Borate-Based Ultraviolet and Deep-Ultraviolet Nonlinear Optical Crystals
Abstract
:1. Introduction
2. Results and Discussion
2.1. Borate Crystals in Which the B-O Groups Are (BO3)3− Groups Only
2.1.1. KBBF-Type Crystals
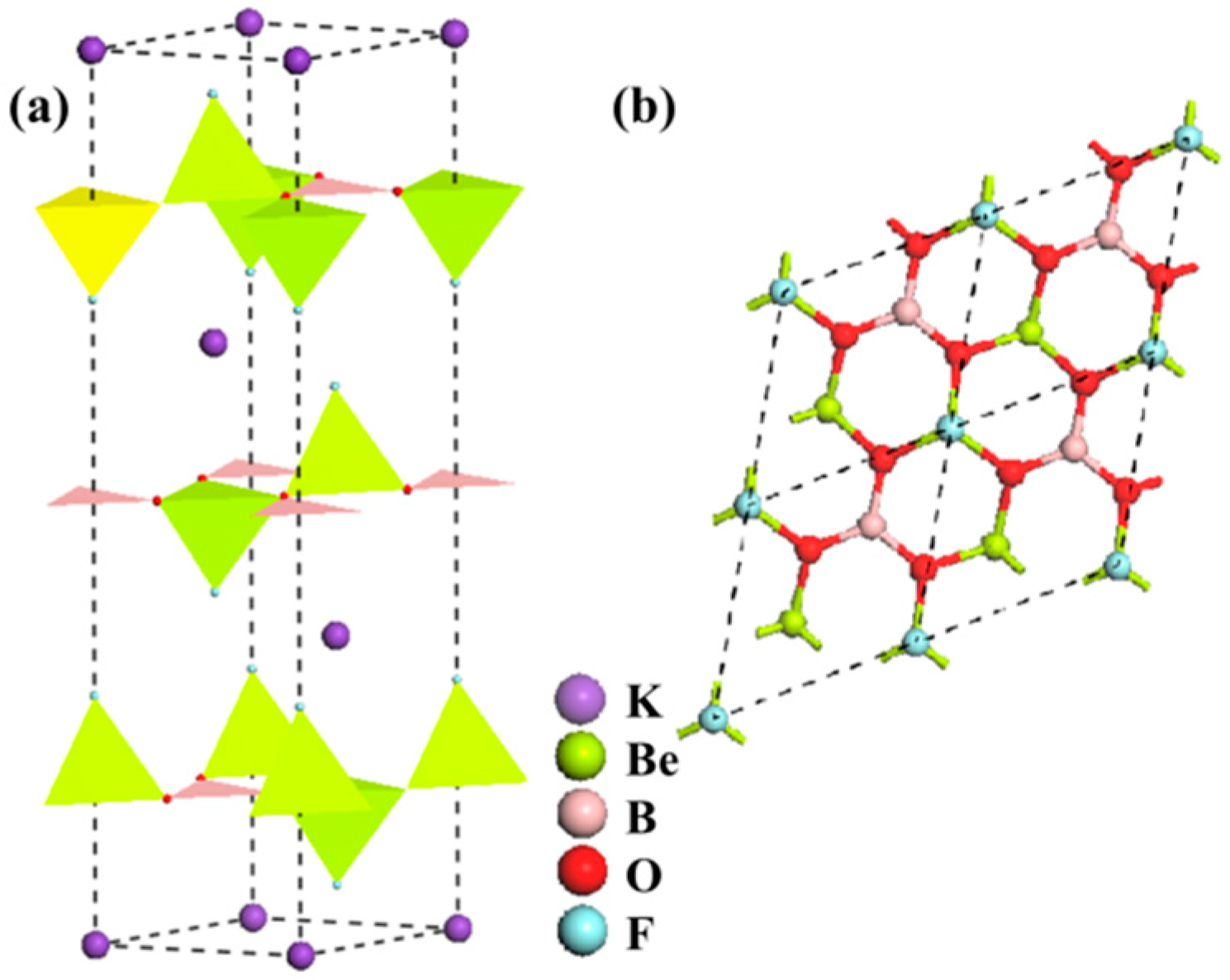
BaBe2BO3F3 (BBBF):
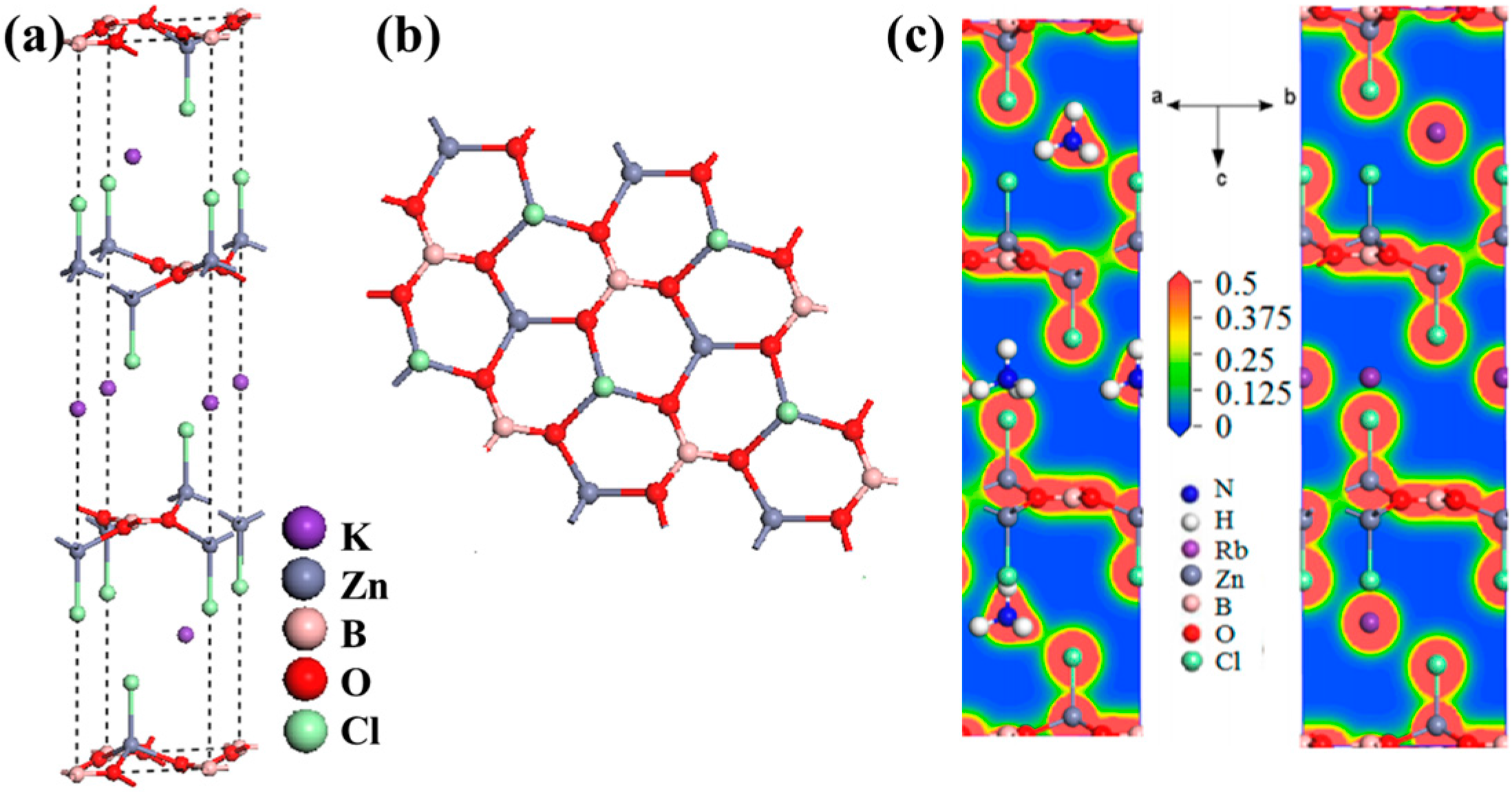
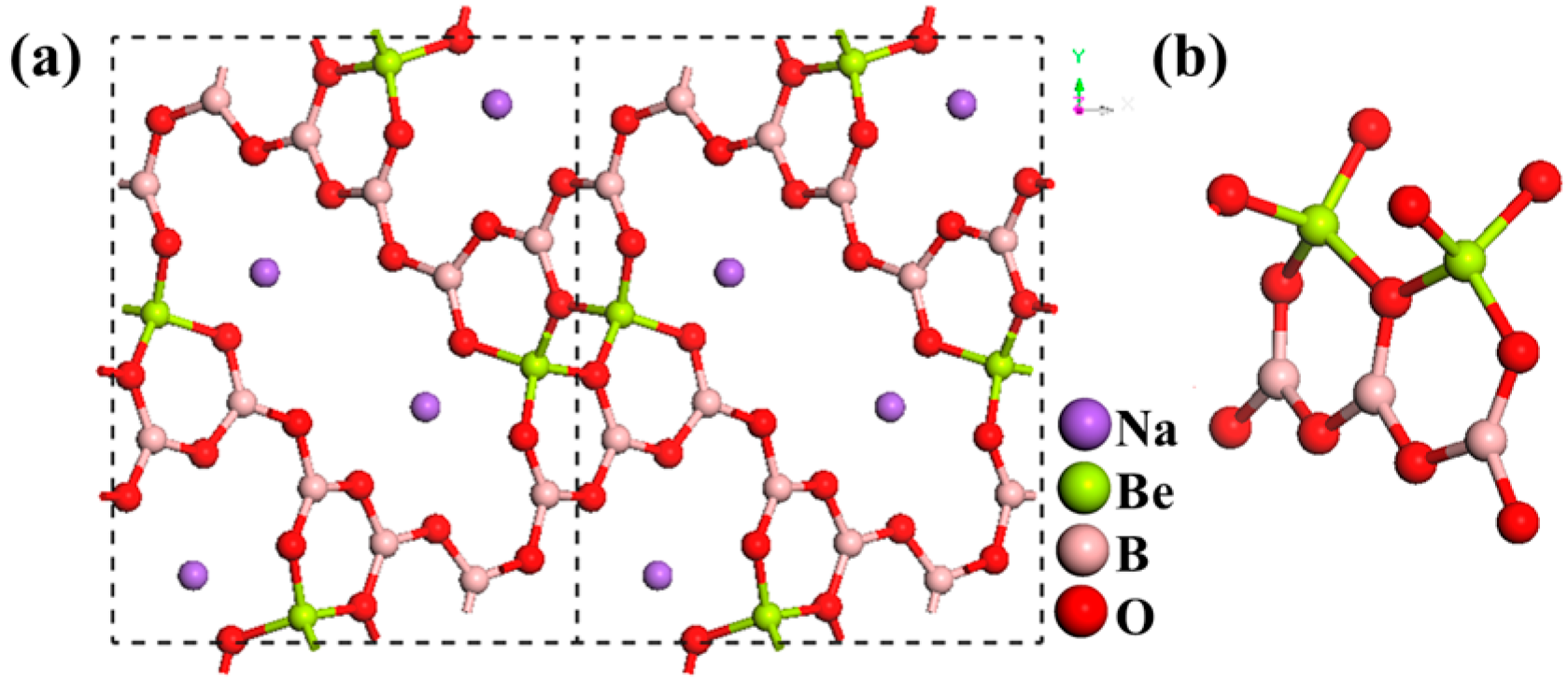
AZn2BO3X2 (A = K, Rb, NH4; X = Cl, Br)
2.1.2. SBBO-Type Crystals
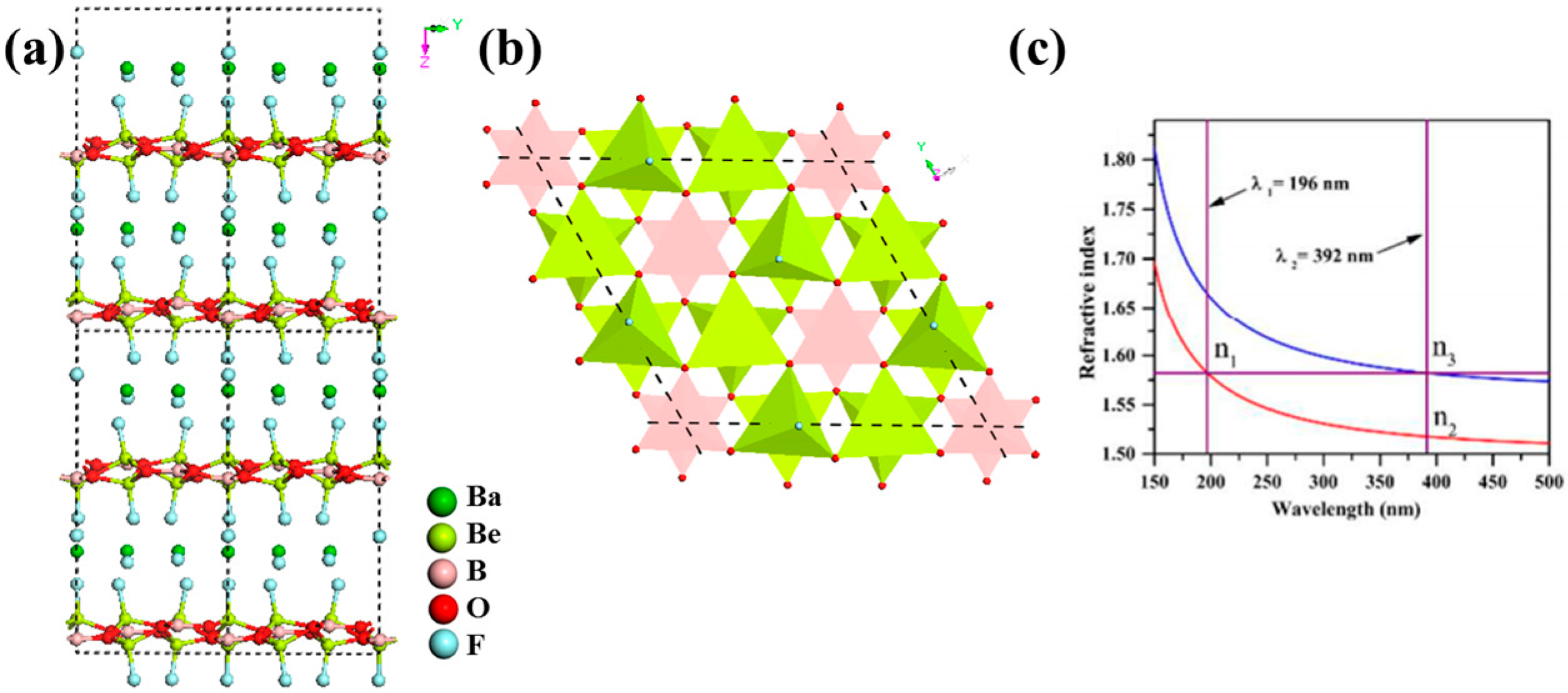
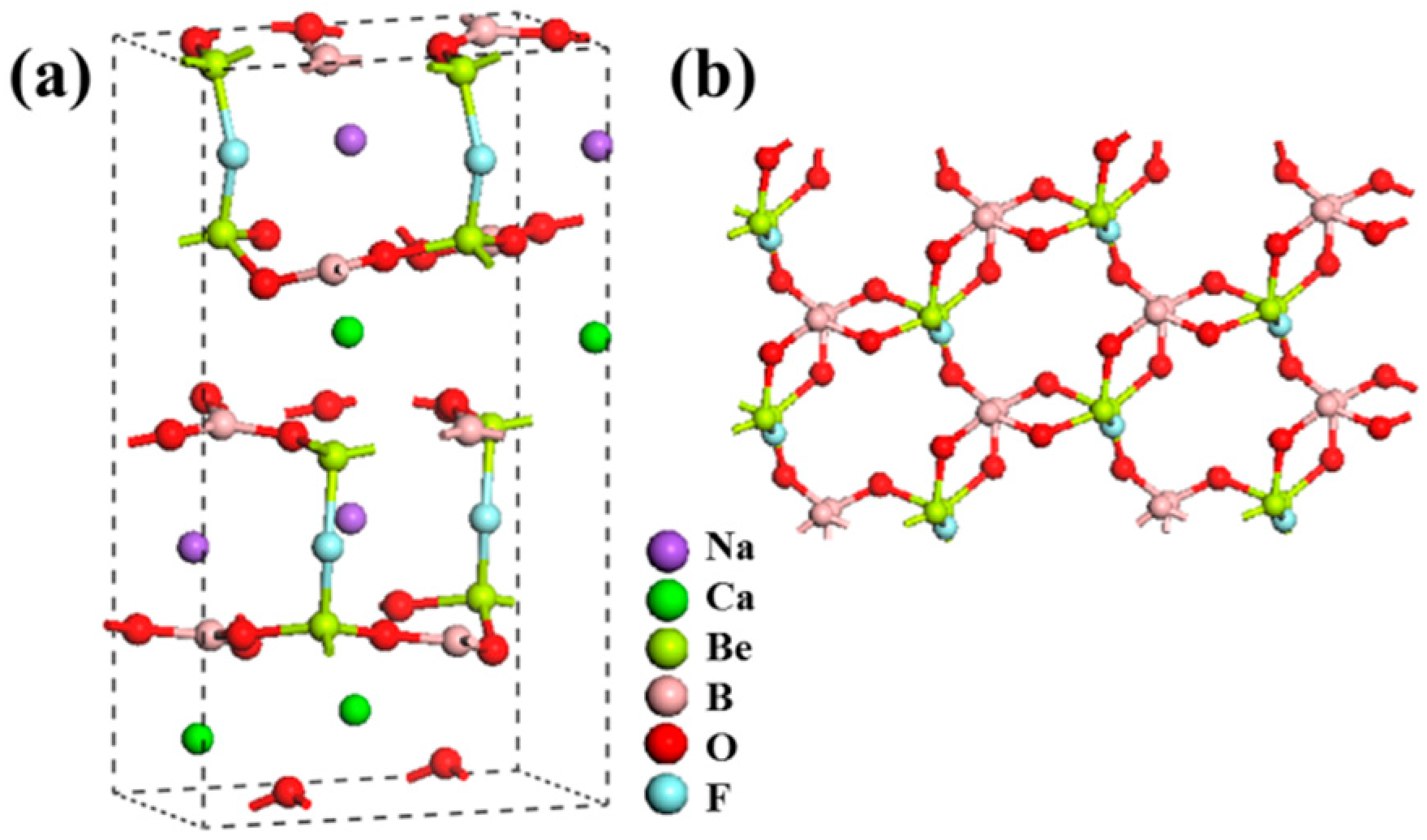
NaCaBe2B2O6F
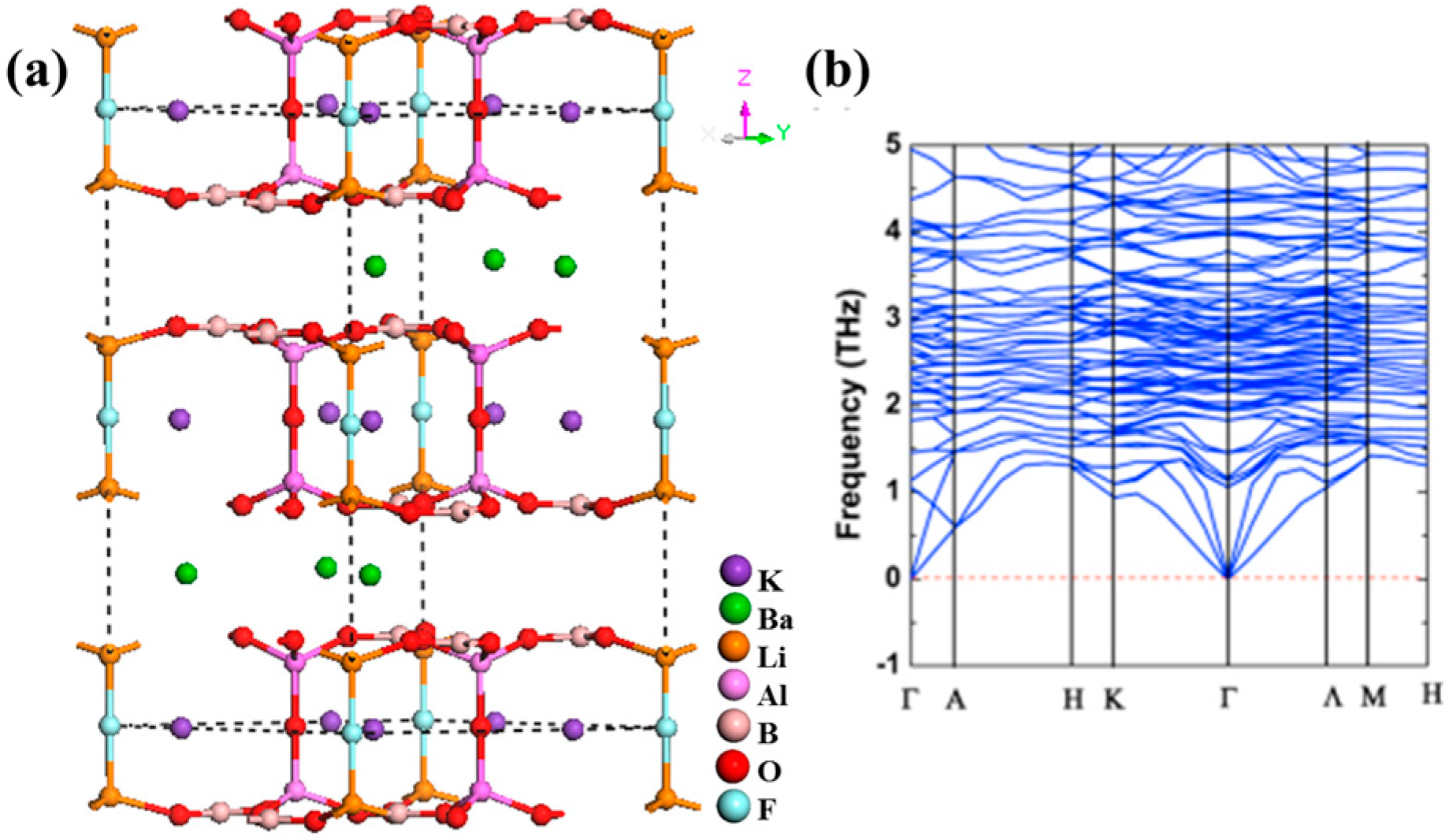
K3Ba3Li2Al4B6O20F
Rb3Al3B3O10F
2.1.3. Three-Dimensional (3D) Network Crystals
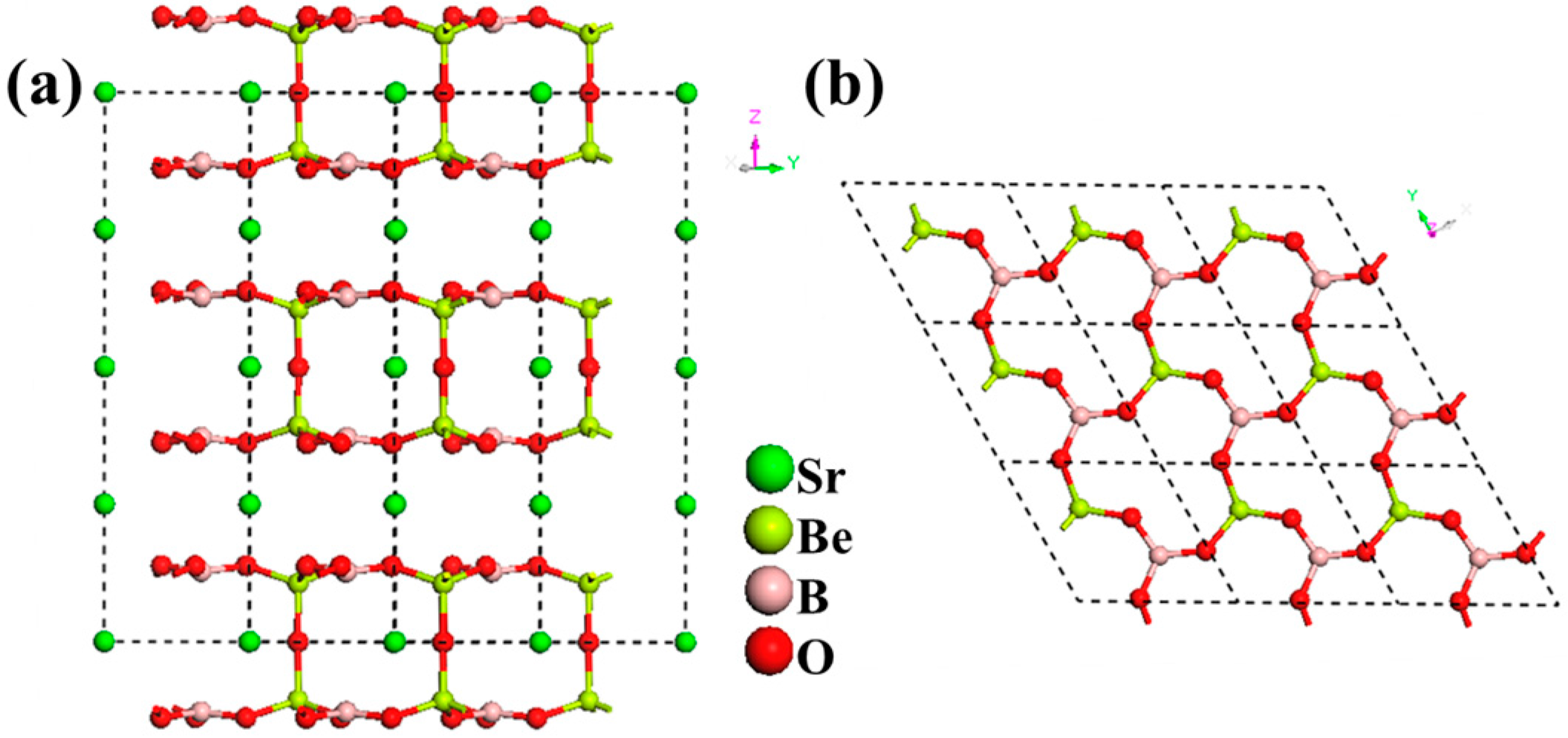
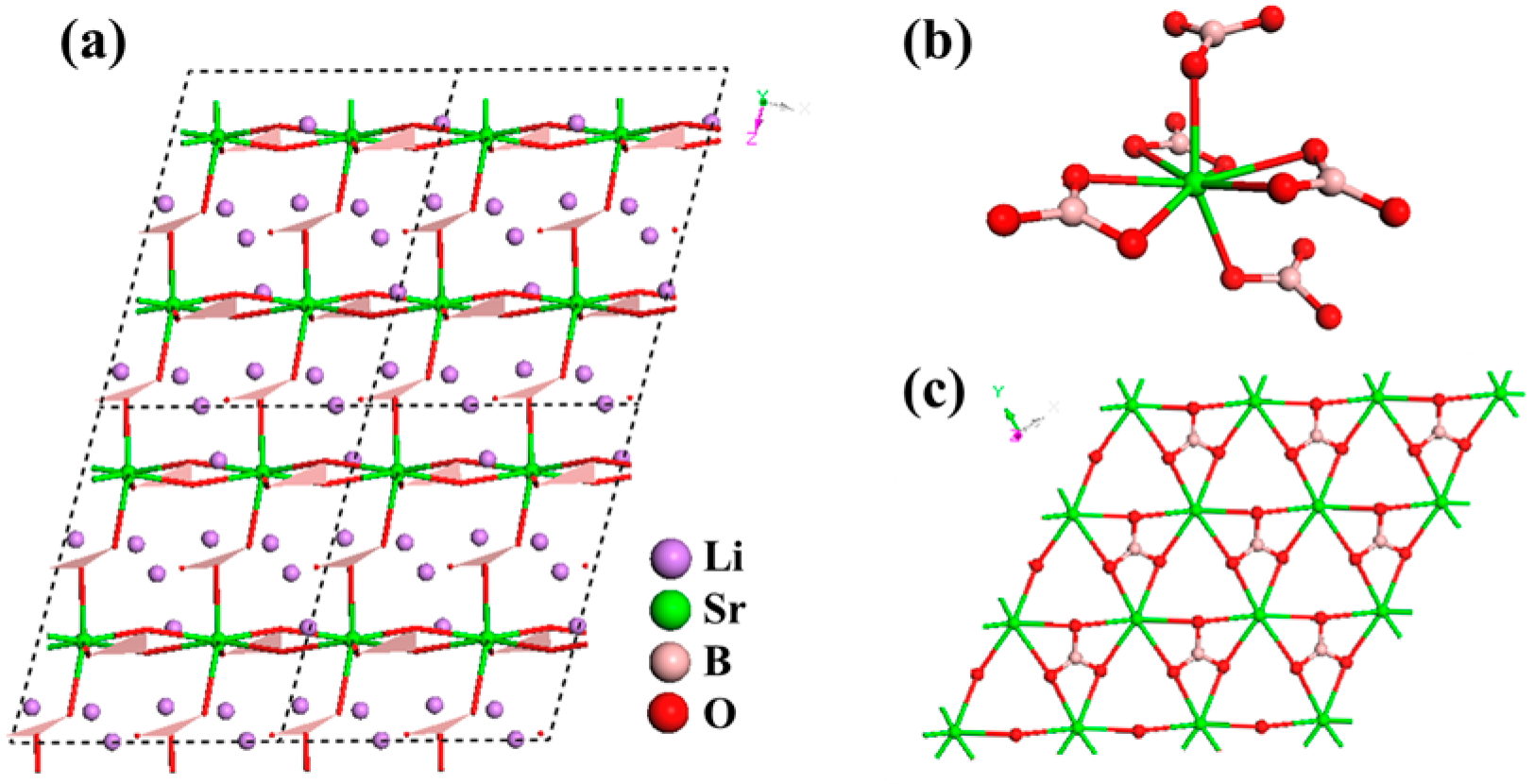
LiSr(BO3)2
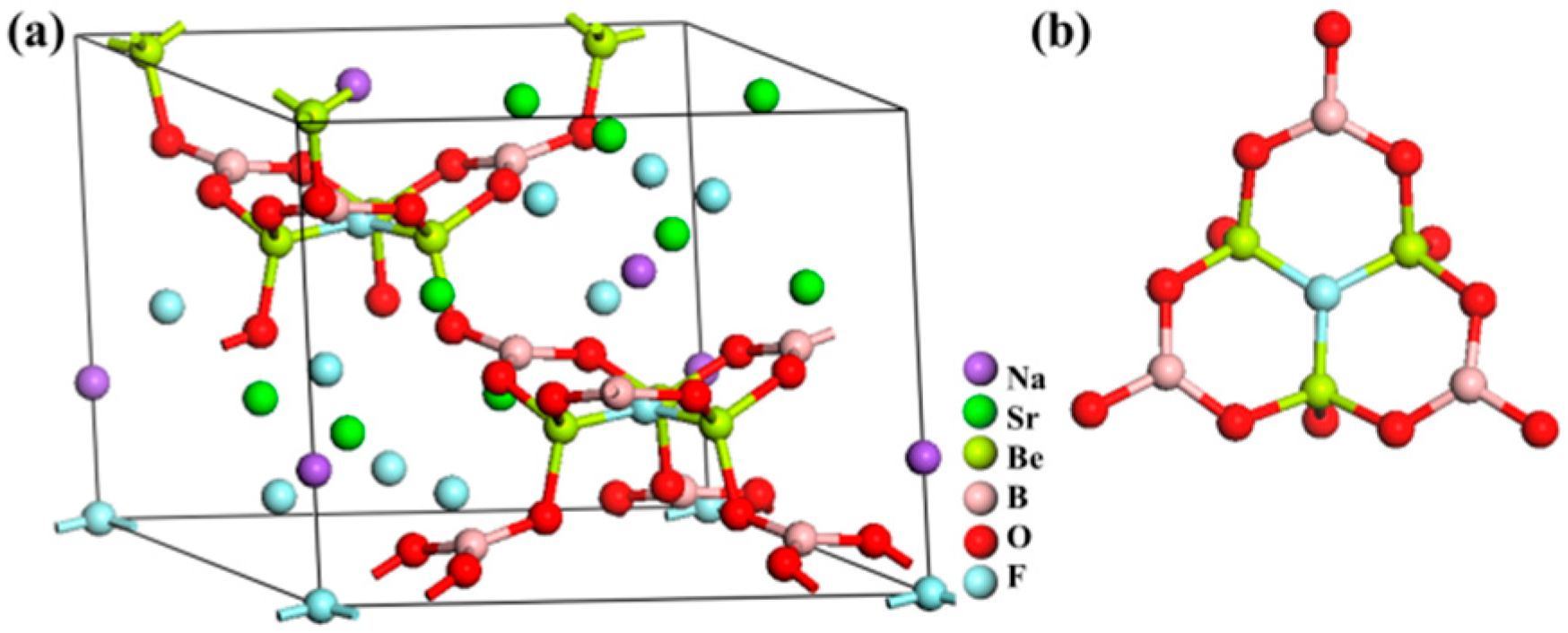
NaSr3Be3B3O9F4
NaBeB3O6
Na2Be4B4O11 and LiNa5Be12B12O33
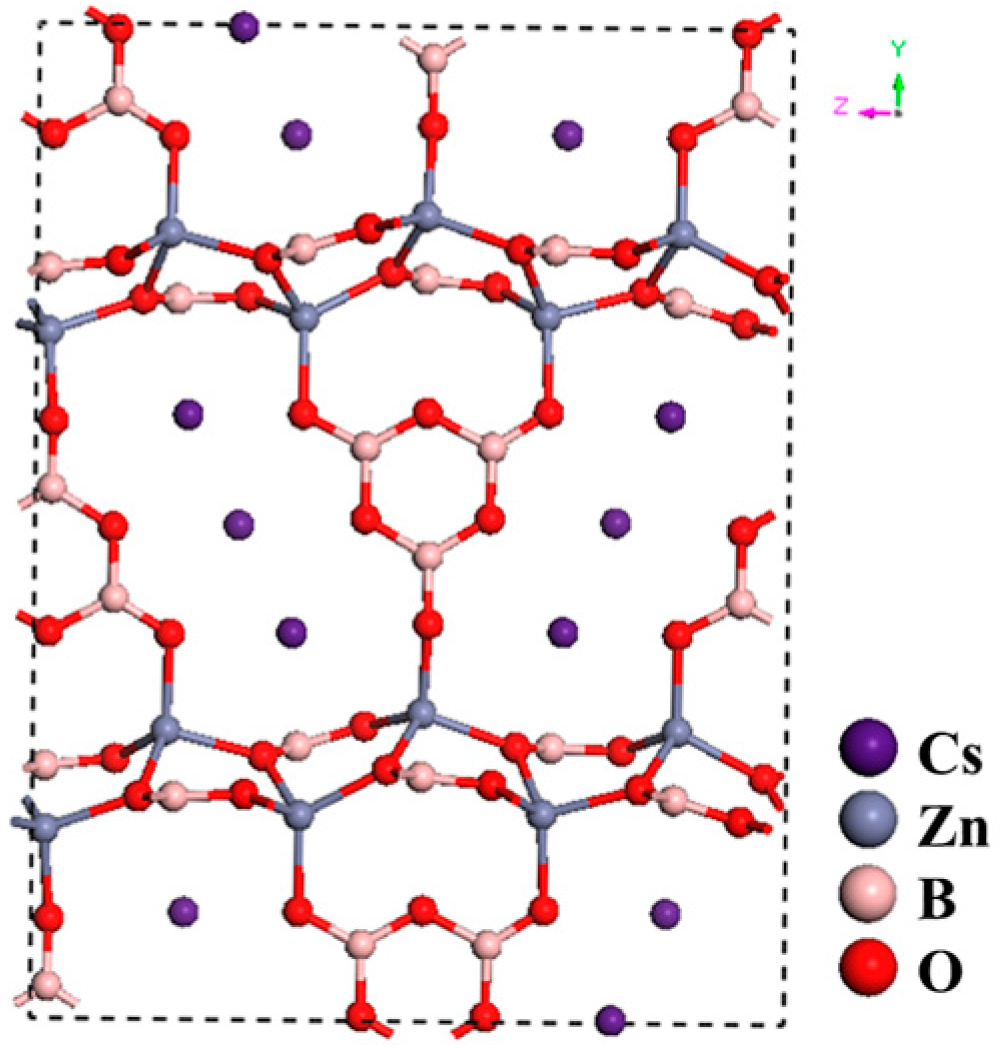
CsZn2B3O7
2.2. Borate Crystals in Which the B-O Groups Are (BO4)5− Groups Only
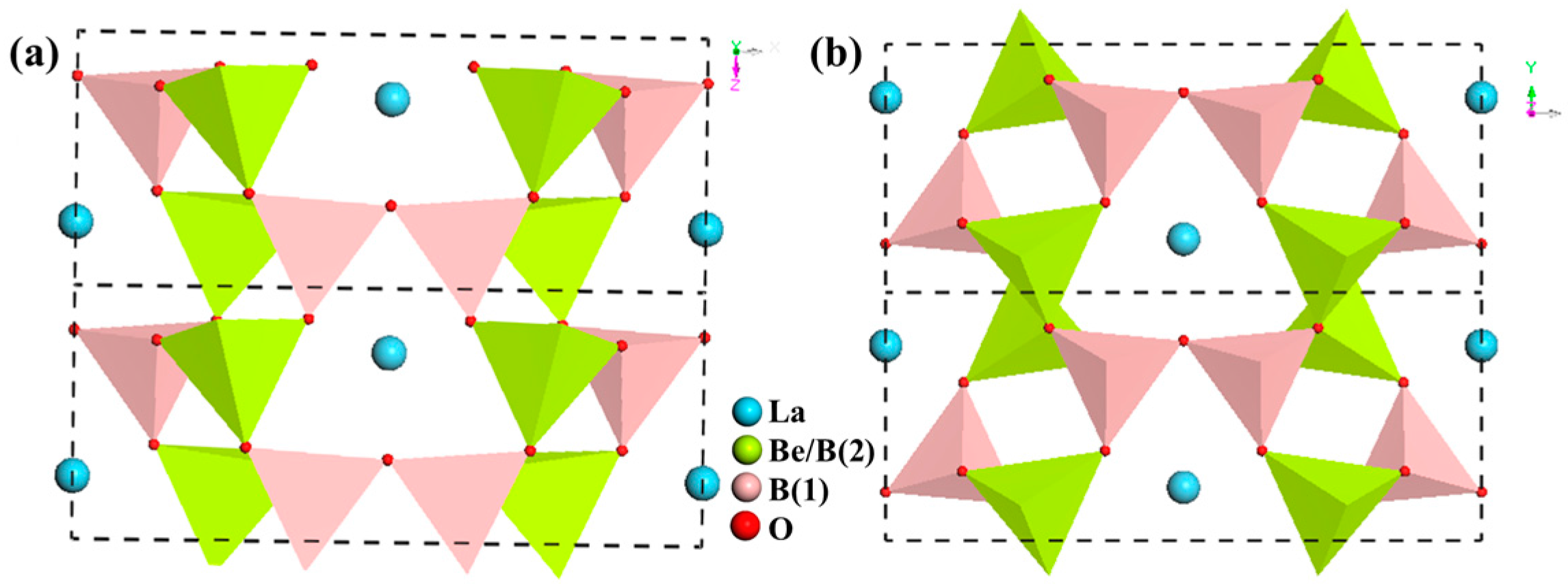
LaBeB3O7
2.3. Borate Crystals Containing the B-O Combinational Groups
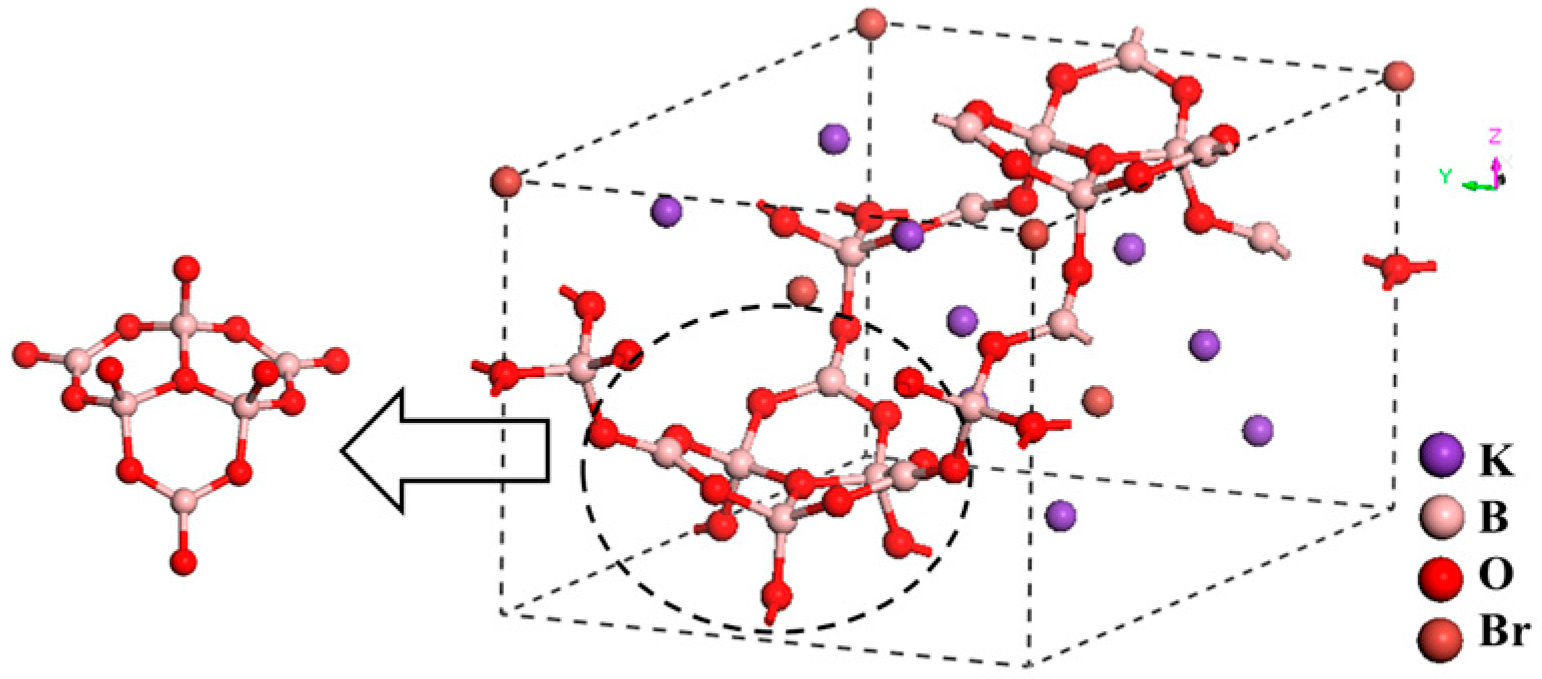
K3B6O10Br and K3B6O10Cl with (B6O13)8−
Ba3B6O11F2 and Sr3B6O11F2 with (B6O14)10−
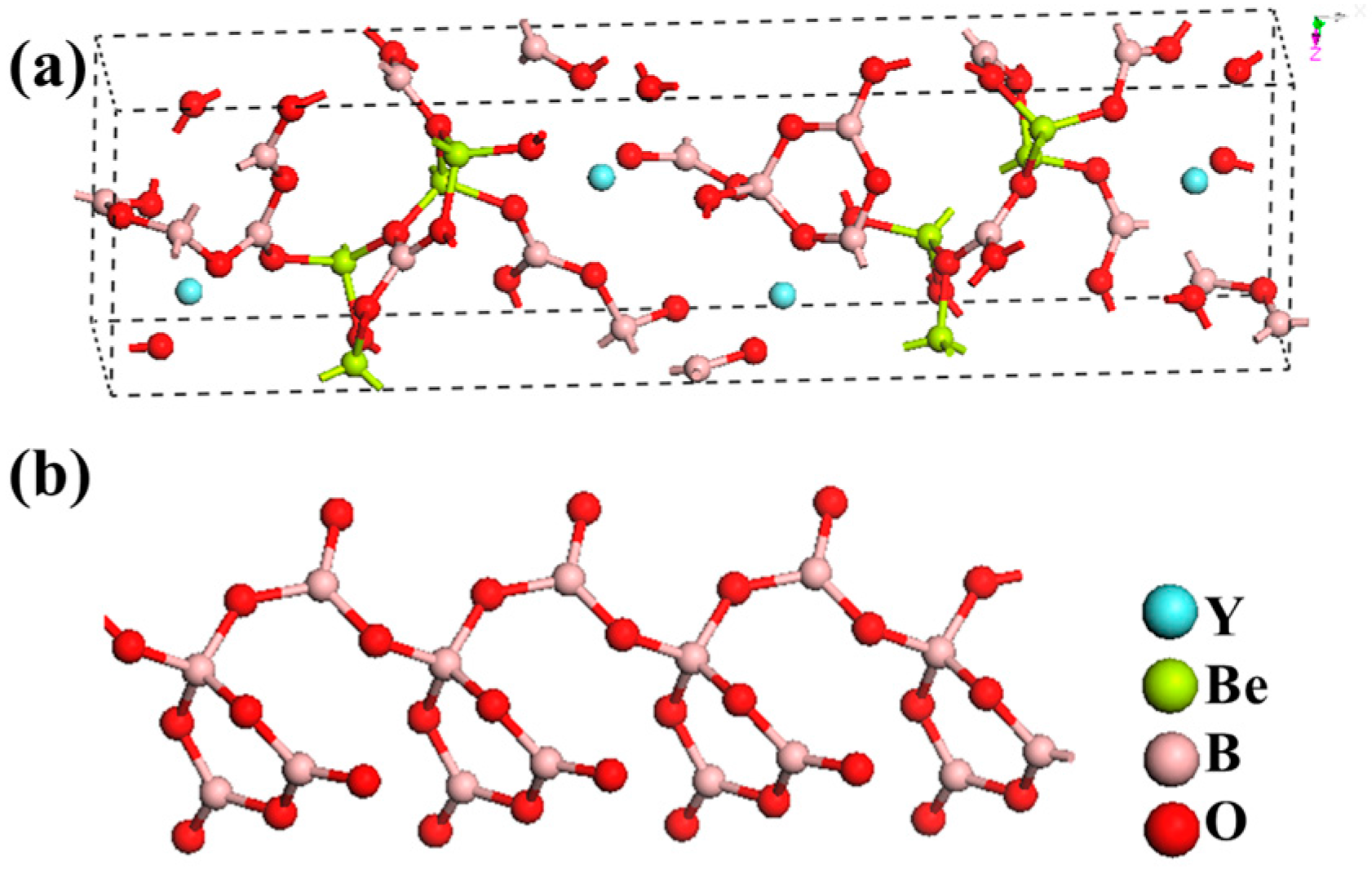
ReBe2B5O11 (Re = Y, Gd) with (B4O8)4− chains
2.4. The Borates Containing B-O Groups and Other NLO Active Groups
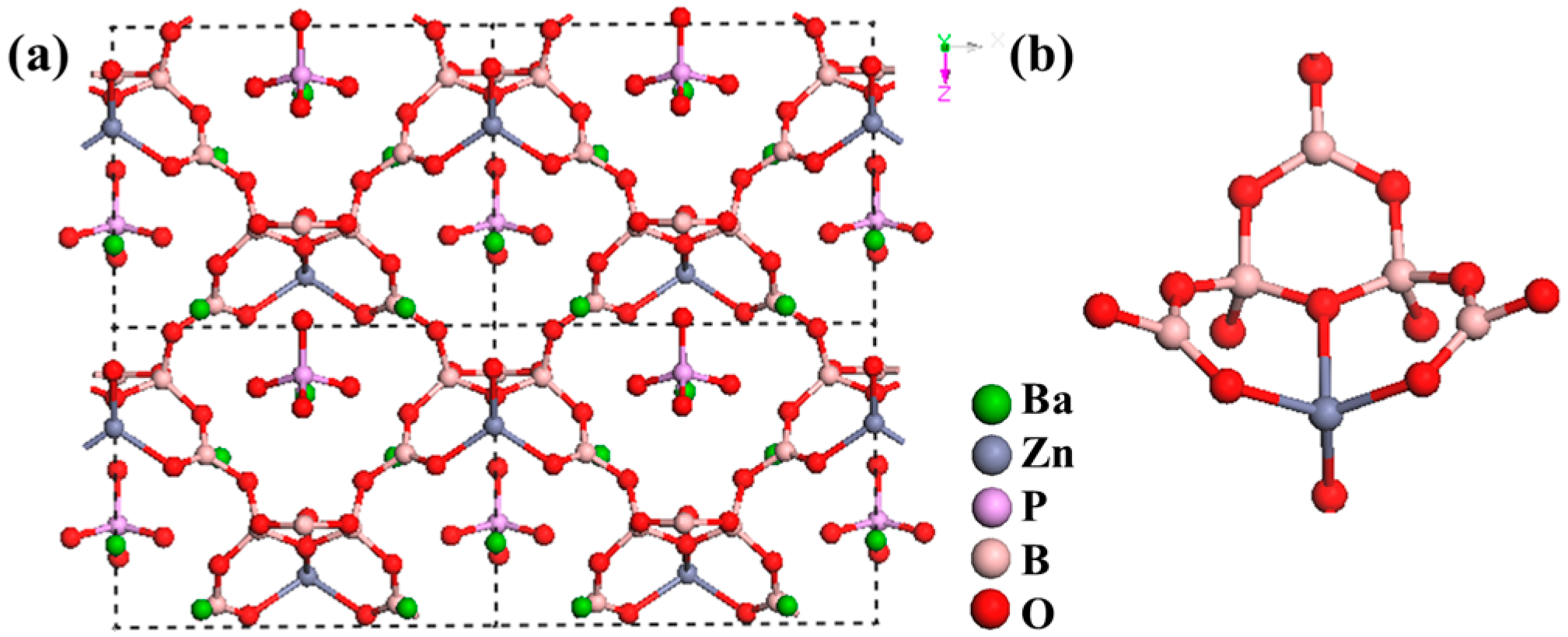
Ba3(ZnB5O10)PO4
Cs2B4SiO9
3. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Franken, P.A.; Weinreich, G.; Peters, C.W.; Hill, A.E. Generation of optical harmonics. Phys. Rev. Lett. 1961, 7, 118. [Google Scholar] [CrossRef]
- Xu, Y.M.; Huang, Y.B.; Cui, X.Y.; Razzoli, E.; Radovic, M.; Shi, M.; Chen, G.F.; Zheng, P.; Wang, N.L.; Zhang, C.L.; et al. Observation of a ubiquitous three-dimensional superconducting gap function in optimally doped Ba0.6K0.4Fe2As2. Nat. Phys. 2011, 7, 198–202. [Google Scholar] [CrossRef]
- Neil, S. Ultraviolet lasers. Nat. Photon. 2007, 1, 83–85. [Google Scholar]
- Burland, D.M.; Miller, R.D.; Walsh, C.A. Second-Order Nonlinearity in Poled-Polymer Systems. Chem. Rev. 1994, 94, 31–75. [Google Scholar] [CrossRef]
- Chiaverini, J.; Leibfried, D.; Schaetz, T.; Barrett, M.D.; Blakestad, R.B.; Britton, J.; Itano, W.M.; Jost, J.D.; Knill, E.; Langer, C.; et al. Nonlinear optics in the extreme ultraviolet. Nature 2004, 432, 605–608. [Google Scholar]
- Kiss, T.; Kanetaka, F.; Yokoya, T.; Shimojima, T.; Kanai, K.; Shin, S.; Onuki, Y.; Togashi, T.; Zhang, C.; Chen, C.T.; et al. Photoemission spectroscopic evidence of gap anisotropy in an f-electron superconductor. Phys. Rev. Lett. 2005, 94, 057001. [Google Scholar] [CrossRef] [PubMed]
- Yatsenko, L.P.; Shore, B.W.; Halfmann, T.; Bergmann, K. Source of metastable H(2s) atoms using the Stark chirped rapid-adiabatic-passage technique. Phys. Rev. A 1999, 94, R4237. [Google Scholar] [CrossRef]
- Kiss, T.; Shimojima, T.; Kanaia, T.; Yokoya, T.; Shin, S.; Onuki, Y.; Togashi, T.; Zhang, C.; Chen, C.T. Ultrahigh-resolution photoemission spectroscopy of superconductors using a VUV laser. J. Electron Spectrosc. 2005, 144, 953–956. [Google Scholar] [CrossRef]
- Meng, J.; Liu, G.; Zhang, W.; Zhao, L.; Liu, H.; Jia, X.; Mu, D.; Liu, S.; Dong, X.; Zhang, J.; et al. Coexistence of Fermi arcs and Fermi pockets in a high-T(c) copper oxide superconductor. Nature 2009, 462, 335–338. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, G.; Hu, Y.; Nielsen, S.B.; Spiro, T.G. Tunable kHz Deep Ultraviolet(193–210 nm) laser for raman applications. Appl. Spectrosc. 2005, 59, 776–781. [Google Scholar] [CrossRef] [PubMed]
- Becker, P. Borate Materials in Nonlinear Optics. Adv. Mater. 1998, 10, 979–992. [Google Scholar] [CrossRef]
- Chen, C.T.; Ye, N.; Lin, J.; Jiang, J.; Zeng, W.R.; Wu, B.C. Computer-assisted search for nonlinear optical crystals. Adv. Mater. 1999, 11, 1071–1078. [Google Scholar] [CrossRef]
- Kurimura, S.; Harada, M.; Muramatsu, K.-i.; Ueda, M.; Adachi, M.; Yamada, T.; Ueno, T. Quartz revisits nonlinear optics: Twinned crystal for quasi-phase matching[Invited]. Opt. Mater. Express 2011, 1, 1367–1375. [Google Scholar] [CrossRef]
- Jiang, X.; Luo, S.; Kang, L.; Gong, P.; Huang, H.; Wang, S.; Lin, Z.; Chen, C. First-Principles Evaluation of the Alkali and/or Alkaline Earth Beryllium Borates in Deep Ultraviolet Nonlinear Optical Applications. ACS Photonics 2015, 2, 1183–1191. [Google Scholar] [CrossRef]
- Chen, C.T.; Sasaki, T.; Li, R.; Wu, Y.; Lin, Z.; Mori, Y.; Hu, Z.; Wang, J.; Aka, G.; Yoshimura, M.; et al. Nonlinear Optical Borate Crystals Principals and Applications; Wiley-VCH: New York, NY, USA, 2012. [Google Scholar]
- Chen, C.T.; Wu, B.C.; Jiang, A.D.; You, G.M. A new-type ultraviolet SHG crystal β-BaB2O4. Sci. Sin. 1985, 28, 235–243. [Google Scholar]
- Eimerl, D.; Davis, L.; Velsko, S.; Graham, E.K.; Zalkin, A. Optical, mechanical and thermal-properties of barium borate. J. Appl. Phys. 1987, 62, 1968–1983. [Google Scholar] [CrossRef]
- Chen, C.T.; Wu, Y.C.; Jiang, A.D.; Wu, B.C.; You, G.M.; Li, R.K.; Lin, S.J. New nonlinear-optical crystal-LiB3O5. J. Opt. Soc. Am. B Opt. Phys. 1989, 6, 616–621. [Google Scholar] [CrossRef]
- Chen, C.; Xu, Z.; Deng, D.; Zhang, J.; Wong, G.K. L.; Wu, B.; Ye, N.; Tang, D. The vacuum ultraviolet phase-matching characteristics of nonlinear optical KBe2BO3F2 crystal. Appl. Phys. Lett. 1996, 68, 2930. [Google Scholar] [CrossRef]
- Mei, L.; Huang, X.; Wang, Y.; Wu, Q.; Wu, B.; Chen, C. Crystal-structure of KBe2BO3F2. ZKri 1995, 210, 93–95. [Google Scholar] [CrossRef]
- Wu, B.C.; Tang, D.Y.; Ye, N.; Chen, C.T. Linear and nonlinear optical properties of the KBe2BO3F2 (KBBF) crystal. Opt. Mater. 1996, 5, 105–109. [Google Scholar] [CrossRef]
- Chen, C. Recent advances in deep and vacuum-UV harmonic generation with KBBF crystal. Opt. Mater. 2004, 26, 425–429. [Google Scholar] [CrossRef]
- Chen, C.T.; Luo, S.; Wang, X.; Wang, G.L.; Wen, X.; Wu, H.; Zhang, X.; Xu, Z.Y. Deep UV nonlinear optical crystal: RbBe2BO3F2. J. Opt. Soc. Am. B 2009, 26, 1519–1525. [Google Scholar] [CrossRef]
- Huang, H.; Chen, C.; Wang, X.; Zhu, Y.; Wang, G.; Zhang, X.; Wang, L.; Yao, J. Ultraviolet nonlinear optical crystal: CsBe2BO3F2. J. Opt. Soc. Am. B 2011, 28, 2186–2190. [Google Scholar] [CrossRef]
- Kang, L.; Luo, S.; Huang, H.; Zheng, T.; Lin, Z.S.; Chen, C.T. Ab initio studies on the optical effects in the deep ultraviolet nonlinear optical crystals of the KBe2BO3F2 family. J. Phys. Condens. Matter. 2012, 24, 335503. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.T.; Wang, G.L.; Wang, X.Y.; Xu, Z.Y. Deep-UV nonlinear optical crystal KBe2BO3F2-discovery, growth, optical properties and applications. Appl. Phys. B 2009, 97, 9–25. [Google Scholar] [CrossRef]
- Togashi, T.; Kanai, T.; Sekikawa, T.; Watanabe, S.; Chen, C.T.; Zhang, C.; Xu, Z.; Wang, J. Generation of vacuum-ultraviolet light by an optically contacted, prism-coupled KBe2BO3F2 crystal. Opt. Lett. 2003, 28, 254–256. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Wang, X.; Zhou, Y.; Chen, Y.; Li, C.; Zhu, Y.; Xu, Z.; Chen, C. 12.95 mW sixth harmonic generation with KBe2BO3F2 crystal. Appl. Phys. B 2008, 91, 95–97. [Google Scholar] [CrossRef]
- Chen, C.T.; Lu, J.H.; Togashi, T.; Suganuma, T.; Sekikawa, T.; Watanabe, S.; Xu, Z.; Wang, J. Second-harmonic generation from a KBe2BO3F2 crystal in the deep ultraviolet. Opt. Lett. 2002, 27, 637–639. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Jiang, X.; Liu, L.; Xia, M.; Fang, Z.; Wang, X.; Lin, Z.; Chen, C. BaBe2BO3F3: A KBBF-Type Deep-Ultraviolet Nonlinear Optical Material with Reinforced [Be2BO3F2]∞ Layers and Short Phase-Matching Wavelength. Chem. Mater. 2016, 28, 8871–8875. [Google Scholar] [CrossRef]
- Huang, Q.; Liu, L.; Wang, X.; Li, R.; Chen, C. Beryllium-Free KBBF Family of Nonlinear-Optical Crystals: AZn2BO3X2 (A = Na, K, Rb; X = Cl, Br). Inorg. Chem. 2016, 55, 12496–12499. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gong, P.; Lin, Z.; Ye, N. AZn2BO3X2(A = K, Rb, NH4; X = Cl, Br): New Members of KBBF Family Exhibiting Large SHG Response and the Enhancement of Layer Interaction by Modified Structures. Chem. Mater. 2016, 28, 9122–9131. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Y.B.; Wu, B.C.; Wu, K.C.; Zeng, W.L.; Yu, L.H. Design and synthesis of an ultraviolet-transparent nonlinear-optical crystal Sr2Be2B2O7. Nature 1995, 373, 322–324. [Google Scholar] [CrossRef]
- Chen, C.T.; Wang, Y.B.; Xia, Y.N.; Wu, B.C.; Tang, D.Y.; Wu, K.C.; Zeng, W.R.; Yu, L.H.; Mei, L.F. New development of nonlinear optical crystals for the ultraviolet region with molecular engineering approach. J. Appl. Phys. 1995, 77, 2268–2272. [Google Scholar] [CrossRef]
- Meng, X.Y.; Wen, X.H.; Liu, G.L. Structure and Stacking Faults in Sr2Be2B2O7 Crystal. J. Korean Phys. Soc. 2008, 52, 1277. [Google Scholar] [CrossRef]
- Chen, C.; Lin, Z.; Wang, Z. The development of new borate-based UV nonlinear optical crystals. Appl. Phys. B 2005, 80, 1–25. [Google Scholar] [CrossRef]
- Huang, H.; Yao, J.; Lin, Z.; Wang, X.; He, R.; Yao, W.; Zhai, N.; Chen, C. Molecular Engineering Design to Resolve the Layering Habit and Polymorphism Problems in Deep UV NLO Crystals: New Structures in MM’Be2B2O6F (M = Na, M’ = Ca; M=K, M’ = Ca, Sr). Chem. Mater. 2011, 23, 5457–5463. [Google Scholar] [CrossRef]
- Zhao, S.; Kang, L.; Shen, Y.; Wang, X.; Asghar, M.A.; Lin, Z.; Xu, Y.; Zeng, S.; Hong, M.; Luo, J. Designing a Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material without a Structural Instability Problem. J. Am. Chem. Soc. 2016, 138, 2961–2964. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gong, P.; Luo, S.; Liu, S.; Li, L.; Asghar, M.A.; Khan, T.; Hong, M.; Lin, Z.; Luo, J. Beryllium-Free Rb3Al3B3O10F with Reinforced Inter layer Bonding as a Deep-Ultraviolet Nonlinear Optical Crystal. J. Am. Chem. Soc. 2015, 137, 2207–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Gong, P.; Bai, L.; Xu, X.; Zhang, S.; Sun, Z.; Lin, Z.; Hong, M.; Chen, C.; Luo, J. Beryllium-free Li4Sr(BO3)2 for deep-ultraviolet nonlinear optical applications. Nat. Commun. 2014, 5, 4019. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yao, J.; Lin, Z.; Wang, X.; He, R.; Yao, W.; Zhai, N.; Chen, C. NaSr3Be3B3O9F4: A promising deep-ultraviolet nonlinear optical material resulting from the cooperative alignment of the [Be3B3O12F]10− anionic group. Angew. Chem. Int. Ed. 2011, 50, 9141–9044. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.C.; Ye, N.; Li, W.; Zhao, D. Alkaline Beryllium Borate NaBeB3O6 and ABe2B3O7 (A = K, Rb) as UV Nonlinear Optical Crystals. J. Am. Chem. Soc. 2010, 132, 8779–8786. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.W.; Liu, L.J.; Jin, S.F.; Yao, W.J.; Zhang, Y.H.; Chen, C.T. Deep-Ultraviolet Nonlinear Optical Materials: Na2Be4B4O11 and LiNa5Be12B12O33. J. Am. Chem. Soc. 2013, 135, 18319–18322. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Ye, N. Na2CsBe6B5O15: An Alkaline Beryllium Borate as a Deep-UV Nonlinear Optical Crystal. J. Am. Chem. Soc. 2011, 133, 11458–11461. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, H.; Pan, S.; Yang, Z.; Hou, X.; Su, X.; Jing, Q.; Poeppelmeier, K.R.; Rondinelli, J.M. Cs3Zn6B9O21: A chemically benign member of the KBBF family exhibiting the largest second harmonic generation response. J. Am. Chem. Soc. 2014, 136, 1264–1267. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Zhang, J.; Zhang, S.Q.; Sun, Z.; Lin, Z.; Wu, Y.; Hong, M.; Luo, J. A new UV nonlinear optical material CsZn2B3O7: ZnO4 tetrahedra double the efficiency of second-harmonic generation. Inorg. Chem. 2014, 53, 2521–2527. [Google Scholar] [CrossRef] [PubMed]
- Tien, T.Y.; Hummel, F.A. Studies in Lithium Oxide Systems: XI, Li2O-B2O3-P2O5. J. Am. Ceram. Soc. 1961, 44, 390–394. [Google Scholar] [CrossRef]
- Schmidt, M.; Ewald, B.; Prots, Y.; Cardoso-Gil, R.; Armbrüster, M.; Loa, I.; Zhang, L.; Huang, Y.-X.; Schwarz, U.; Kniep, R. Growth and Characterization of BPO4 Single Crystals. Z. Anorg. Allg. Chem. 2004, 630, 655–662. [Google Scholar] [CrossRef]
- Li, Z.H.; Lin, Z.H.; Wu, Y.C.; Fu, P.Z.; Wang, Z.Z.; Chen, C.T. Crystal growth, optical properties measurement, and theoretical calculation of BPO4. Chem. Mater. 2004, 16, 2906–2908. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, L.R.; Zhang, S.F.; Wang, G.L.; Zhao, S.E.; Zhu, Y.; Wu, Y.C.; Chen, C.T. Optical properties of the vacuum-ultraviolet nonlinear optical crystal-BPO4. J. Opt. Soc. Am. B Opt. Phys. 2011, 28, 2236–2239. [Google Scholar] [CrossRef]
- Oseledchik, Y.S.; Prosvirnin, A.L.; Starshenko, V.V.; Osadchuk, V.V.; Pisarevsky, A.I.; Belokrys, S.P.; Korol, A.S.; Svitanko, N.V.; Selevich, A.F.; Krikunov, S.A. Crystal growth and properties of strontium tetraborate. J. Cryst. Growth 1994, 135, 372–376. [Google Scholar] [CrossRef]
- Oseledchik, Y.S.; Prosvirnin, A.L.; Pisarevskiy, A.I.; Starshenko, V.V.; Osadchuk, V.V.; Belokrys, S.P.; Svitanko, N.V.; Korol, A.S.; Krikunov, S.A.; Selevich, A.F. New nonlinear optical crystals: Strontium and lead tetraborates. Opt. Mater. 1995, 4, 669–674. [Google Scholar] [CrossRef]
- Petrov, V.; Noack, F.; Shen, D.Z.; Pan, F.; Shen, G.Q.; Wang, X.Q.; Komatsu, R.; Alex, V. Application of the nonlinear crystal SrB4O7 for ultrafast diagnostics converting to wavelengths as short as 125 nm. Opt. Lett. 2004, 29, 373–375. [Google Scholar] [CrossRef] [PubMed]
- Zaitsev, A.I.; Aleksandrovskii, A.S.; Zamkov, A.V.; Sysoev, A.M. Nonlinear optical, piezoelectric, and acoustic properties of SrB4O7. Inorg. Mater. 2006, 42, 1360–1362. [Google Scholar] [CrossRef]
- Yan, X.; Luo, S.; Lin, Z.; Yue, Y.; Wang, X.; Liu, L.; Chen, C. LaBeB3O7: A new phase-matchable nonlinear optical crystal exclusively containing the tetrahedral XO4 (X = B and Be) anionic groups. J. Mater. Chem. C 2013, 1, 3616. [Google Scholar] [CrossRef]
- Al-Ama, A.G.; Belokoneva, E.L.; Stefanovich, S.Y.; Dimitrova, O.V.; Mochenova, N.N. Potassium bromo-borate K3B6O10Br-A new nonlinear optical material. Cryst. Rep. 2006, 51, 225–230. [Google Scholar] [CrossRef]
- Wu, H.; Pan, S.; Poeppelmeier, K.R.; Li, H.; Jia, D.; Chen, Z.; Fan, X.; Yang, Y.; Rondinelli, J.M.; Luo, H. K3B6O10Cl: A new structure analogous to perovskite with a large second harmonic generation response and deep UV absorption edge. J. Am. Chem. Soc. 2011, 133, 7786–7790. [Google Scholar] [CrossRef] [PubMed]
- Xia, M.J.; Xu, B.; Li, R.K. Growth and nonlinear optical properties of K3B6O10Br crystal. J. Cryst. Growth 2014, 404, 65–68. [Google Scholar] [CrossRef]
- Zhang, M.; Su, X.; Pan, S.L.; Wang, Z.; Zhang, H.; Yang, Z.H.; Zhang, B.B.; Dong, L.Y.; Wang, Y.; Zhang, F.F.; Yang, Y. Linear and Nonlinear Optical Properties of K3B6O10Br Single Crystal: Experiment and Calculation. J. Phys. Chem. C 2014, 118, 11849–11856. [Google Scholar] [CrossRef]
- Wu, H.P.; Pan, S.L.; Yu, H.W.; Jia, D.Z.; Chang, A.M.; Li, H.Y.; Zhang, F.F.; Huang, X. Growth, thermal and optical properties of a novel nonlinear optical material K3B6O10Cl. CrystEngComm 2012, 14, 799–803. [Google Scholar] [CrossRef]
- Xu, B.; Xia, M.J.; Wang, X.Y.; Li, R.K.; Chen, C.T. Second-harmonic generation at 532 nm in K3B6O10Br crystal. Opt. Lett. 2015, 40, 1073–1076. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Hou, Z.Y.; Xia, M.J.; Liu, L.J.; Wang, X.Y.; Li, R.K.; Chen, C.T. High average power third harmonic generation at 355 nm with K3B6O10Br crystal. Opt. Express 2016, 24, 10345–10351. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Wu, H.P.; Pan, S.L.; Yang, Z.H.; Su, X.; Zhang, F.F. A novel deep UV nonlinear optical crystal Ba3B6O11F2, with a new fundamental building block, B6O14 group. J. Mater. Chem. 2012, 22, 9665–9670. [Google Scholar] [CrossRef]
- Huang, Z.; Su, X.; Pan, S.; Dong, X.; Han, S.; Yu, H.; Zhang, M.; Yang, Y.; Cui, S.; Yang, Z. Sr3B6O11F2: A promising polar fluoroborate with short UV absorption edge and moderate second harmonic generation response. Scr. Mater. 2013, 69, 449–452. [Google Scholar] [CrossRef]
- Yan, X.; Luo, S.; Lin, Z.; Yao, J.; He, R.; Yue, Y.; Chen, C. ReBe2B5O11 (Re = Y, Gd): Rare-earth beryllium borates as deep-ultraviolet nonlinear-optical materials. Inorg. Chem. 2014, 53, 1952–1954. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.W.; Zhang, W.G.; Young, J.; Rondinelli, J.M.; Halasyamani, P.S. Design and Synthesis of the Beryllium-Free Deep-Ultraviolet Nonlinear Optical Material Ba3(ZnB5O10)PO4. Adv. Mater. 2015, 27, 7380–7385. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.P.; Yu, H.W.; Pan, S.L.; Huang, Z.J.; Yang, Z.H.; Su, X.; Poeppelmeier, K.R. Cs2B4SiO9: A deep-ultraviolet nonlinear optical crystal. Angew. Chem. Int. Ed. Engl. 2013, 52, 3406–3410. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Zhang, C.Q.; Chen, C.T.; Xu, Z.Y.; Wang, J.Y. Determination of nonlinear optical coefficients of KBe2BO3F2 crystals. Chin. Phys. Lett. 2003, 20, 243–245. [Google Scholar]




| Chemical Formula (Abbreviation) | Space Group | UV Edge (nm) | SHG Coefficient (pm/V) or Powder SHG Efficiency | ∆n(@1064 nn) |
|---|---|---|---|---|
| Borate crystal in which B-O groups are (BO3)3− only | ||||
| KBe2BO3F2 (KBBF) [20] | R32 | 147 [26] | d11 = 0.49 [68] | 0.077 [36] |
| CsBe2BO3F2 (CBBF) [24] | R32 | 151 | d11 = 0.50 | 0.058 |
| RbBe2BO3F2 (RBBF) [23] | R32 | 152 | d11 = 0.45 ± 0.01 | 0.073(@694.3 nm) |
| BaBe2BO3F3 (BBBF) [30] | P63 | 147(cal) | 0.32 × KDP | 0.081(cal@200 nm) |
| NH4Zn2BO3Cl2 [32] | R32 | 190 | 2.82 × KDP | - |
| KZn2BO3Cl2 [32] | R32 | 194 [32] (~200) [31] | 3.01 × KDP [32] 1.3 × KDP [31] | - |
| RbZn2BO3Cl2 [31,32] | R32 | 198 [32] | 2.85 × KDP [32] 1.17 × KDP [31] | - |
| KZn2BO3Br2 [31,32] | R32 | 209 [32] | 2.68 × KDP [32] | - |
| RbZn2BO3Br2 [31,32] | R32 | 214 [32] | 2.53 × KDP [32] | - |
| Sr2Be2B2O7 (SBBO) [33] | P-6c2 | 155 | d22 = 2.0–2.48 | 0.062(@589 nm) |
| NaCaBe2B2O6F [37] | Cc | 190 | 1/3 × KDP | - |
| K3Ba3Li2Al4B6O20F [38] | P-62c | 190 | 1.5 × KDP | 0.052(cal@532 nm) |
| Rb3Al3B3O10F [39] | P31c | < 200 | 1.2 × KDP | - |
| LiSr (BO3)2 [40] | Cc | 186 | deff = 0.76 2 × KDP | 0.056(cal) |
| NaSr3Be3B3O9F4 [41] | R3m | 170 | 5 × KDP | 0.06 |
| NaBeB3O6 [42] | Pna21 | 170(cal) [14] | deff = 0.62 1.6 × KDP | |
| Na2Be4B4O11 [43] | P1 | 171 | 1.3 × KDP | - |
| LiNa5Be12B12O33 [43] | Pc | 169 | 1.4 × KDP | - |
| γ-KBe2B3O7 [42] | P21 | 186(cal) [14] | deff = 0.27 0.68 × KDP | |
| β-KBe2B3O7 [42] | Pmn21 | 187(cal) [14] | deff = 0.29 0.75 × KDP | |
| RbBe2B3O7 [42] | Pmn21 | 179(cal) [14] | deff = 0.31 0.79 × KDP | |
| Na2CsBe6B5O15 [44] | C2 | 192(cal) [14] | 1.17 × KDP | - |
| CsZn2B3O7 [45,46] | Cmc21 | 218 [46] (<200 [45]) | 1.5 × KDP [46] (3.3 × KDP) [45] | 0.056(cal) [46] |
| Borate crystals in which the B-O groups are (BO4)5− groups only | ||||
| LaBeB3O7 [55] | Pnm21 | 220 | 1~2 × KDP | ~0.03(cal) |
| Borate crystals containing the B-O combinational groups | ||||
| K3B6O10Cl [57] | R3m | 180 [57,60] | 4 × KDP [57] | ~0.05 (404–694 nm) [60] |
| K3B6O10Br [56] | R3m | 182 [58] | d22 = −1.23 ± 0.01 d33 = 0.43 ± 0.01 [58] (d22 = 0.83 d33 = 0.51 [59]) | 0.045 (0.046) [59] |
| Ba3B6O11F2 [63] | P21 | <190 | 3 × KDP | - |
| Sr3B6O11F2 [64] | P21 | <190 | 2.5 × KDP | 0.04–0.047(cal) (1052–302 nm) |
| GdBe2B5O11 [65] | Pna2 | <200 | 1 × KDP | - |
| YBe2B5O11 [65] | Pna2 | <200 | 1 × KDP | - |
| The borates containing B-O groups and other NLO active groups | ||||
| Ba3 (ZnB5O10)PO4 [66] | Pmn21 | 180 [66] | dpowder = 4 × KDP [66] | 0.033 (cal@532 nm) [66] |
| Cs2B4SiO9 [67] | I-4 | 190 | 4.6 × KDP | - |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Jiang, X.; Lin, Z.; Wu, Y. Borate-Based Ultraviolet and Deep-Ultraviolet Nonlinear Optical Crystals. Crystals 2017, 7, 95. https://doi.org/10.3390/cryst7040095
Yang Y, Jiang X, Lin Z, Wu Y. Borate-Based Ultraviolet and Deep-Ultraviolet Nonlinear Optical Crystals. Crystals. 2017; 7(4):95. https://doi.org/10.3390/cryst7040095
Chicago/Turabian StyleYang, Yi, Xingxing Jiang, Zheshuai Lin, and Yicheng Wu. 2017. "Borate-Based Ultraviolet and Deep-Ultraviolet Nonlinear Optical Crystals" Crystals 7, no. 4: 95. https://doi.org/10.3390/cryst7040095






