Theoretical Study on Electronic, Optical Properties and Hardness of Technetium Phosphides under High Pressure
Abstract
:1. Introduction
2. Computational Methods and Details
3. Results and Discussions
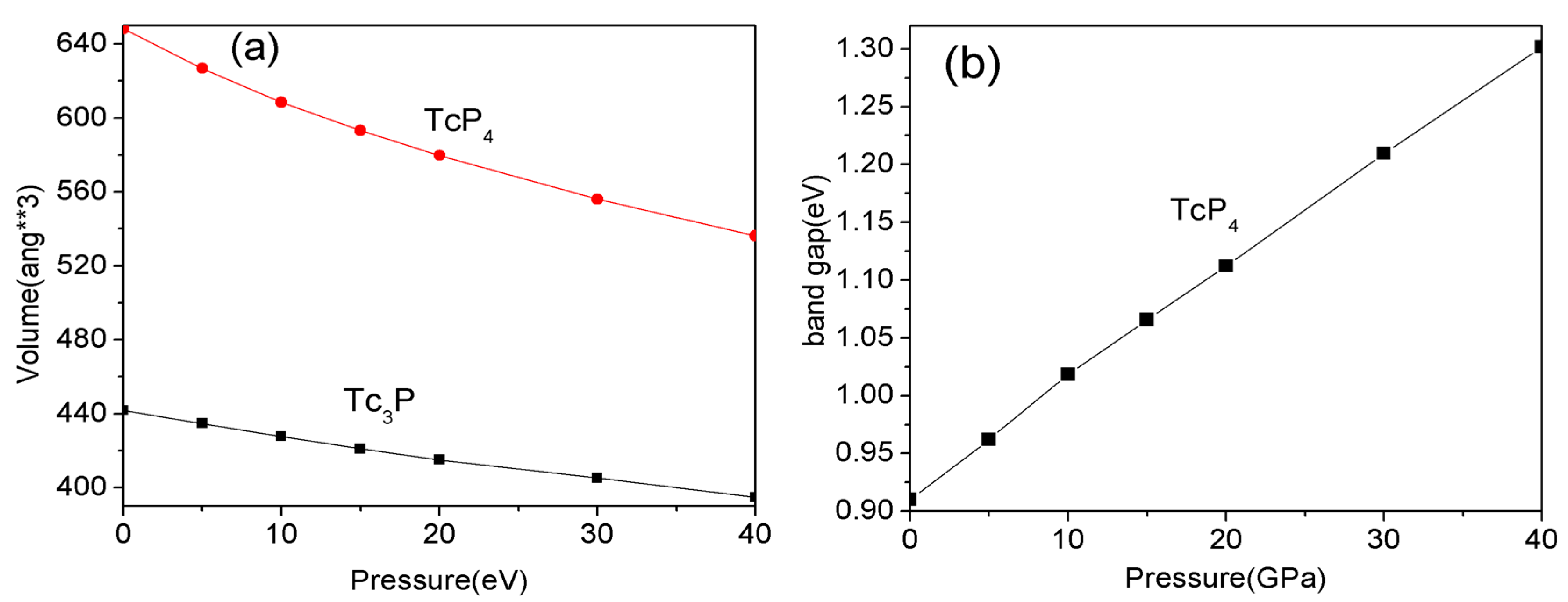
3.1. Structure Properties
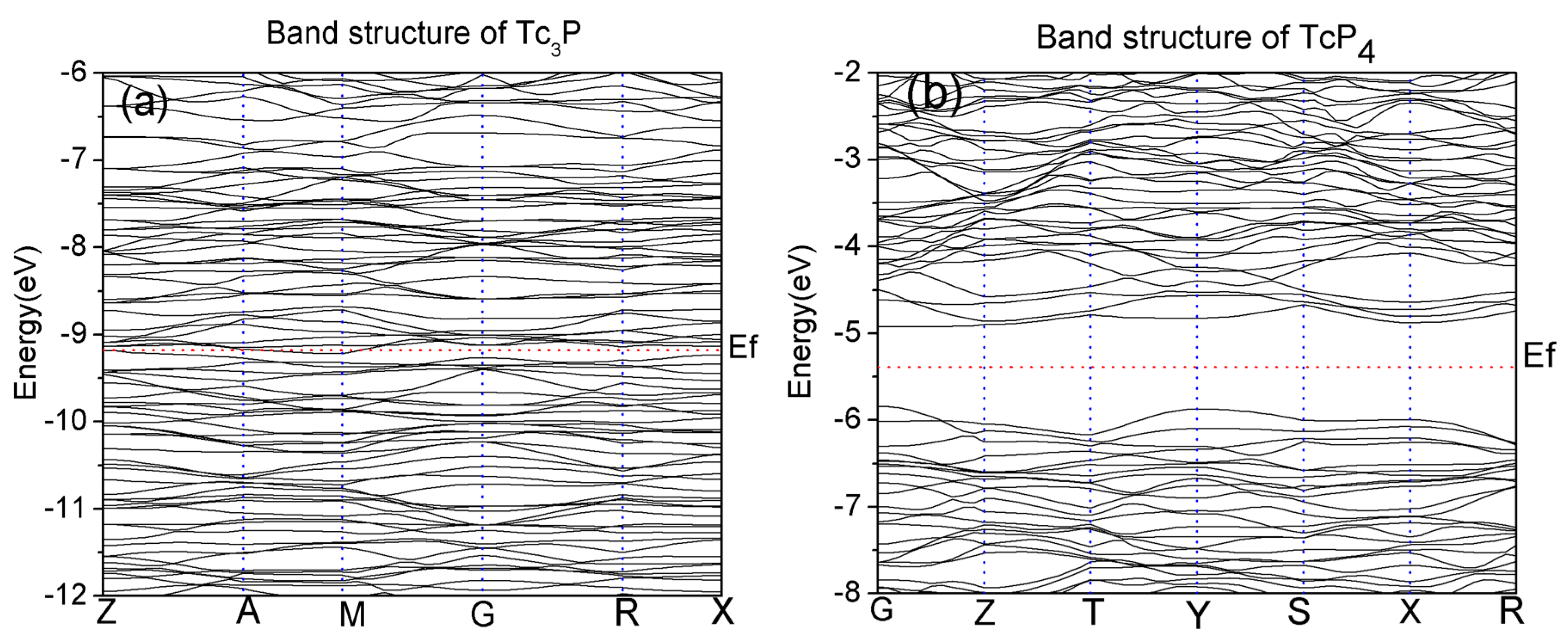
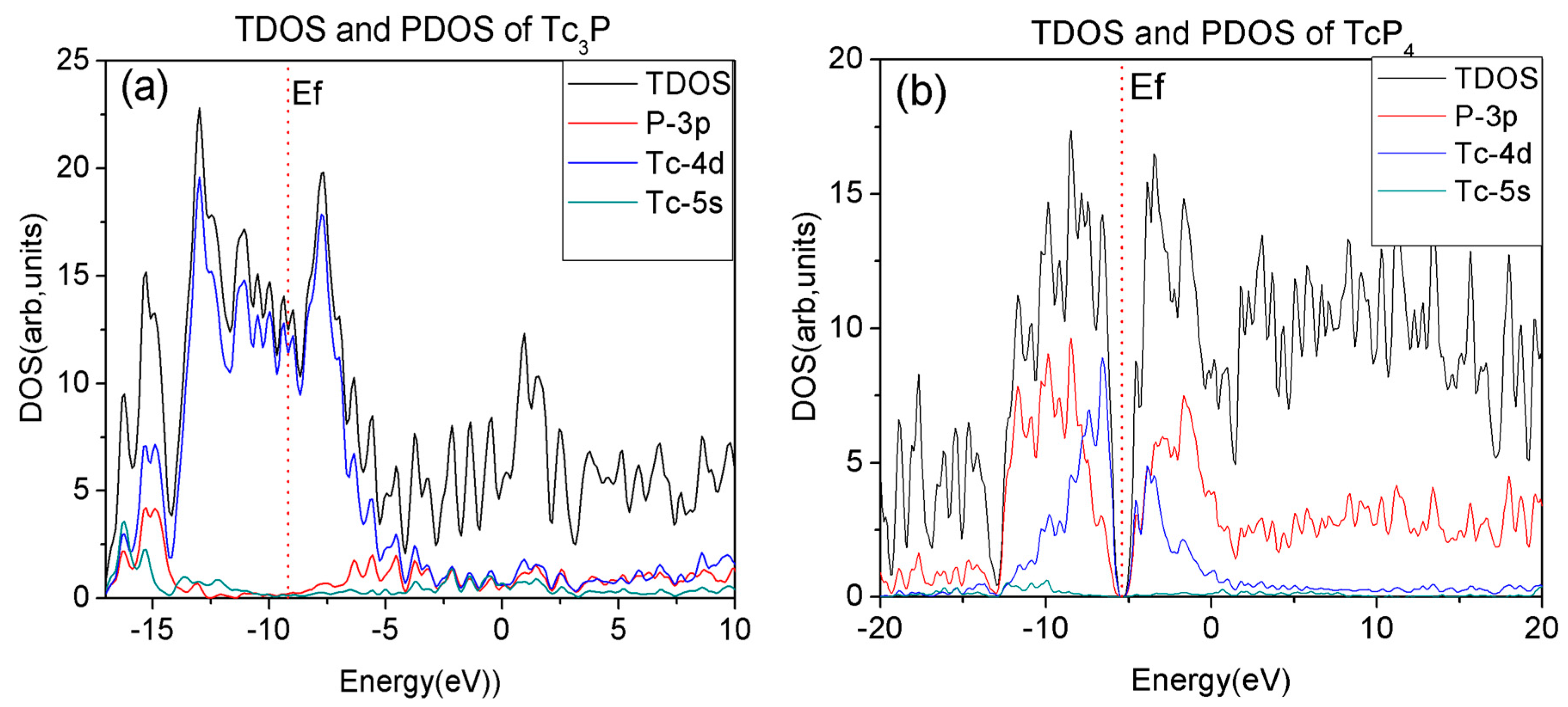
3.2. Electronic Properties
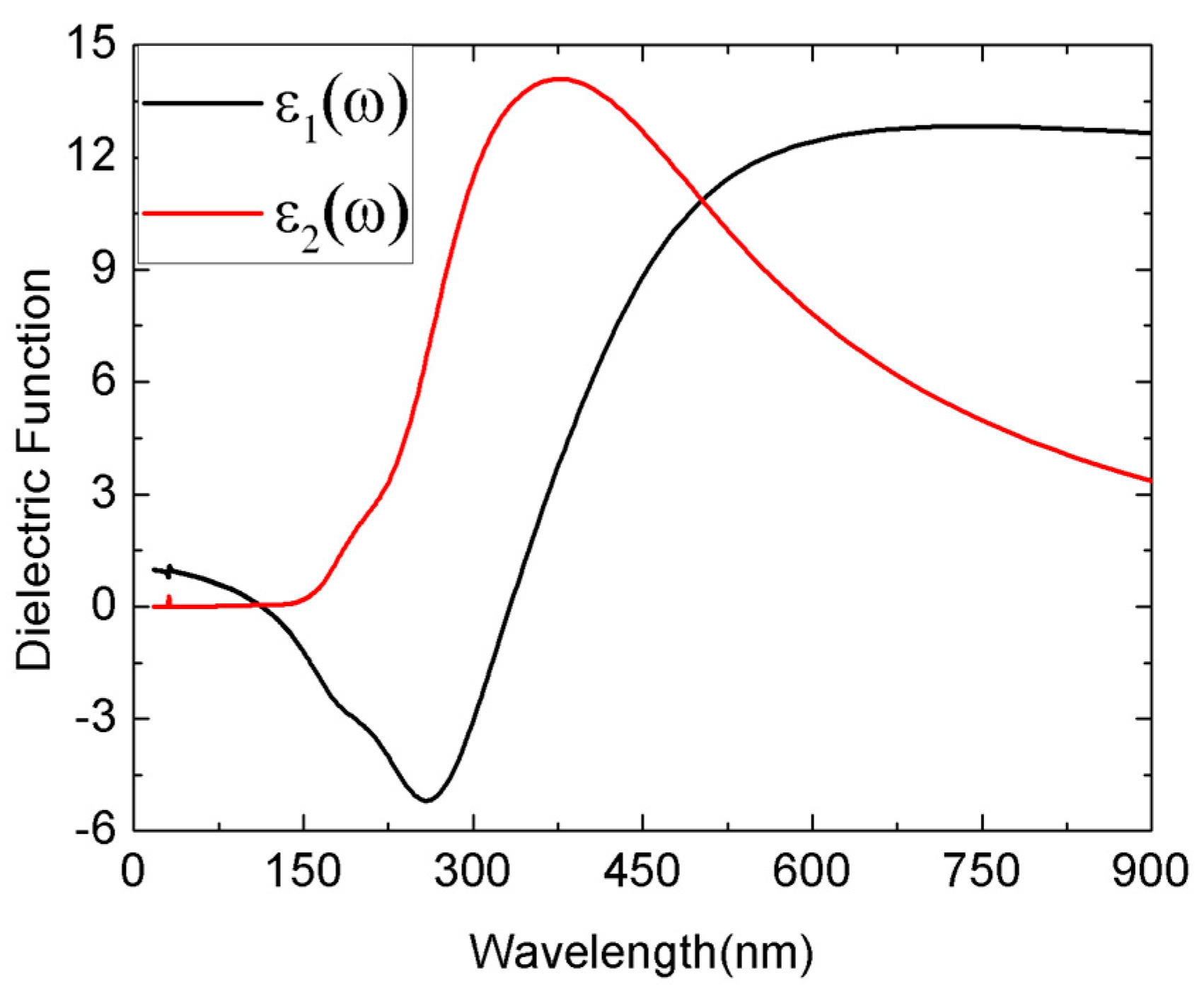
3.3. Optical Properties
3.4. Hardness
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sumiya, H.; Toda, N.; Satoh, S. Mechanical Properties of Synthetic Type IIa Diamond Crystal. Diam. Relat. Mater. 1997, 6, 1841–1846. [Google Scholar] [CrossRef]
- Komanduri, R.; Shaw, M.C. Wear of synthetic diamond when grinding ferrous materials. Nature 1975, 255, 211–213. [Google Scholar] [CrossRef]
- Yang, S.; Liang, C.; Prins, R. Preparation and hydrotreating activity of unsupported nickel phosphide with high surface area. J. Catal. 2006, 241, 465–469. [Google Scholar] [CrossRef]
- Muetterties, E.L.; Sauer, J.C. Catalytic Properties of Metal Phosphides: I. Qualitative Assay of Catalytic Properties. J. Am. Chem. Soc. 1974, 96, 3410–3415. [Google Scholar] [CrossRef]
- Dmitruk, N.L.; Zuev, V.A.; Stepanova, M.A. Spectral distribution of the photoconductivity of cadmium diphosphide. Russ. Phys. J. 1991, 34, 642–644. [Google Scholar]
- Kushnir, O.S.; Bevz, O.A.; Polovinko, I.I.; Sveleba, S.A. Temperature dependence of optical activity and circular dichroism in α-ZnP2 crystals. Phys. Status Solidi B 2003, 238, 92–101. [Google Scholar] [CrossRef]
- Lazarev, V.B.; Shevchenko, V.Y.; Grinberg, L. K.; Sobolev, V.V. Semiconducting II-V Compounds; Nauka: Moscow, Russia, 1976. [Google Scholar]
- Morozova, V.A.; Marenkin, S.F.; Koshelev, O.G.; Trukhan, V.M. Optical absorption in monoclinic zinc diphosphide. Inorg. Mater. 2006, 42, 221. [Google Scholar] [CrossRef]
- Sheleg, A.U.; Zaretskii, V.V. X-ray study of the commensurate—Incommensurate phase transitions in α-ZnP2. Phys. Status Solidi A 1984, 86, 517–523. [Google Scholar] [CrossRef]
- Slobodyanyuk, A.V.; Schaack, G. Measurement of Raman scattered intensities in media with natural or field-induced optical activity. J. Raman Spectrosc. 1987, 18, 561–568. [Google Scholar] [CrossRef]
- Feng, S.Q.; Wang, L.L.; Jiang, X.X.; Li, H.N.; Cheng, X.L.; Su, L. High-pressure dynamic, thermodynamic properties, and hardness of CdP2. Chin. Phys. B 2017, 4, 046301. [Google Scholar] [CrossRef]
- Feng, S.Q.; Yang, Y.; Li, J.Y.; Jiang, X.X.; Li, H.N.; Cheng, X.L. Pressure effect on the hardness of diamond and W2B5: First-principle. Mod. Phys. Lett. B 2017, 31, 1750137. [Google Scholar] [CrossRef]
- Ruehl, R.; Jeitschko, W.; Schwochau, K. Preparation and Crystal Structures of Technetium Phosphides. J. Solid. State. Chem. 1982, 44, 134–140. [Google Scholar] [CrossRef]
- Ordejón, P.; Artacho, E.; Soler, J.M. Self-consistent order-N density-functional calculations for very large systems. Phys. Rev. B 1996, 53, R10441. [Google Scholar] [CrossRef]
- Barth, U.; Von Hedin, L. A local exchange-correlation potential for the spin polarized case: I. J. Phys. C. Solid State Phys. 1972, 5, 1629. [Google Scholar] [CrossRef]
- Kohn, W.; Sham, L.J. Self-Consistent Equations Including Exchange and Correlation Effects. Phys. Rev. 1965, 140, A1133. [Google Scholar] [CrossRef]
- Strobel, R.; Maciejewski, M.; Pratsinis, S.E.; Baiker, A. Unprecedented formation of metastable monoclinic BaCO3 nanoparticles. Therm. Acta 2006, 445, 23–26. [Google Scholar] [CrossRef]
- Staroverov, V.N.; Scuseria, G.E. High-density limit of the Perdew-Burke-Ernzerhof generalized gradient approximation and related density functionals. Phys. Rev. A 2006, 74, 044501. [Google Scholar] [CrossRef]
- Wu, Z.G.; Cohen, R.E. More accurate generalized gradient approximation for solids. Phys. Rev. B 2006, 73, 235116. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Zunger, A. Self-interaction correction to density-functional approximations for many-electron systems. Phys. Rev. B 1981, 23, 5075. [Google Scholar] [CrossRef]
- Kleinman, L.; Bylander, D.M. Efficacious Form for Model Pseudopotentials. Phys. Rev. Lett. 1982, 48, 1425. [Google Scholar] [CrossRef]
- Okoye, C.M.I. Theoretical study of the electronic structure, chemical bonding and optical properties of KNbO3 in the paraelectric cubic phase. J. Phys. Condens. Matter. 2003, 15, 5945–5958. [Google Scholar] [CrossRef]
- Gao, F. Theoretical model of intrinsic hardness. Phys. Rev. B 2006, 73, 132104. [Google Scholar] [CrossRef]
- Fan, C.L.; Cheng, X.L.; Zhang, H. First-principles study of the structural and electronic properties of the alpha modification of zinc diphosphide. Phys. Status Solidi B 2009, 246, 77–81. [Google Scholar] [CrossRef]
- Feng, S.Q.; Cheng, X.L. Theoretical study on electronic properties and pressure-induced phase transition in β-CdP2. Comput. Theor. Chem. 2011, 966, 149–153. [Google Scholar] [CrossRef]
- Feng, S.Q.; Li, X.D.; Su, L.; Li, H.N.; Yang, H.Y.; Cheng, X.L. Ab initio Study on Structural, Electronic Properties, and Hardness of Re-doped W2B5. Solid. Stat. Commun. 2016, 245, 60–64. [Google Scholar] [CrossRef]
- Feng, S.Q.; Guo, F.; Li, J.Y.; Wang, Y.Q.; Zhang, L.M.; Cheng, X.L. Theoretical investigations of physical stability, electronic properties and hardness of transition-metal tungsten borides WBx (x = 2.5, 3). Chem. Phys. Lett. 2015, 635, 205–209. [Google Scholar] [CrossRef]
- Liu, Z.T.; Gall, Y.; Khare, S.V.D. Electronic and bonding analysis of hardness in pyrite-type transition-metal pernitrides. Phys. Rev. B 2014, 90, 134102. [Google Scholar] [CrossRef]
- Tian, Y.J.; Xu, B.; Zhao, Z.S. Microscopic theory of hardness and design of novel superhard crystals. Int. J. Refract. Met. H. 2012, 33, 93–106. [Google Scholar] [CrossRef]
- Zhong, M.M.; Kuang, X.Y.; Wang, Z.H.; Shao, P.; Ding, L.P.; Huang, X.F. Phase Stability, Physical Properties, and Hardness of Transition-Metal Diborides MB2 (M = Tc, W, Re, and Os): First-Principles Investigations. J. Phys. Chem. C 2013, 117, 10643. [Google Scholar] [CrossRef]
- Gu, Q.F.; Krauss, G.; Steurer, W. Transition metal borides: Superhard versus ultra-incompressihle. Adv. Mater. 2008, 20, 3620–3626. [Google Scholar] [CrossRef]

| Lattice Parameter | Tc3P | TcP4 | ||||
|---|---|---|---|---|---|---|
| Theoretical Results | Experimental Values [13] | Theoretical Results | Experimental Values [13] | |||
| GGA | LDA | GGA | LDA | |||
| a0 (Å) | 9.490 | 9.376 | 9.568 | 6.341 | 6.280 | 6.238 |
| b0 (Å) | 9.490 | 9.376 | 9.568 | 9.339 | 9.241 | 9.215 |
| c0 (Å) | 4.907 | 4.856 | 4.736 | 10.949 | 10.827 | 10.837 |
| V0 (Å3) | 441.9 | 426.9 | 433.6 | 648.3 | 628.3 | 623.0 |
| Compounds | Bond | dμ (Å) | Pμ | Ω (Å3) | vbμ (Å3) | fm (10−3) | Hvμ (GPa) | Hv Tian | Hv Gao | Hv exp |
|---|---|---|---|---|---|---|---|---|---|---|
| WB2-WB2 | B-B (1) | 1.727 | 0.76 | 2.521 | 0 | 28.8 | 25.6 [31] | 27.7 [32] | ||
| B-B (2) | 1.838 | 0.65 | 3.038 | 0 | ||||||
| W-B (1) | 2.335 | 0.26 | 6.229 | 1.787 | ||||||
| ReB2-ReB2 | B-B | 1.807 | 0.64 | 1.846 | 0 | 43.4 | 39.1 [31] | 39.3 [32] | ||
| Re-B | 2.240 | 0.25 | 3.515 | 1.311 | ||||||
| TcP4 | P-P | 2.178 | 0.58 | 621.7 | 5.362 | 0 | 24.68 | 19.7 | 20.1 | |
| P-P | 2.190 | 0.56 | 5.451 | 0 | 23.19 | |||||
| P-P | 2.191 | 0.51 | 5.458 | 0 | 21.07 | |||||
| P-P | 2.203 | 0.60 | 5.549 | 0 | 24.12 | |||||
| P-P | 2.252 | 0.51 | 5.927 | 0 | 18.36 | |||||
| Tc-P | 2.342 | 0.52 | 6.667 | 0.929 | 14.39 | |||||
| Tc-P | 2.356 | 0.33 | 6.787 | 0.929 | 8.86 | |||||
| Tc-P | 2.358 | 0.54 | 6.804 | 0.929 | 14.44 | |||||
| Tc-P | 2.377 | 0.53 | 6.970 | 0.929 | 13.62 | |||||
| Tc-P | 2.425 | 0.57 | 7.401 | 0.929 | 13.25 | |||||
| Tc-P | 2.523 | 0.30 | 8.335 | 0.929 | 5.72 | |||||
| Tc-Tc | 3.000 | 0.63 | 14.012 | 0.929 | 5.05 |
| Compounds | 0 GPa | 10 GPa | 20 GPa | 40 GPa |
|---|---|---|---|---|
| Hardness (GPa) | 20.14 | 22.35 | 24.88 | 25.74 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, S.; Cheng, X.; Cheng, X.; Yue, J.; Li, J. Theoretical Study on Electronic, Optical Properties and Hardness of Technetium Phosphides under High Pressure. Crystals 2017, 7, 176. https://doi.org/10.3390/cryst7060176
Feng S, Cheng X, Cheng X, Yue J, Li J. Theoretical Study on Electronic, Optical Properties and Hardness of Technetium Phosphides under High Pressure. Crystals. 2017; 7(6):176. https://doi.org/10.3390/cryst7060176
Chicago/Turabian StyleFeng, Shiquan, Xuerui Cheng, Xinlu Cheng, Jinsheng Yue, and Junyu Li. 2017. "Theoretical Study on Electronic, Optical Properties and Hardness of Technetium Phosphides under High Pressure" Crystals 7, no. 6: 176. https://doi.org/10.3390/cryst7060176




