A Reversible Single-Crystal to Single-Crystal Thermal Phase Transformation of 3-(2-Bromo-4-(1-methylethyl)phenyl)-1,1-dimethyl urea
Abstract
:1. Introduction
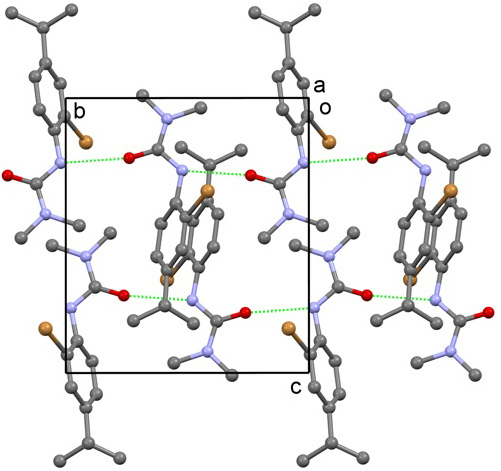
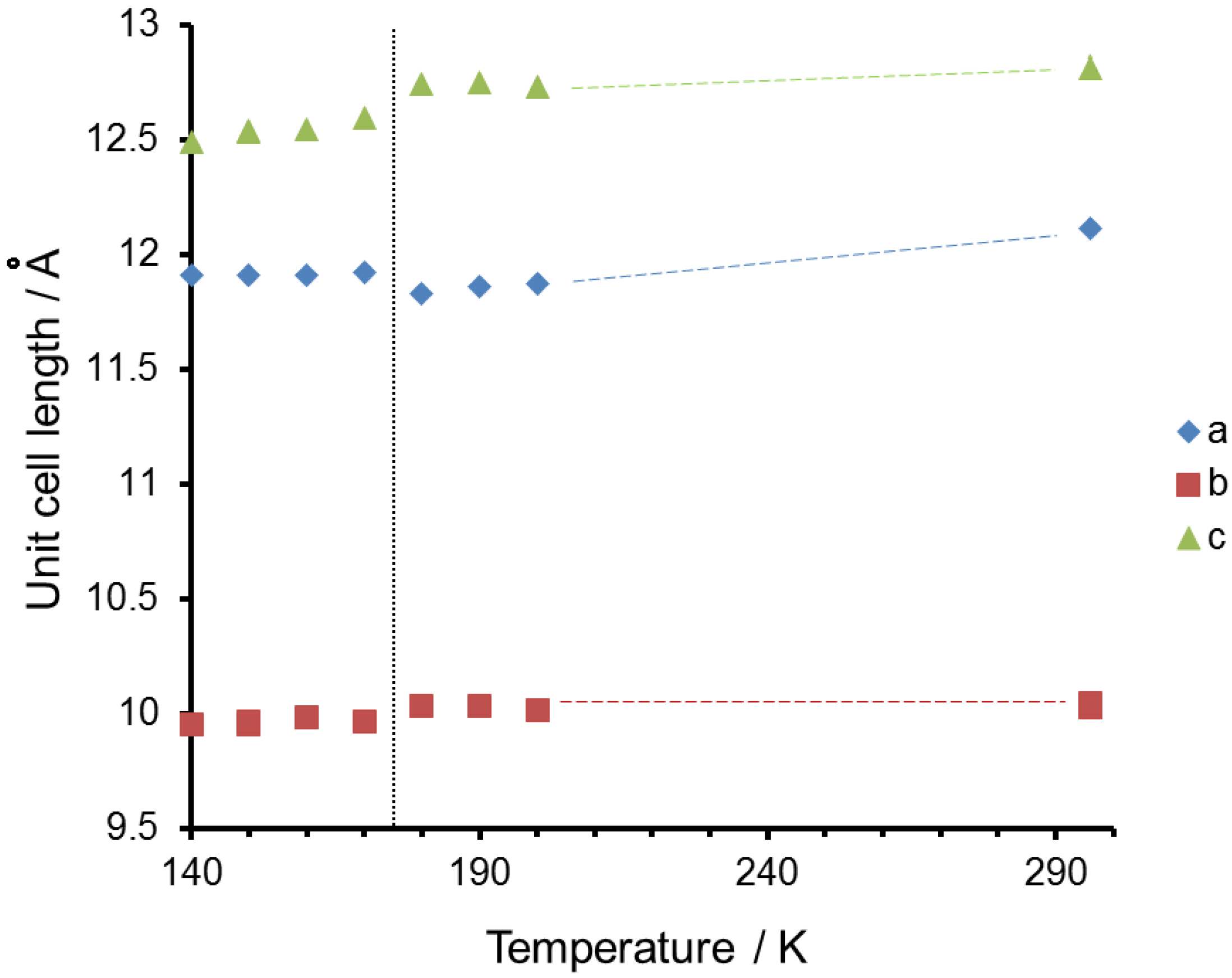
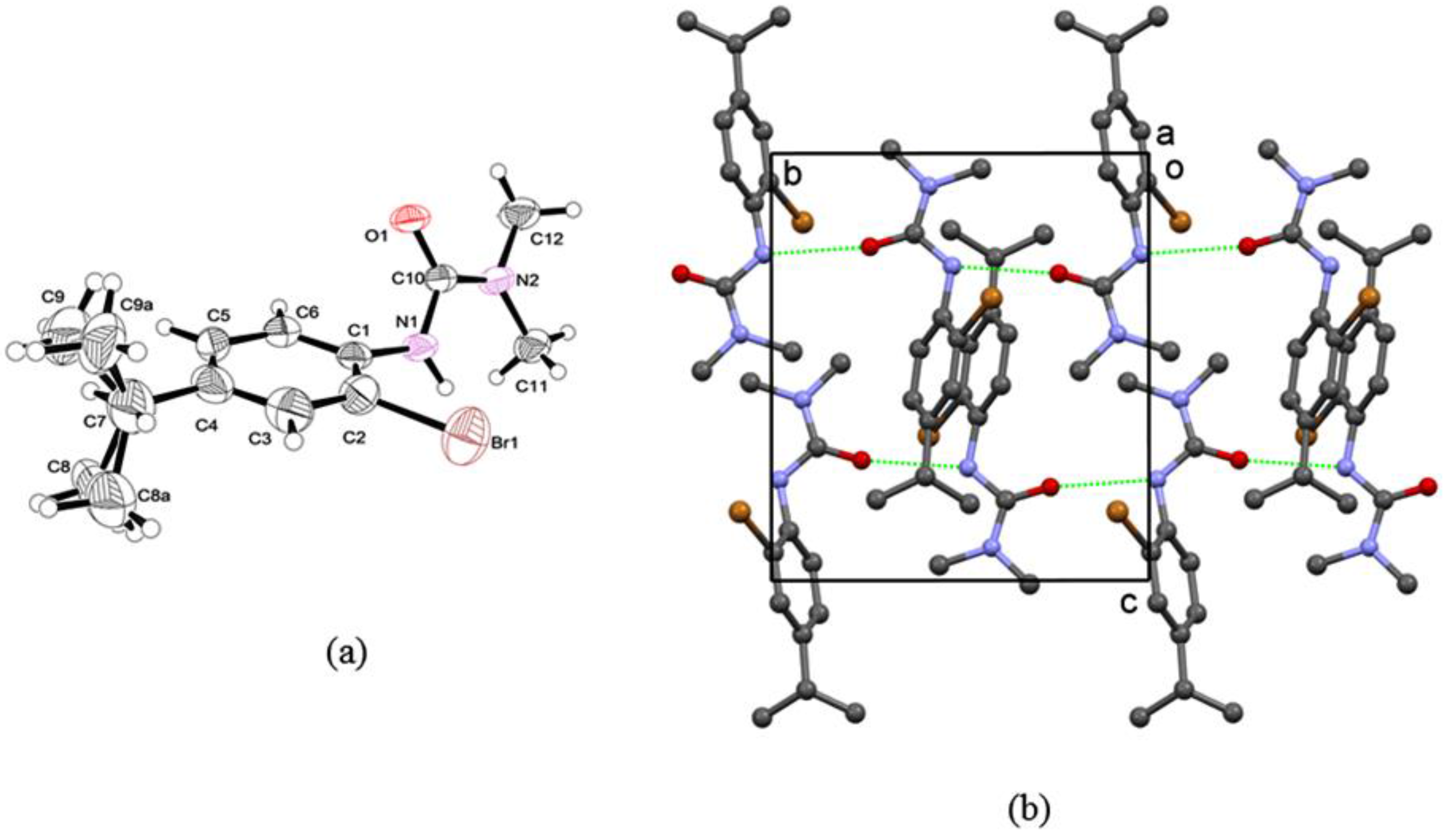
2. Results and Discussion
3. Experimental Section
3.1. General
3.2. Synthesis of 3-(2-Bromo-4-(1-methylethyl)phenyl)-1,1-dimethylurea
3.3. Structure Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kocyigit-Kaymakcioglu, B.; Celen, A.O.; Tabanca, N.; Ali, A.; Khan, S.I.; Khan, I.A.; Wedge, D.E. Synthesis and biological activity of substituted urea and thiourea derivatives containing 1,2,4-triazole moieties. Molecules 2013, 18, 3562–3576. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.M.; Saeed, S.; Ali, M.; Gohar, M.; Zahid, J.; Khan, A.; Perveen, S.; Choudhary, M.I. Unsymmetrically disubstituted urea derivatives: A potent class of antiglycating agents. Bioorg. Med. Chem. 2009, 17, 2447–2451. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, L.; Lima, L.A.; Cechinel-Filho, V.; Correa, R.; Buzzi, F.d.C.; Nunes, R.J. Synthesis of new 1-phenyl-3-{4-[(2E)-3-phenylprop-2-enoyl]phenyl}-thiourea and urea derivatives with anti-nociceptive activity. Bioorg. Med. Chem. 2008, 16, 8526–8534. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Zhang, J.; Nan, F.; Petukhov, P.A.; Grajkowska, E.; Wroblewski, J.T.; Yamamoto, T.; Bzdega, T.; Wroblewska, B.; Neale, J.H. Synthesis of urea-based inhibitors as active site probes of glutamate carboxypeptidase II: Efficacy as analgesic agents. J. Med. Chem. 2004, 47, 1729–1738. [Google Scholar] [CrossRef] [PubMed]
- Hemantha, H.P.; Chennakrishnareddy, G.; Vishwanatha, T.M.; Sureshbabu, V.V. One-pot synthesis of ureido peptides and urea-tethered glycosylated amino acids employing Deoxo-Fluor and TMSN3. Synlett 2009, 407–410. [Google Scholar] [CrossRef]
- Carnaroglio, D.; Martina, K.; Palmisano, G.; Penoni, A.; Domini, C.; Cravotto, G. One-pot sequential synthesis of isocyanates and urea derivatives via a microwave-assisted Staudinger-aza-Wittig reaction. Beilstein J. Org. Chem. 2013, 9, 2378–2386. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Porco, J.A., Jr. Synthesis of carbamates and ureas using Zr(IV)-catalyzed exchange processes. Org. Lett. 2007, 9, 1517–1520. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Cheng, H.; Liu, R.; Wang, Q.; Hao, Y.; Yu, Y.; Zhao, F. Synthesis of urea derivatives from amines and CO2 in the absence of catalyst and solvent. Green Chem. 2010, 12, 1811–1816. [Google Scholar] [CrossRef]
- Marinescu, L.; Thinggaard, J.; Thomsen, I.B.; Bols, M. Radical azidonation of aldehydes. J. Org. Chem. 2003, 68, 9453–9455. [Google Scholar] [CrossRef] [PubMed]
- Padiya, K.J.; Gavade, S.; Kardile, B.; Tiwari, M.; Bajare, S.; Mane, M.; Gaware, V.; Varghese, S.; Harel, D.; Kurhade, S. Unprecedented “in water” imidazole carbonylation: paradigm shift for preparation of urea and carbamate. Org. Lett. 2012, 14, 2814–2817. [Google Scholar] [CrossRef] [PubMed]
- Vasantha, B.; Hemantha, H.P.; Sureshbabu, V.V. 1-Propanephosphonic acid cyclic anhydride (T3P) as an efficient promoter for the Lossen rearrangement: Application to the synthesis of urea and carbamate derivatives. Synthesis 2010, 2990–2996. [Google Scholar] [CrossRef]
- Dube, P.; Nathel, N.F.F.; Vetelino, M.; Couturier, M.; Aboussafy, C.L.; Pichette, S.; Jorgensen, M.L.; Hardink, M. Carbonyldiimidazole-mediated Lossen rearrangement. Org. Lett. 2009, 11, 5622–5625. [Google Scholar] [CrossRef] [PubMed]
- Paz, J.; Perez-Balado, C.; Iglesias, B.; Munoz, L. Different reactivity of hydroxylamine with carbamoyl azides and carbamoyl cyanides: Synthesis of hydroxyureas and carbamoyl amidoximes. J. Org. Chem. 2010, 75, 8039–8047. [Google Scholar] [CrossRef] [PubMed]
- Spyropoulos, C.; Kokotos, C.G. One-pot synthesis of ureas from Boc-protected amines. J. Org. Chem. 2014, 79, 4477–4483. [Google Scholar] [CrossRef] [PubMed]
- Artuso, E.; Degani, I.; Fochi, R.; Magistris, C. Preparation of mono-, di-, and trisubstituted ureas by carbonylation of aliphatic amines with S,S-dimethyl dithiocarbonate. Synthesis 2007, 3497–3506. [Google Scholar] [CrossRef]
- Mizuno, T.; Nakai, T.; Mihara, M. Synthesis of unsymmetrical ureas by sulfur-assisted carbonylation with carbon monoxide and oxidation with molecular oxygen under mild conditions. Synthesis 2009, 2492–2496. [Google Scholar] [CrossRef]
- Kim, S.H.; Hong, S.H. Ruthenium-catalyzed urea synthesis using methanol as the C1 source. Org. Lett. 2016, 18, 212–215. [Google Scholar] [CrossRef] [PubMed]
- Thalluri, K.; Manne, S.R.; Dev, D.; Mandal, B. Ethyl 2-cyano-2-(4-nitrophenylsulfonyloxyimino)acetate-mediated Lossen rearrangement: Single-pot racemization-free synthesis of hydroxamic acids and ureas from carboxylic acids. J. Org. Chem. 2014, 79, 3765–3775. [Google Scholar] [CrossRef] [PubMed]
- Vinogradova, E.V.; Fors, B.P.; Buchwald, S.L. Palladium-catalyzed cross-coupling of aryl chlorides and triflates with sodium cyanate: A practical synthesis of unsymmetrical ureas. J. Am. Chem. Soc. 2012, 134, 11132–11135. [Google Scholar] [CrossRef] [PubMed]
- Le, H.V.; Ganem, B. Trifluoroacetic anhydride-catalyzed oxidation of isonitriles by DMSO: A rapid, convenient synthesis of isocyanates. Org. Lett. 2011, 13, 2584–2585. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; El-Hiti, G.A.; Alshammari, M.B. Directed lithiation of N′-[2-(4-methoxyphenyl)ethyl]-N,N-dimethylurea and tert-butyl [2-(4-methoxyphenyl)ethyl]carbamate. Synthesis 2014, 46, 394–402. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Alshammari, M.B.; Fekri, A. Control of site of lithiation of 3-(aminomethyl)pyridine derivatives. Synthesis 2013, 45, 3426–3434. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Alshammari, M.B. Lithiation and substitution of N′-(ω-phenylalkyl)-N,N-dimethylureas. Synthesis 2012, 44, 2013–2022. [Google Scholar] [CrossRef]
- Smith, K.; El-Hiti, G.A.; Alshammari, M.B. Variation in the site of lithiation of 2-(2-methylphenyl)ethanamine derivatives. J. Org. Chem. 2012, 77, 11210–11215. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; El-Hiti, G.A.; Hegazy, A.S. Lateral lithiation of N′-(2-methylbenzyl)-N,N-dimethylurea and N-(2-methylbenzyl)pivalamide: Synthesis of tetrahydroisoquinolines. Synthesis 2010, 1371–1380. [Google Scholar] [CrossRef]
- Taylor, R.G.D.; Yeo, B.R.; Hallett, A.J.; Kariuki, B.M.; Pope, S.J.A. An organometallic complex revealing an unexpected, reversible, temperature induced SC-SC transformation. CrystEngComm 2014, 16, 4641–4652. [Google Scholar] [CrossRef]
- Khoj, M.A.; Hughes, C.E.; Harris, K.D.M.; Kariuki, B.M. Polymorphism in a trans-cinnamic acid derivative exhibiting two distinct β-type phases: Structural properties, [2 + 2] photodimerization reactions, and polymorphic phase transition behavior. Cryst. Growth Des. 2013, 13, 4110–4117. [Google Scholar] [CrossRef]
- Yates, J.L.R.; Sparkes, H.A. 4-Bromo-trans-cinnamic acid: Structural characterisation and crystallographic investigation into the solid state [2 + 2] cycloaddition reaction and temperature induced phase transition. CrystEngComm 2013, 15, 3547–3553. [Google Scholar] [CrossRef]
- Palmer, B.A.; Kariuki, B.M.; Morte-Rodenas, A.; Harris, K.D.M. Structural rationalization of the phase transition behavior in a solid organic inclusion compound: bromocyclohexane/thiourea. Cryst. Growth Des. 2012, 12, 577–582. [Google Scholar] [CrossRef]
- Pete, U.D.; Dikundwar, A.G.; Sharma, V.M.; Gejji, S.P.; Bendre, R.S.; Guru Row, T.N. Partial rotation of the isopropyl group in the solid state: Single-crystal-to-single-crystal phase transformation in a carvacrol derivative. CrystEngComm 2015, 17, 7482–7485. [Google Scholar] [CrossRef]
- Rubin-Preminger, J.M.; Bernstein, J.; Harris, R.K.; Evans, I.R.; Ghi, P.Y. [R,S]-ethambutol dihydrochloride: Variable-temperature studies of a dimorphic system with very similar packing. J. Pharm. Sci. 2004, 93, 2810–2819. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, A.M.; Simonsen, O. The structures of (R,S)-2,2′-(l,2-ethanediyldiimino)bis-1-butanol dihydroehlodde at 295 and 333 K. A reversible phase transition. Acta Crystallogr. C 1989, 45, 4. [Google Scholar] [CrossRef]
- Das, D.; Engel, E.; Barbour, L.J. Reversible single-crystal to single-crystal polymorphic phase transformation of an organic crystal. Chem. Commun. (Cambridge UK) 2010, 46, 1676–1678. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Englert, U. Crystal-to-crystal transformation from a chain polymer to a two-dimensional network at low temperatures. Angew. Chem. Int. Ed. 2005, 44, 2281–2283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.-P.; Lin, Y.-Y.; Zhang, W.-X.; Chen, X.-M. Temperature- or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer. J. Am. Chem. Soc. 2005, 127, 14162–14163. [Google Scholar] [CrossRef] [PubMed]
- Dunitz, J.D. Phase transitions in molecular crystals from a chemical viewpoint. Pure Appl. Chem. 1991, 63, 177–185. [Google Scholar] [CrossRef]
- Meijer, M.D.; Gebbink, R.J.M.K.; van Koten, G. Solid-gas interactions between small gaseous molecules and transition metals in the solid state. Toward sensor applications. Perspect. Supramol. Chem. 2003, 7, 375–386. [Google Scholar]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]


| Identification Code | 1HT | 1LT | 2 |
|---|---|---|---|
| Empirical formula | C12H17BrN2O | C12H17BrN2O | C12H17BrN2O |
| Formula weight | 285.18 | 285.18 | 285.18 |
| Temperature (K) | 296(2) | 200(2) | 140(2) |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| Crystal system | Monoclinic | Monoclinic | Triclinic |
| Space group | P21/c | P21/c | P |
| a (Å) | 12.117(2) | 11.8696(12) | 11.8916 |
| b (Å) | 10.0335(10) | 10.0171(7) | 9.9293(6) |
| c (Å) | 12.816(2) | 12.7306(12) | 12.4631(7) |
| α (°) | 90 | 90 | 92.720(5) |
| β (°) | 117.82(2) | 117.199(13) | 116.190(5) |
| γ (°) | 90 | 90 | 81.000(5) |
| Volume (Å3) | 1377.9(5) | 1346.3(3) | 1303.86(14) |
| Z | 4 | 4 | 4 |
| σcal (Mg/m3) | 1.375 | 1.407 | 1.453 |
| μ (mm−1) | 2.967 | 3.037 | 3.135 |
| F(000) | 584 | 584 | 584 |
| Crystal size (mm3) | 0.272 × 0.177 × 0.122 | 0.309 × 0.170 × 0.106 | 0.301 × 0.209 × 0.099 |
| Reflections collected | 4752 | 7310 | 11649 |
| Independent reflections | 2718 | 3229 | 11030 |
| R(int) | 0.0622 | 0.0284 | 0.0751 |
| Data/restraints/parameters | 2718/109/166 | 3229/110/170 | 11,649/0/297 |
| Goodness-of-fit on F2 | 0.813 | 1.036 | 0.913 |
| R1[I > 2σ(I)] | 0.0614 | 0.0673 | 0.0446 |
| wR2 | 0.1619 | 0.1644 | 0.1029 |
| R1 (all data) | 0.1744 | 0.1117 | 0.0850 |
| wR2 | 0.1827 | 0.1961 | 0.1081 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kariuki, B.M.; El-Hiti, G.A. A Reversible Single-Crystal to Single-Crystal Thermal Phase Transformation of 3-(2-Bromo-4-(1-methylethyl)phenyl)-1,1-dimethyl urea. Crystals 2017, 7, 75. https://doi.org/10.3390/cryst7030075
Kariuki BM, El-Hiti GA. A Reversible Single-Crystal to Single-Crystal Thermal Phase Transformation of 3-(2-Bromo-4-(1-methylethyl)phenyl)-1,1-dimethyl urea. Crystals. 2017; 7(3):75. https://doi.org/10.3390/cryst7030075
Chicago/Turabian StyleKariuki, Benson M., and Gamal A. El-Hiti. 2017. "A Reversible Single-Crystal to Single-Crystal Thermal Phase Transformation of 3-(2-Bromo-4-(1-methylethyl)phenyl)-1,1-dimethyl urea" Crystals 7, no. 3: 75. https://doi.org/10.3390/cryst7030075
APA StyleKariuki, B. M., & El-Hiti, G. A. (2017). A Reversible Single-Crystal to Single-Crystal Thermal Phase Transformation of 3-(2-Bromo-4-(1-methylethyl)phenyl)-1,1-dimethyl urea. Crystals, 7(3), 75. https://doi.org/10.3390/cryst7030075