SRG Inscription in Supramolecular Liquid Crystalline Polymer Film: Replacement of Mesogens
Abstract
:1. Introduction
2. Results and Discussion
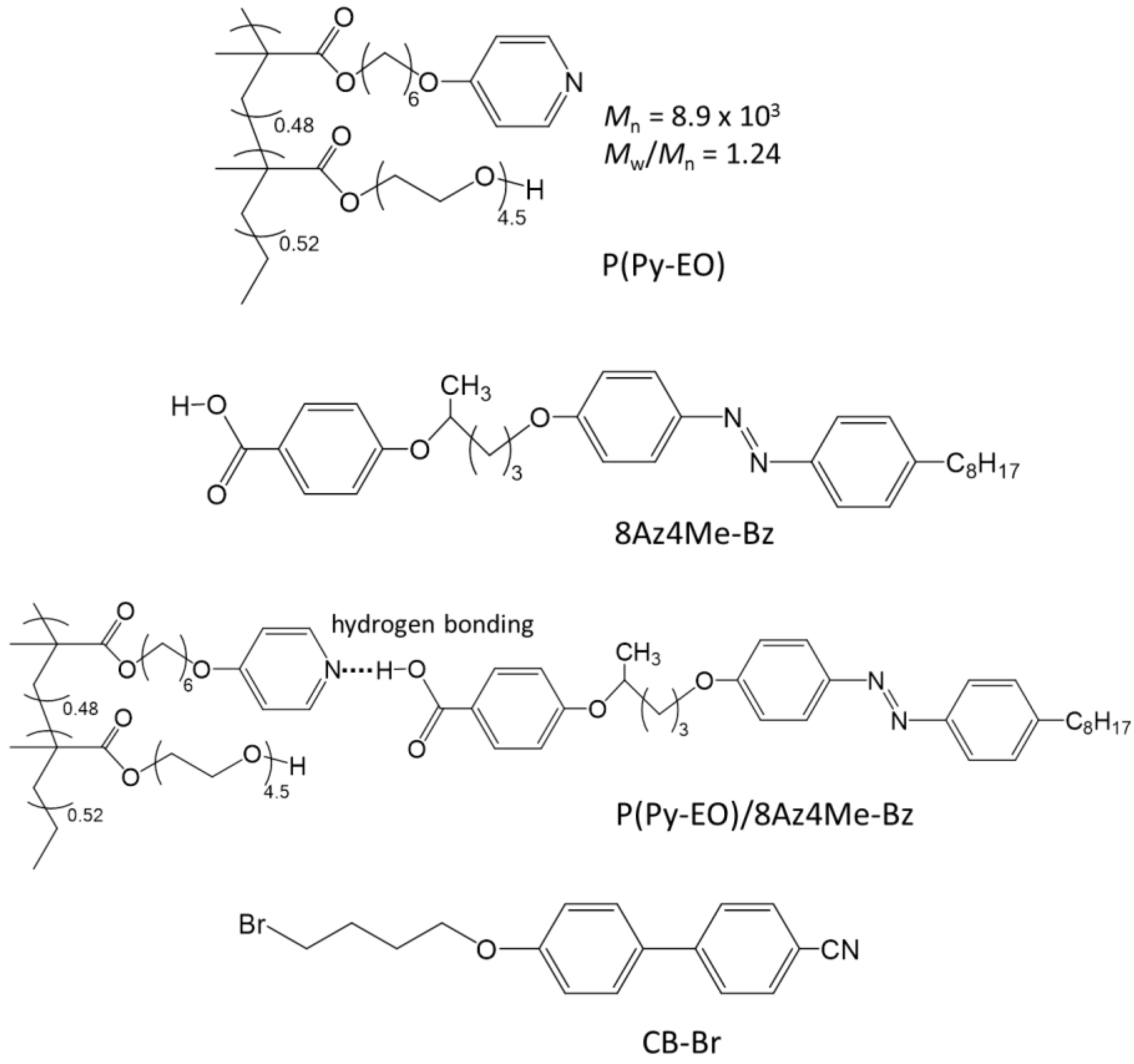
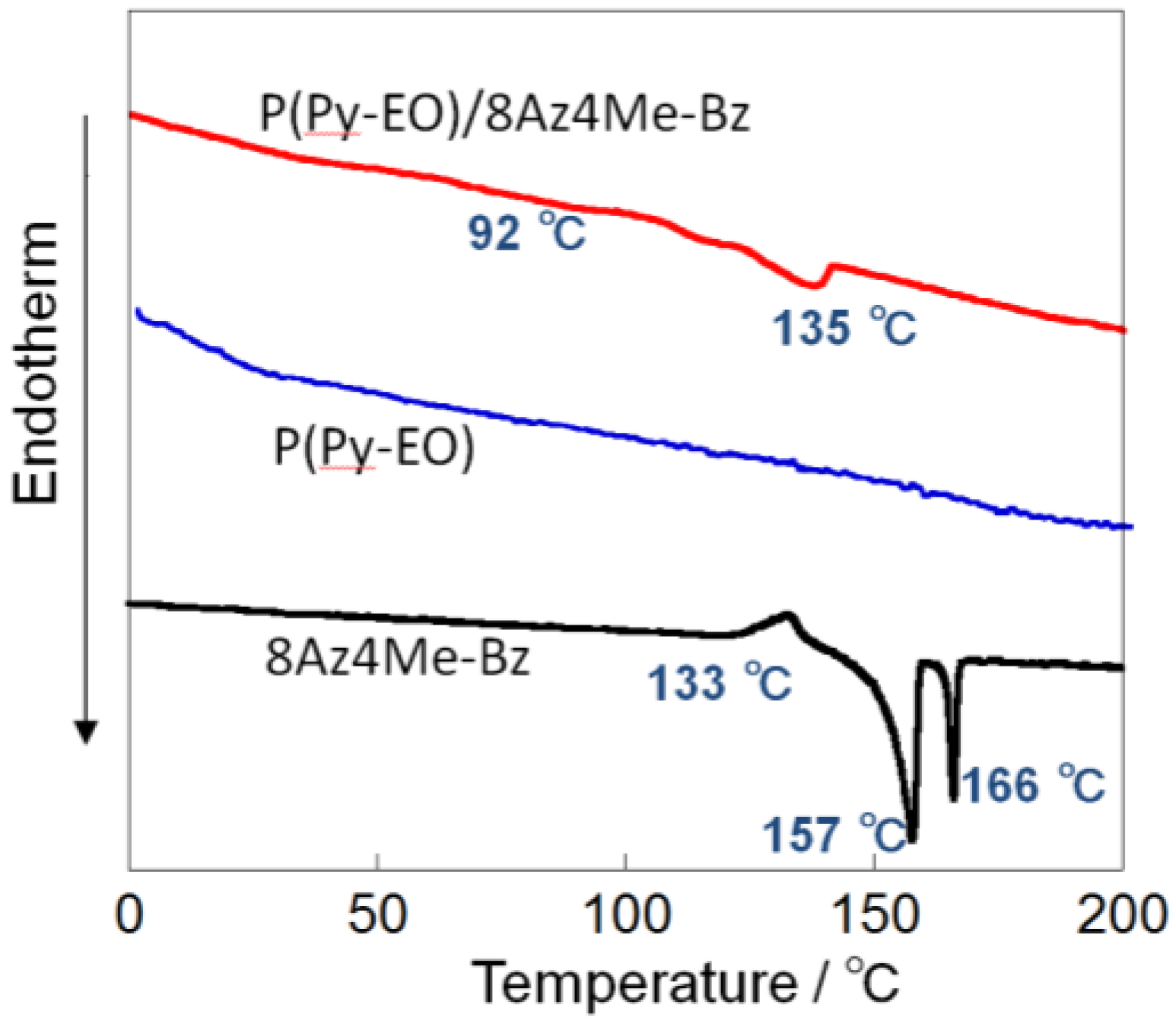
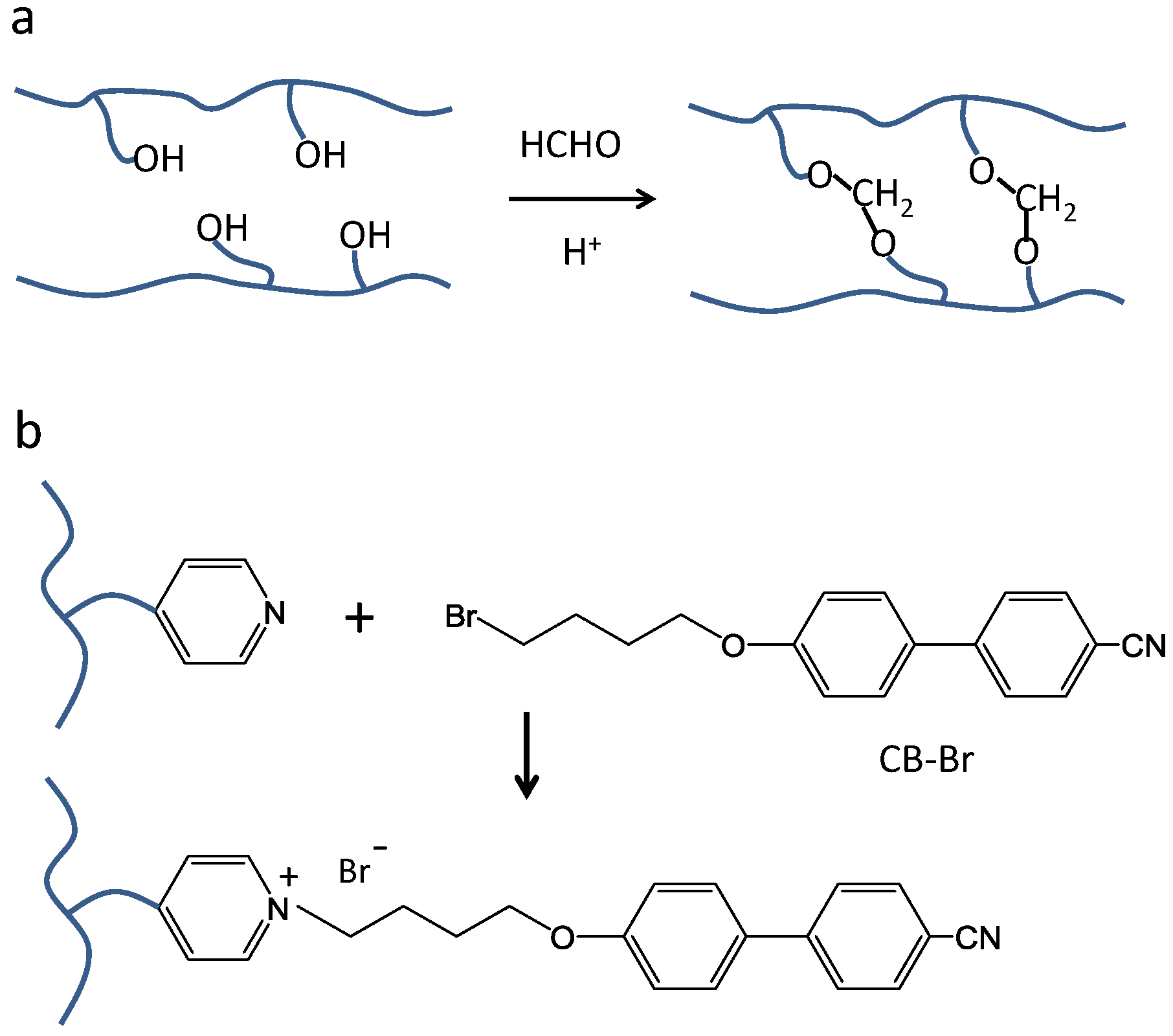
2.1. Characterizations of P(Py-EO)-Az
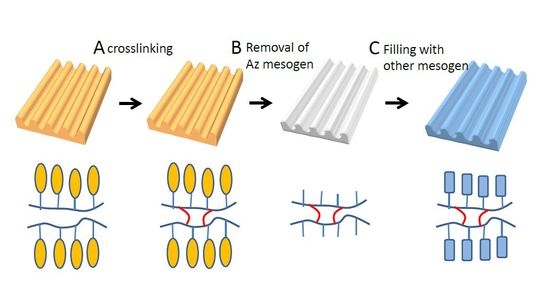
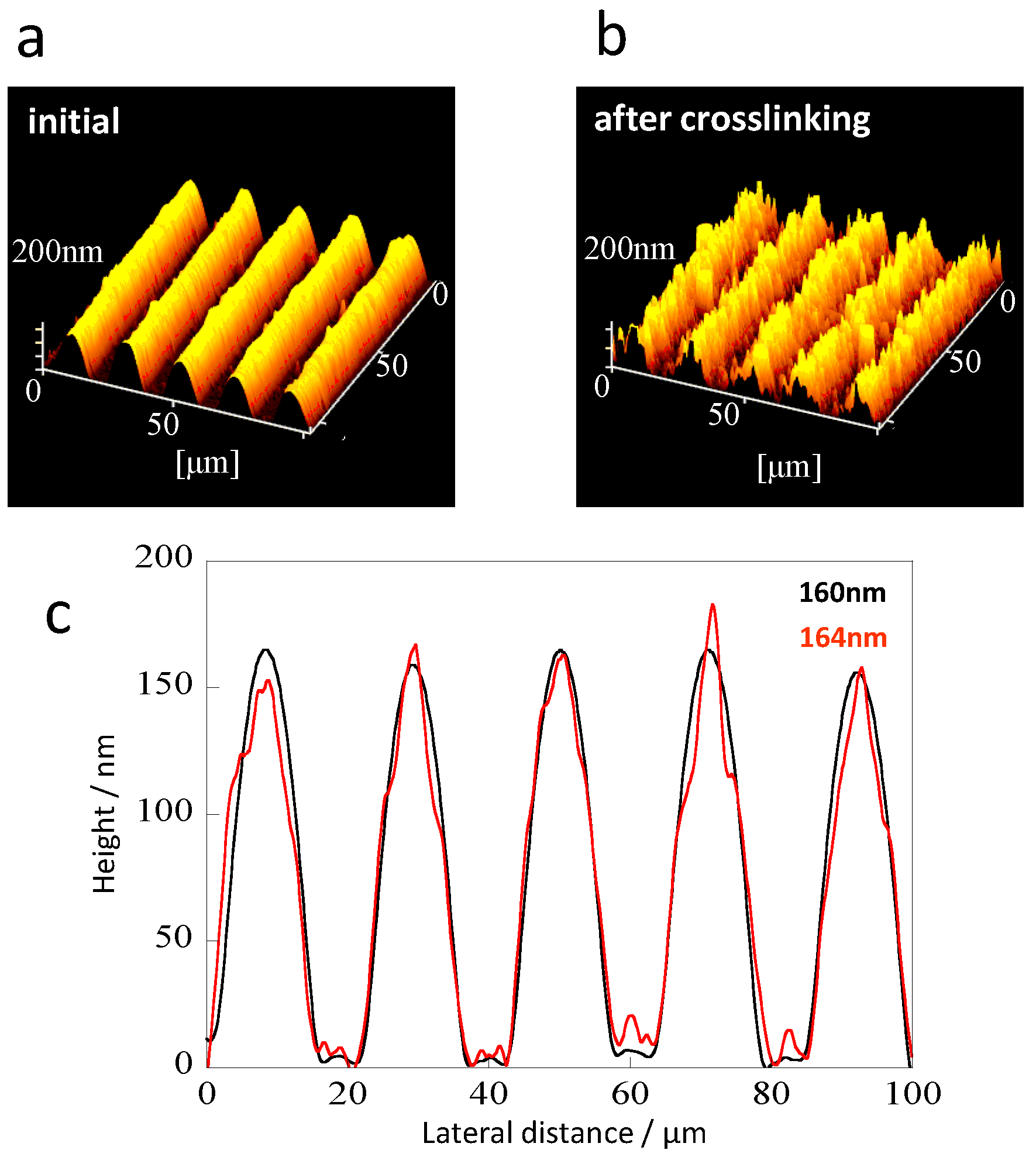
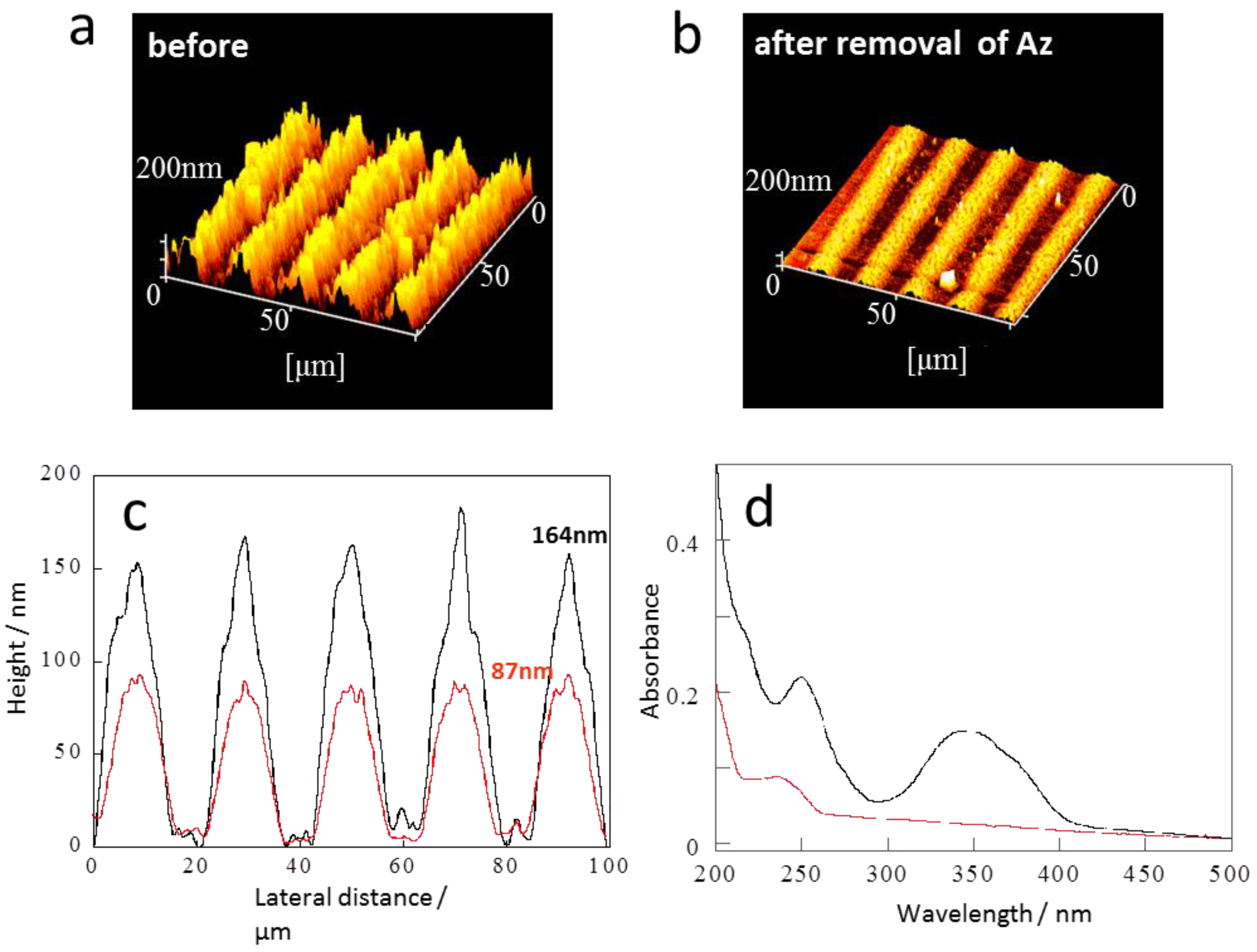
2.2. SRG Inscription and Replacement of Mesogens
3. Materials and Methods
3.1. Materials
3.2. Methods and Measurements
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yager, K.G.; Barrett, C.J. All-optical patterning of azo polymer films. Curr. Opin. Solid State Mater. Sci. 2001, 5, 487–494. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced Motions in Azo-Containing Polymers. Chem. Rev. 2002, 102, 4139–4176. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, K.N.; Kim, D.Y.; Bian, S.; Williams, J.; Liu, W.; Li, L.; Samuelson, L.; Kumar, J.; Tripathy, K.S. Surface relief structures on azo polymer films. J. Mater. Chem. 1999, 9, 1941–1955. [Google Scholar] [CrossRef]
- Lee, S.; Kang, H.S.; Park, J.K. Directional photofluidization lithography: Micro/nanostructural evolution by photofluidic motions of azobenzene materials. Adv. Mater. 2012, 24, 2069–2103. [Google Scholar] [CrossRef] [PubMed]
- Shimamura, A.; Priimagi, A.; Mamiya, J.; Ikeda, T.; Yu, Y.; Barrett, C.J.; Shishido, A. Simultaneous analysis of optical and mechanical properties of cross-linked azobenzene-containing liquid-crystalline polymer films. ACS Appl. Mater. Interfaces 2011, 3, 4190–4196. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Meso- and Microscopic Motions in Photoresponsive Liquid Crystalline Polymer Films. Macromol. Rapid Commun. 2014, 35, 271–290. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Photoresponsive self-assembly motions in polymer thin films. Curr. Opin. Solid State Mater. Sci. 2006, 10, 241–248. [Google Scholar] [CrossRef]
- Kawatsuki, N.; Hasegawa, T.; Ono, H.; Tamoto, T. Formation of Polarization Gratings and Surface Relief Gratings in Photocrosslinkable Polymer Liquid Crystals by Polarization Holography. Adv. Mater. 2003, 15, 991–994. [Google Scholar] [CrossRef]
- Ubukata, T.; Seki, T.; Ichimura, K. Surface Relief Gratings in Host–Guest Supramolecular Materials. Adv. Mater. 2000, 12, 1675–1678. [Google Scholar] [CrossRef]
- Zettsu, N.; Ubukata, T.; Seki, T.; Ichimura, K. Soft Crosslinkable Azo Polymer for Rapid Surface Relief Formation and Persistent Fixation. Adv. Mater. 2001, 13, 1693–1697. [Google Scholar] [CrossRef]
- Isayama, J.; Nagano, S.; Seki, T. Phototriggered Mass Migrating Motions in Liquid Crystalline Azobenzene Polymer Films with Systematically Varied Thermal Properties. Macromolecules 2010, 43, 4105–4112. [Google Scholar] [CrossRef]
- Zettsu, N.; Seki, T. Highly Efficient Photogeneration of Surface Relief Structure and Its Immobilization in Cross-Linkable Liquid Crystalline Azobenzene Polymers. Macromolecules 2004, 37, 8692–8698. [Google Scholar] [CrossRef]
- Nakano, H.; Takahashi, T.; Kadota, T.; Shirota, Y. Formation of a Surface Relief Grating Using a Novel Azobenzene-Based Photochromic Amorphous Molecular Material. Adv. Mater. 2002, 14, 1157–1160. [Google Scholar] [CrossRef]
- Ando, H.; Tanino, T.; Nakano, H.; Shirota, Y. Photoinduced surface relief grating formation using new polymers containing the same azobenzene chromophore as a photochoromic amorphous molecular material. Mater. Chem. Phys. 2009, 113, 376–381. [Google Scholar] [CrossRef]
- Zettsu, N.; Ogasawara, T.; Arakawa, R.; Nagano, S.; Ubukata, T.; Seki, T. Highly Photosensitive Surface Relief Gratings Formation in a Liquid Crystalline Azobenzene Polymer: New Implications for the Migration Process. Macromolecules 2007, 40, 4607–4613. [Google Scholar] [CrossRef]
- Yu, H.; Ikeda, T. Photocontrollable liquid-crystalline actuators. Adv. Mater. 2011, 23, 2149–2180. [Google Scholar] [CrossRef] [PubMed]
- Seki, T. Light-directed alignment, surface morphing and related processes: recent trends. J. Mater. Chem. C 2016, 4, 7895–7910. [Google Scholar] [CrossRef]
- Zettsu, N.; Ogasawara, T.; Mizoshita, N.; Nagano, S.; Seki, T. Photo-Triggered Surface Relief Grating Formation in Supramolecular Liquid Crystalline Polymer Systems with Detachable Azobenzene Unit. Adv. Mater. 2008, 20, 516–521. [Google Scholar] [CrossRef]
- Goldenberg, L.M.; Kulikovsky, L.; Kulikovska, O.; Stumpe, J. New materials with detachable azobenzene: effective, colourless and extremely stable surface relief gratings. J. Mater. Chem. 2009, 19, 8068–8071. [Google Scholar] [CrossRef]
- Nishizawa, K.; Nagano, S.; Seki, T. Micropatterning of titanium oxide film via phototactic mass transport. J. Mater. Chem. 2009, 19, 7191–7194. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.J. A new approach to mesophase stabilization through hydrogen bonding molecular interactions in binary mixtures. J. Am. Chem. Soc. 1989, 111, 8533–8534. [Google Scholar] [CrossRef]
- Kato, T.; Frechet, J.M.J. Stabilization of a liquid-crystalline phase through noncovalent interaction with a polymer side chain. Macromolecules 1989, 22, 3818–3819. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kanie, K. Hydrogen-Bonded Liquid Crystalline Materials: Supramolecular Polymeric Assembly and the Induction of Dynamic Function. Macromol. Rapid Commun. 2001, 22, 797–814. [Google Scholar] [CrossRef]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional Liquid-Crystalline Assemblies: Self-Organized Soft Materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Ambrožič, G.; Žigon, M. Hydrogen-bonded polyurethane complexes based on 4-alkoxybenzoic acids as the low molar mass components. Polym. Int. 2005, 54, 606–613. [Google Scholar] [CrossRef]
- Li, W.; Nagano, S.; Seki, T. Photo-crosslinkable liquid-crystalline azo-polymer for surface relief gratings and persistent fixation. New J. Chem. 2009, 33, 1343–1348. [Google Scholar] [CrossRef]




© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mitsui, S.; Nagano, S.; Hara, M.; Seki, T. SRG Inscription in Supramolecular Liquid Crystalline Polymer Film: Replacement of Mesogens. Crystals 2017, 7, 52. https://doi.org/10.3390/cryst7020052
Mitsui S, Nagano S, Hara M, Seki T. SRG Inscription in Supramolecular Liquid Crystalline Polymer Film: Replacement of Mesogens. Crystals. 2017; 7(2):52. https://doi.org/10.3390/cryst7020052
Chicago/Turabian StyleMitsui, Shun, Shusaku Nagano, Mitsuo Hara, and Takahiro Seki. 2017. "SRG Inscription in Supramolecular Liquid Crystalline Polymer Film: Replacement of Mesogens" Crystals 7, no. 2: 52. https://doi.org/10.3390/cryst7020052