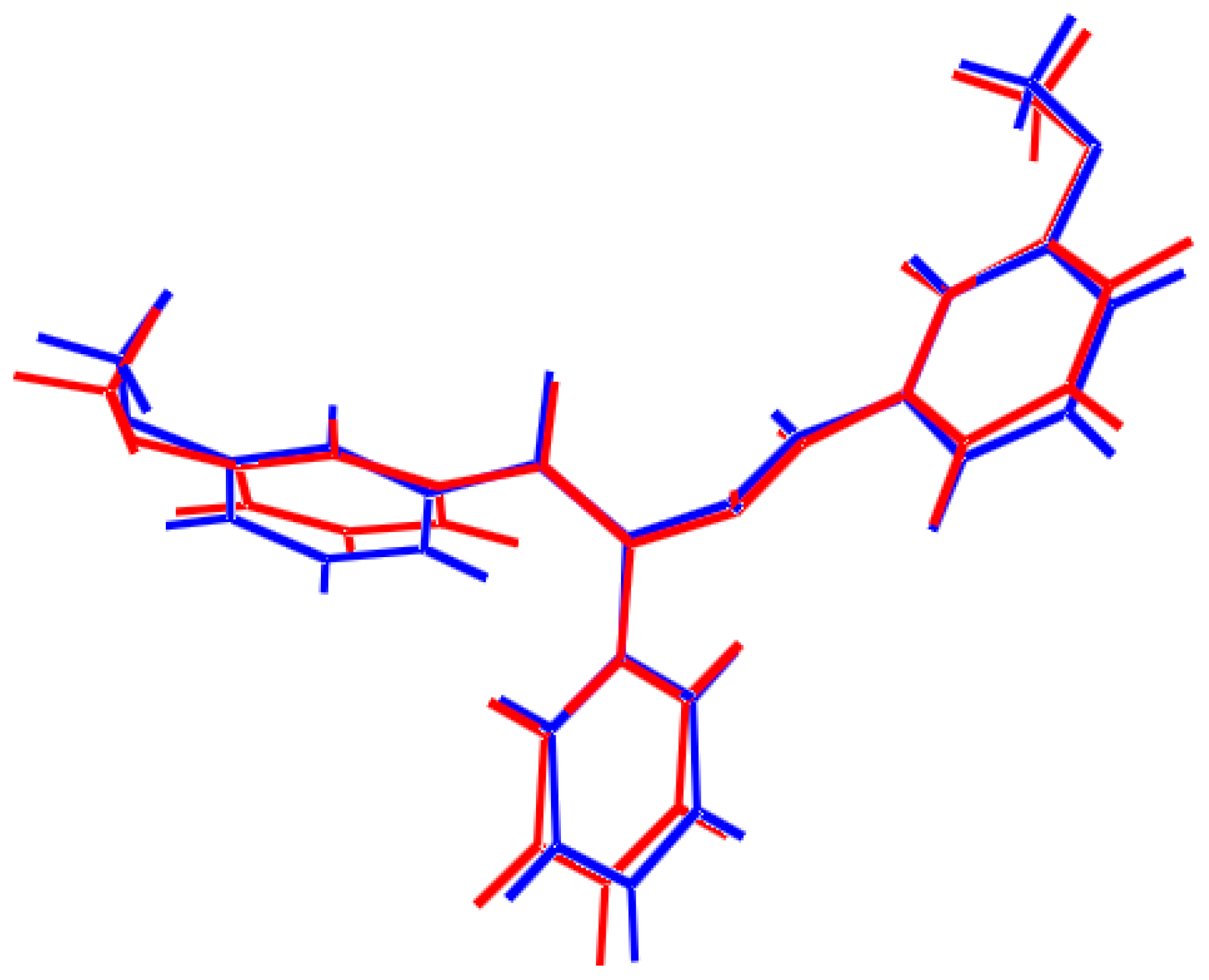
2.2. Optimized Geometry
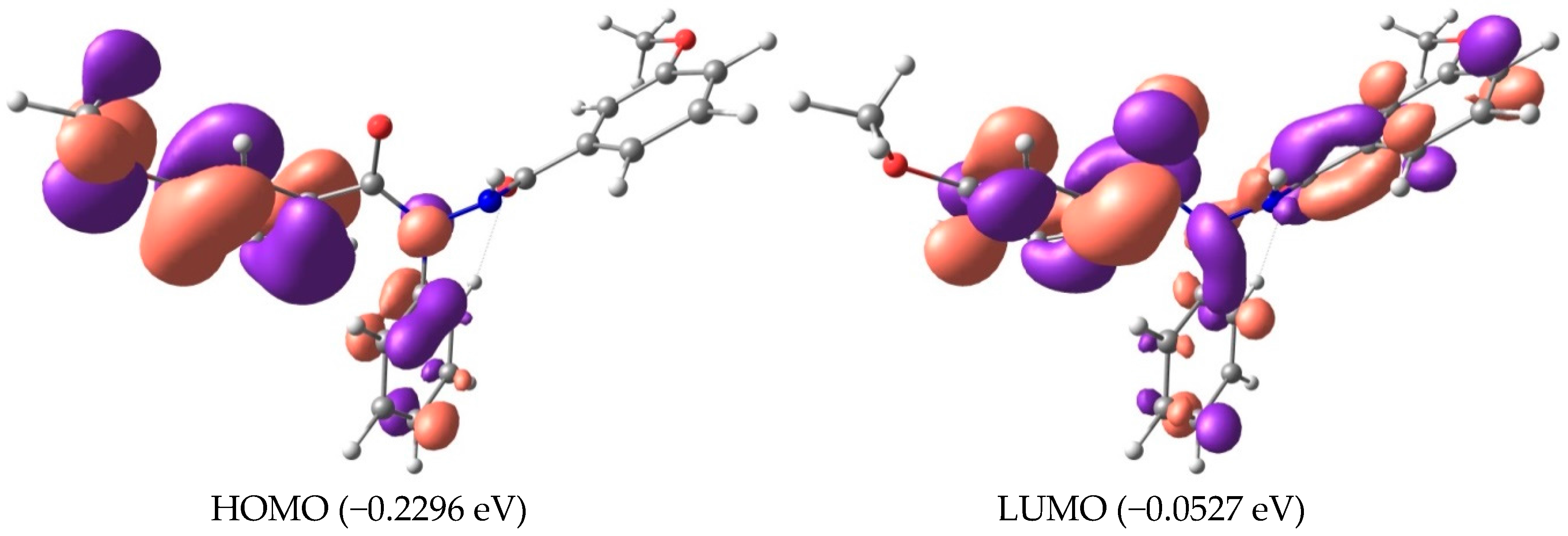
2.3. Frontier Molecular Orbitals
3. Experimental
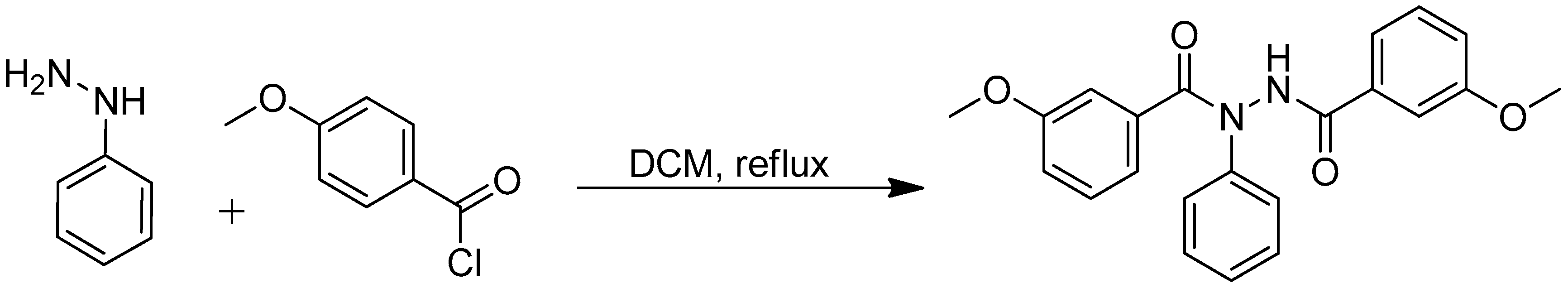
3.1. Synthesis m-Methoxy-N′-(3-methoxybenzoyl)-N-phenylbenzohydrazide
3.2. Computation
3.3. X-ray Crystallography
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cao, S.; Qian, X.; Song, G. N′-tert-Butyl-N′-aroyl-N-(alkoxycarbonylmethyl)-N-aroylhydrazines, a novel nonsteroidal ecdysone agonist: Syntheses, insecticidal activity, conformational, and crystal structure analysis. Can. J. Chem. 2001, 79, 272–278. [Google Scholar] [CrossRef]
- Ke, S.; Qian, X.; Liu, F.; Wang, N.; Fan, F.; Li, Z.; Yang, Q. Diacylhydrazine derivatives as novel potential chitin biosynthesis inhibitors: Design, synthesis, and structure–activity relationship. Eur. J. Med. Chem. 2009, 44, 2985–2993. [Google Scholar] [CrossRef] [PubMed]
- Mao, C.H.; Wang, Q.M.; Huang, R.Q.; Bi, F.C.; Chen, L.; Liu, Y.X.; Shang, J. Synthesis and insecticidal evaluation of novel n-oxalyl derivatives of tebufenozide. J. Agric. Food Chem. 2004, 52, 6737–6741. [Google Scholar] [CrossRef] [PubMed]
- Zotti, M.J.; Christiaens, O.; Rougé, P.; Grutzmacher, A.; Zimmer, P.; Smagghe, G. Structural changes under low evolutionary constraint may decrease the affinity of dibenzoylhydrazine insecticides for the ecdysone receptor in non-lepidopteran insects. Insect Mol. Biol. 2012, 21, 488–501. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.-L.; Li, J.; Yang, G.-F. Synthesis and insecticidal activity of chromanone and chromone analogues of diacylhydrazines. Bioorg. Med. Chem. 2007, 15, 1888–1895. [Google Scholar] [CrossRef] [PubMed]
- Smirle, M.J.; Lowery, D.T.; Zurowski, C.L. Chemical residues and bioactivity of tebufenozide applied to apple foliage. Pest Manag. Sci. 2004, 60, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.P.; Randall, G.E.; Castro, J.R.; Lindberg, R.D. Hypogastric artery infusion and radiation therapy for advanced squamous cell carcinoma of the cervix. Am. J. Roentgenol. Radium Ther. Nucl. Med. 1972, 114, 110–115. [Google Scholar] [PubMed]
- Sundaram, M.; Palli, S.; Smagghe, G.; Ishaaya, I.; Feng, Q.-L.; Primavera, M.; Tomkins, W.; Krell, P.; Retnakaran, A. Effect of RH-5992 on adult development in the spruce budworm, Choristoneura fumiferana. Insect Biochem. Mol. Biol. 2002, 32, 225–231. [Google Scholar] [CrossRef]
- Clarke-Harris, D.; Fleischer, S.J. Sequential sampling and biorational chemistries for management of lepidopteran pests of vegetable amaranth in the caribbean. J. Econ. Entomol. 2003, 96, 798–804. [Google Scholar] [CrossRef] [PubMed]
- Retnakaran, A.; Krell, P.; Feng, Q.; Arif, B. Ecdysone agonists: Mechanism and importance in controlling insect pests of agriculture and forestry. Arch. Insect Biochem. Physiol. 2003, 54, 187–199. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Enríquez, C.-L.; Pineda, S.; Figueroa, J.I.; Schneider, M.-I.; Martínez, A.-M. Toxicity and sublethal effects of methoxyfenozide on spodoptera exigua (lepidoptera: Noctuidae). J. Econ. Entomol. 2010, 103, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Boudjelida, H.; Bouaziz, A.; Soin, T.; Smagghe, G.; Soltani, N. Effects of ecdysone agonist halofenozide against Culex pipiens. Pestic. Biochem. Physiol. 2005, 83, 115–123. [Google Scholar] [CrossRef]
- Mosallanejad, H.; Soin, T.; Swevers, L.; Iatrou, K.; Nakagawa, Y.; Smagghe, G. Non-steroidal ecdysteroid agonist chromafenozide: Gene induction activity, cell proliferation inhibition and larvicidal activity. Pestic. Biochem. Physiol. 2008, 92, 70–76. [Google Scholar] [CrossRef]
- Buzykin, B.; Gubaidullin, A.; Litvinov, I.; Gazetdinova, N.; Sysoeva, L. 1, 2-diacyl-1-arylhydrazines-ii. Molecular and crystalline structure of 1, 2-dibenzoyl-1-phenyl-2-acetylhydrazines and 1-salicyloyl-1-(4-nitrophenyl)-2-acetylhydrazines. Russ. J. Gen. Chem. 1998, 68, 1972–1976. [Google Scholar]
- Abbas, A.; Nazir, H.; Naseer, M.M.; Bolte, M.; Hussain, S.; Hafeez, N.; Hasan, A. Synthesis, spectral characterization, self-assembly and biological studies of N-acyl-2-pyrazolines bearing long alkoxy side chains. Spectrochim. Acta Part A 2014, 120, 176–184. [Google Scholar] [CrossRef] [PubMed]
- Saeed, A.; Arshad, M.I.; Bolte, M.; Fantoni, A.C.; Espinoza, Z.Y.D.; Erben, M.F. On the roles of close shell interactions in the structure of acyl-substituted hydrazones: An experimental and theoretical approach. Spectrochim. Acta Part A 2016, 157, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Lide, D.R. A survey of carbon-carbon bond lengths. Tetrahedron 1962, 17, 125–134. [Google Scholar] [CrossRef]
- Allen, F.H.; Kennard, O.; Watson, D.G.; Brammer, L.; Orpen, A.G.; Taylor, R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkin Trans. 2 1987, S1–S19. [Google Scholar] [CrossRef]
- Becker, H. Jan Fleming, Frontier Orbitals and Organic Chemical Reactions., John Wiley u. Sons LTD. J. Praktische Chem. 1978, 320, 879–880. [Google Scholar] [CrossRef]
- Neese, F. The orca program system. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 73–78. [Google Scholar] [CrossRef]
- Allouche, A.R. Gabedit—A graphical user interface for computational chemistry softwares. J. Comput. Chem. 2011, 32, 174–182. [Google Scholar] [CrossRef] [PubMed]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self—Consistent molecular orbital methods. XII. Further extensions of gaussian—type basis sets for use in molecular orbital studies of organic molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of shelx. Acta Crystallogr. Sect. A 2007, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
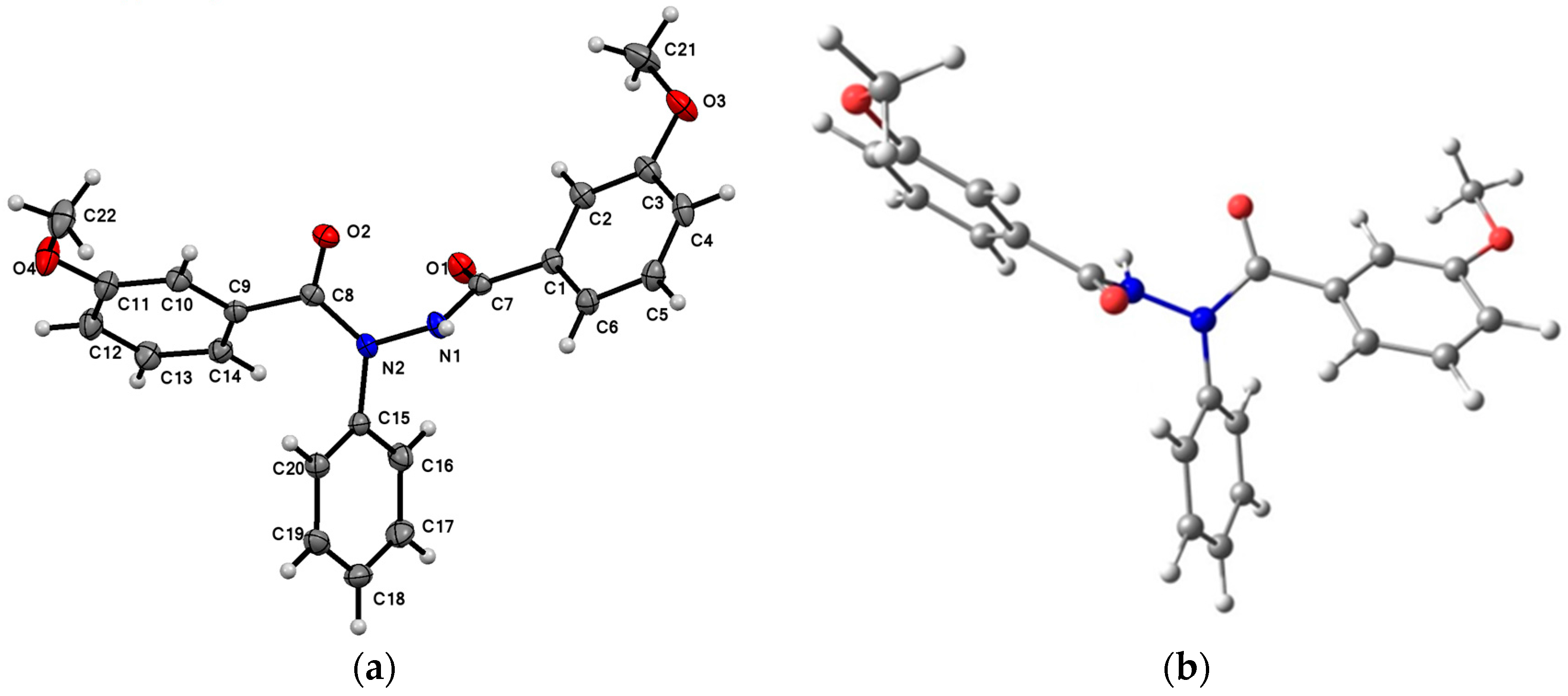
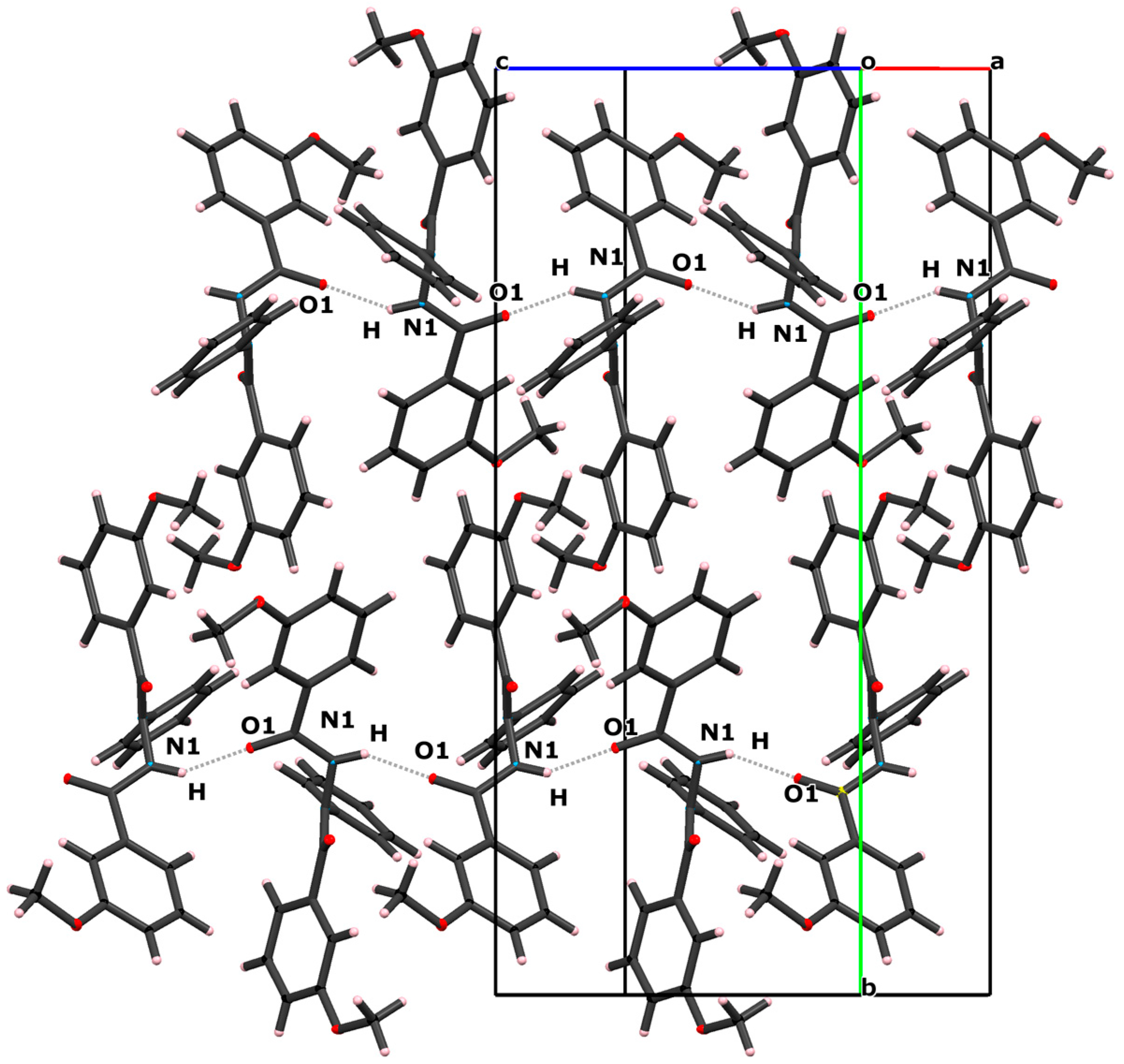
| D–H…A | D–H (Å) | H…A (Å) | D…A (Å) | D–H…A (°) |
|---|---|---|---|---|
| N1–H…O1 i | 0.867 (16) | 1.946 (16) | 2.7089 (11) | 146.1 (14) |
| C21–H21A…O2 | 0.960 | 2.692 | 3.604(2) | 159(1) |
| Bond Lengths (Å) | Experimental | Calculated B3LYP/6-311G | Difference |
|---|---|---|---|
| N2–C8 | 1.375 | 1.398 | 0.023 |
| N2–N1 | 1.395 | 1.411 | 0.016 |
| N2–C15 | 1.437 | 1.445 | 0.008 |
| N1–C7 | 1.347 | 1.386 | 0.039 |
| O1–C7 | 1.221 | 1.249 | 0.028 |
| O2–C8 | 1.221 | 1.253 | 0.032 |
| O3–C3 | 1.369 | 1.391 | 0.022 |
| O3–C21 | 1.427 | 1.445 | 0.018 |
| O4–C11 | 1.364 | 1.392 | 0.028 |
| O4–C22 | 1.427 | 1.454 | 0.027 |
| C1–C7 | 1.494 | 1.492 | −0.002 |
| C8–C9 | 1.499 | 1.489 | −0.01 |
| Angle (°) | Experimental | Calculated | Difference |
| C3–O3–C21 | 117.08 | 118.97 | −1.89 |
| C11–O4–C22 | 116.92 | 118.92 | -2 |
| C8–N2–N1 | 115.62 | 114.64 | 0.98 |
| C8–N2–C15 | 127.8 | 126.42 | 1.38 |
| N1–N2–C15 | 112.92 | 116.85 | −3.93 |
| C7–N1–N2 | 119.55 | 119.83 | −0.28 |
| O2–C8–N2 | 120.87 | 119.23 | 1.64 |
| O2–C8–C9 | 120.59 | 121.47 | −0.88 |
| N2–C8–C9 | 118.46 | 119.3 | −0.84 |
| O3–C3–C2 | 124.12 | 124.24 | −0.12 |
| O3–C3–C4 | 115.69 | 115.43 | 0.26 |
| O1–C7–N1 | 122.89 | 122.22 | 0.67 |
| O1–C7–C1 | 122.55 | 122.38 | 0.17 |
| N1–C7–C1 | 114.56 | 115.39 | −0.83 |
| O4–C11–C10 | 124.14 | 124.24 | −0.1 |
| O4–C11–C12 | 116 | 115.49 | 0.51 |
| C20–C15–N2 | 120.75 | 119.76 | 0.99 |
| Crystal Data | |
|---|---|
| Empirical Formula | C22H20N2O4 |
| Formula weight | 376.40 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 130 |
| a, b, c (Å) | 8.7338(1), 24.5602(3), 9.6929(1) |
| β (°) | 113.186 (2) |
| V (Å3) | 1911.23 (4) |
| Z | 4 |
| Radiation type | CuKα |
| μ (mm−1) | 0.74 |
| Crystal size (mm) | 0.59 × 0.20 × 0.06 |
| Data collection | |
| Diffractometer | SuperNova, Dual, Cu at zero, Atlas diffractometer |
| Tmin, Tmax | 0.809, 1.000 |
| No. of measured, independent and observed [I > 2s(I)] reflections | 9689, 4000, 3668 |
| Rint | 0.018 |
| (sin q/l)max (Å−1) | 0.632 |
| Refinement | |
| R[F2 > 2s(F2)], wR(F2), S | 0.035, 0.094, 1.04 |
| No. of reflections | 4000 |
| No. of parameters | 259 |
| Dñmax, Dñmin (e·Å−3) | 0.24, −0.24 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arshad, I.; Yameen, J.; Saeed, A.; White, J.M.; Albericio, F. Synthesis, Crystal Structure, DFT Study of m-Methoxy-N′-(3-Methoxybenzoyl)-N-Phenylbenzohydrazide. Crystals 2017, 7, 19. https://doi.org/10.3390/cryst7010019
Arshad I, Yameen J, Saeed A, White JM, Albericio F. Synthesis, Crystal Structure, DFT Study of m-Methoxy-N′-(3-Methoxybenzoyl)-N-Phenylbenzohydrazide. Crystals. 2017; 7(1):19. https://doi.org/10.3390/cryst7010019
Chicago/Turabian StyleArshad, Ifzan, Javeria Yameen, Aamer Saeed, Jonathan M. White, and Fernando Albericio. 2017. "Synthesis, Crystal Structure, DFT Study of m-Methoxy-N′-(3-Methoxybenzoyl)-N-Phenylbenzohydrazide" Crystals 7, no. 1: 19. https://doi.org/10.3390/cryst7010019