Synthesis, Crystal Structures, and Properties of a New Supramolecular Polymer Based on Mixed Imidazole and Carboxylate Ligands
Abstract
:1. Introduction
2. Results and Discussion
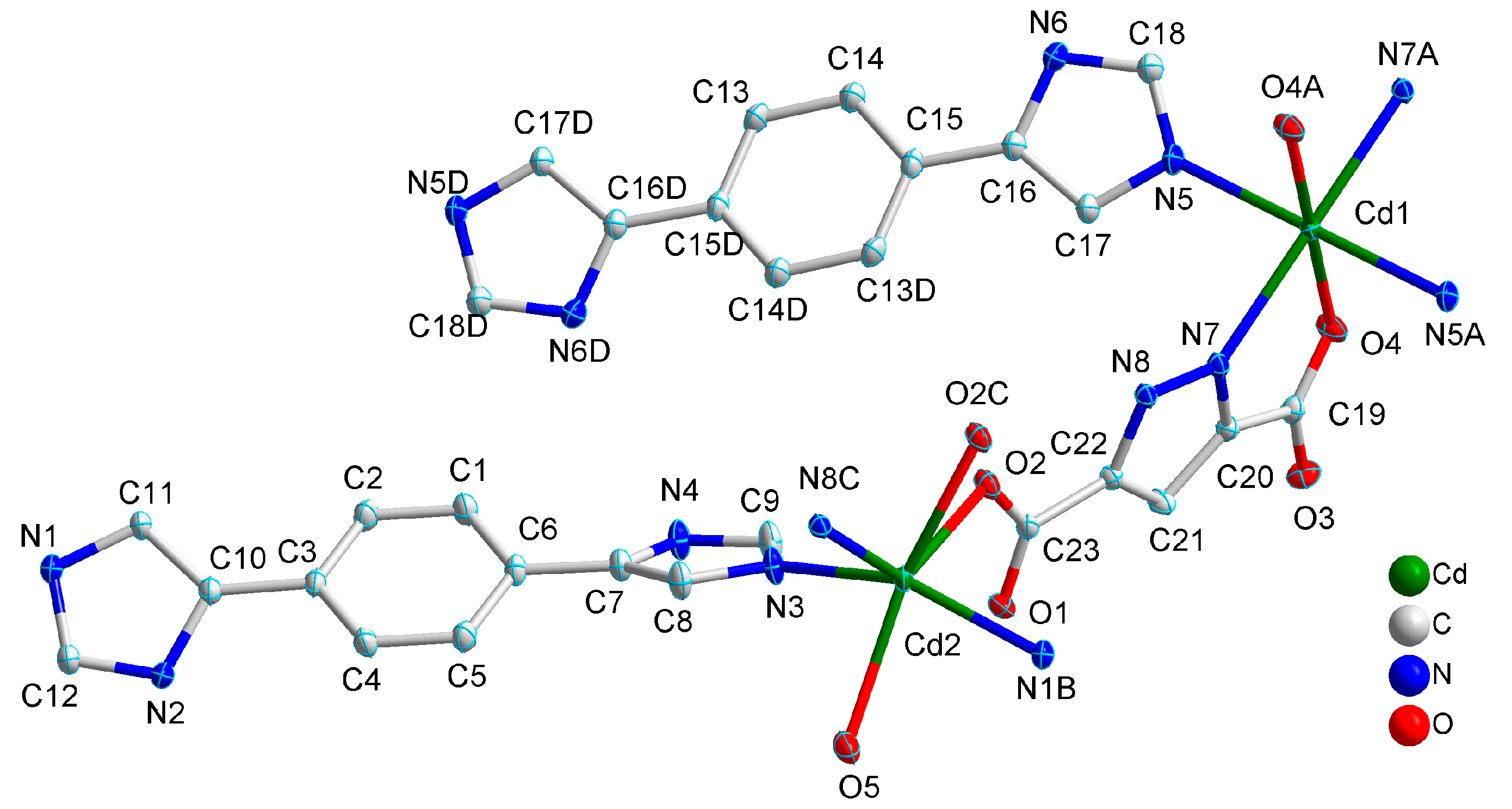
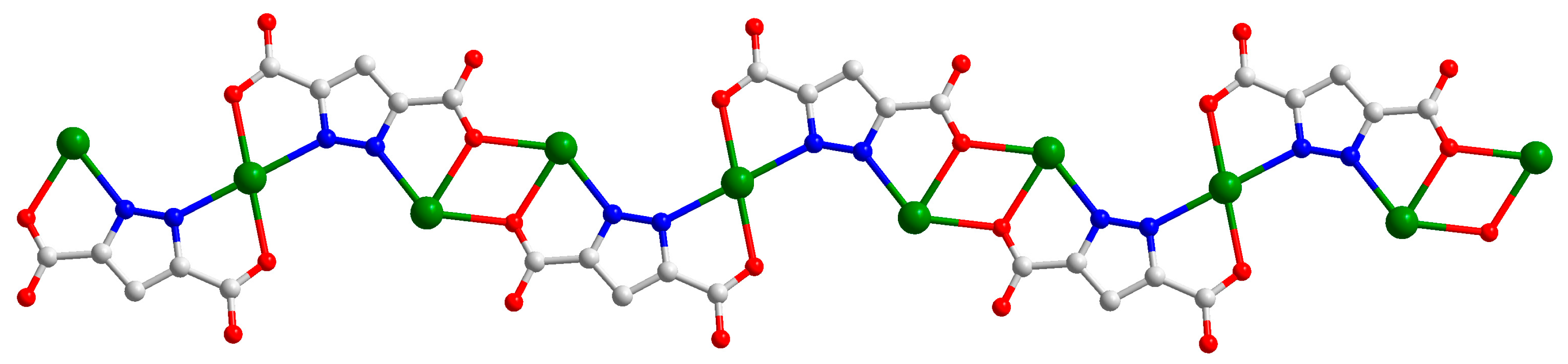
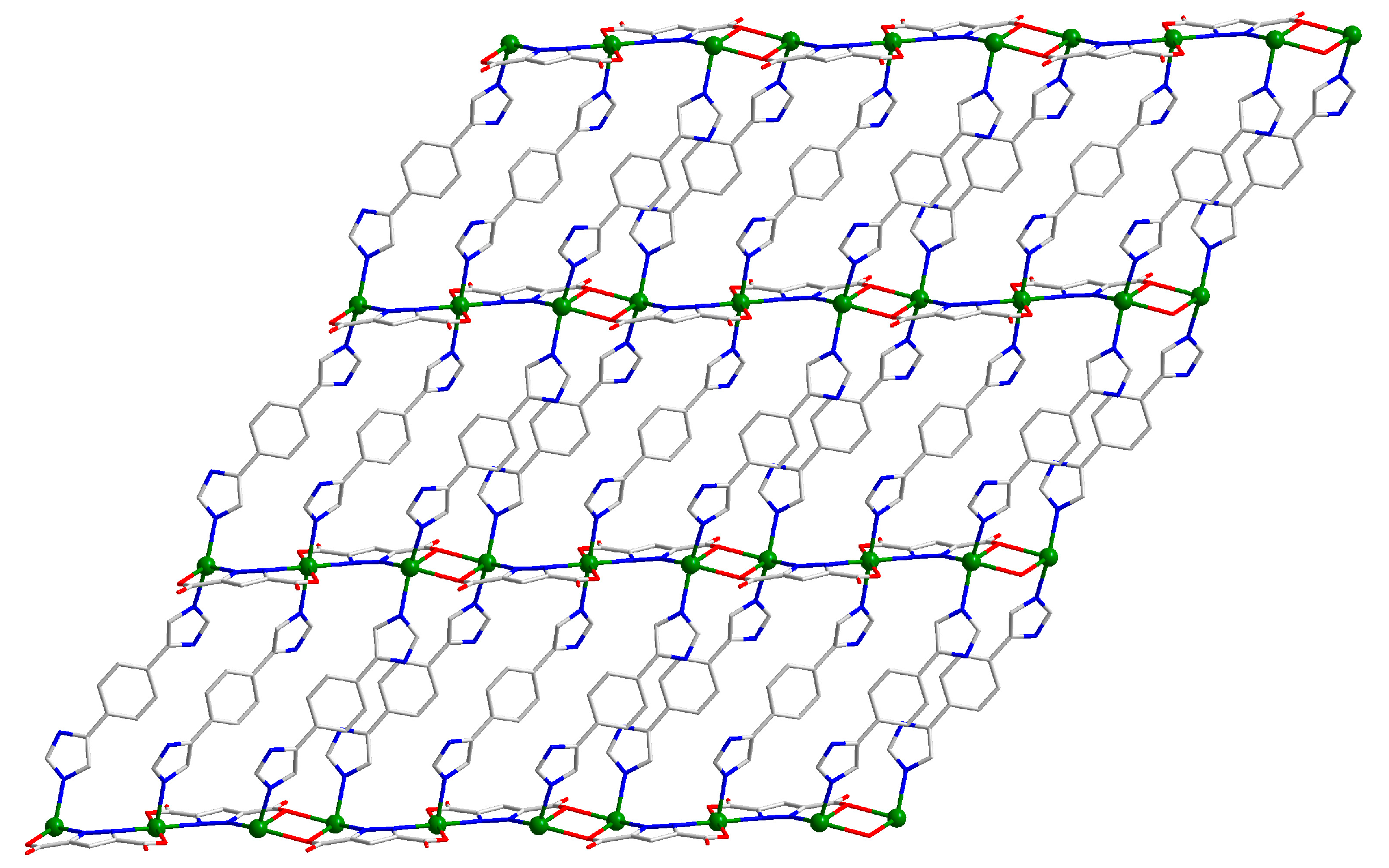
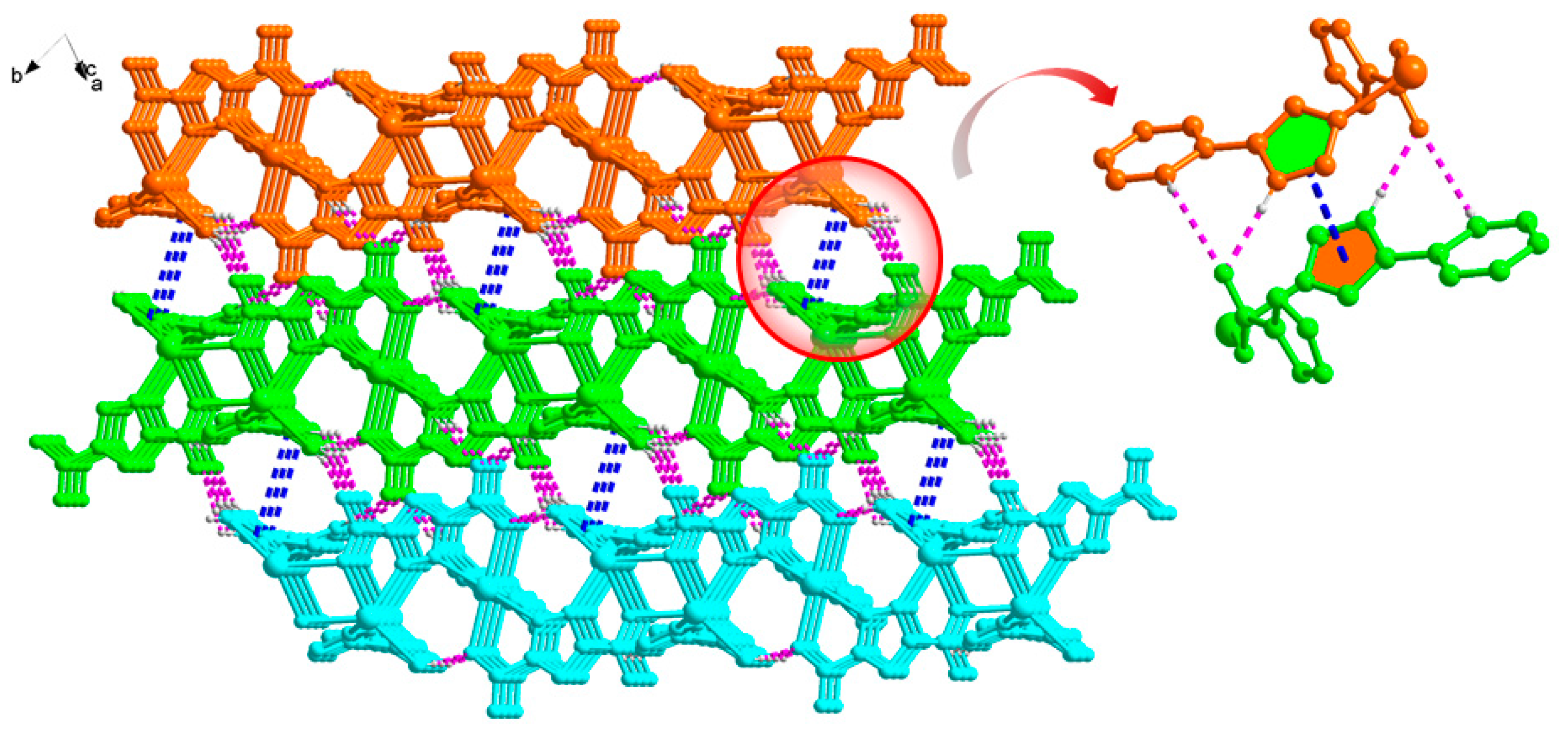
2.1. Structural Description of [Cd3(H2L)3(Pza)2(H2O)2]n (1)
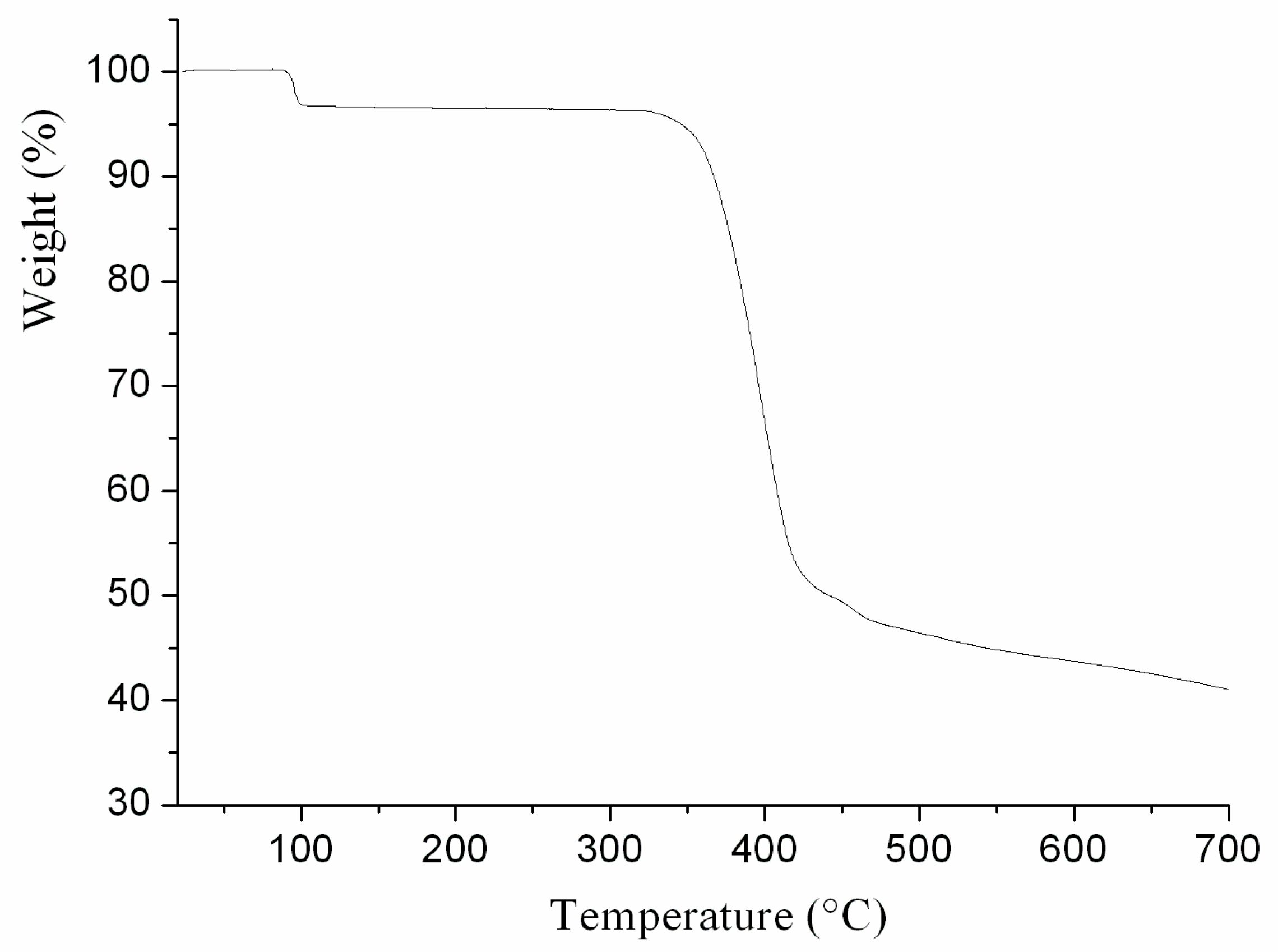
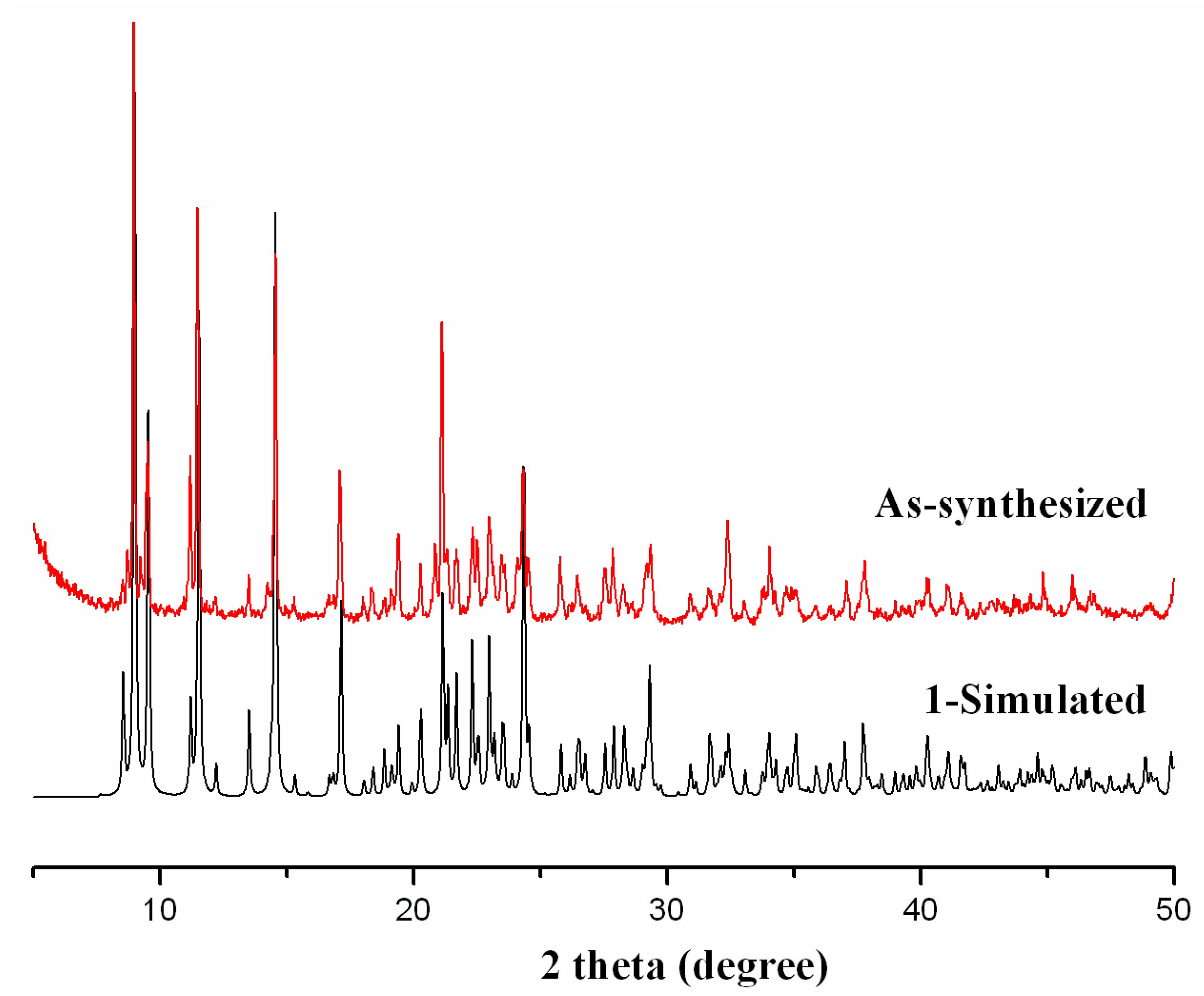
2.2. Thermal Analysis and Powder X-ray Diffraction Analysis
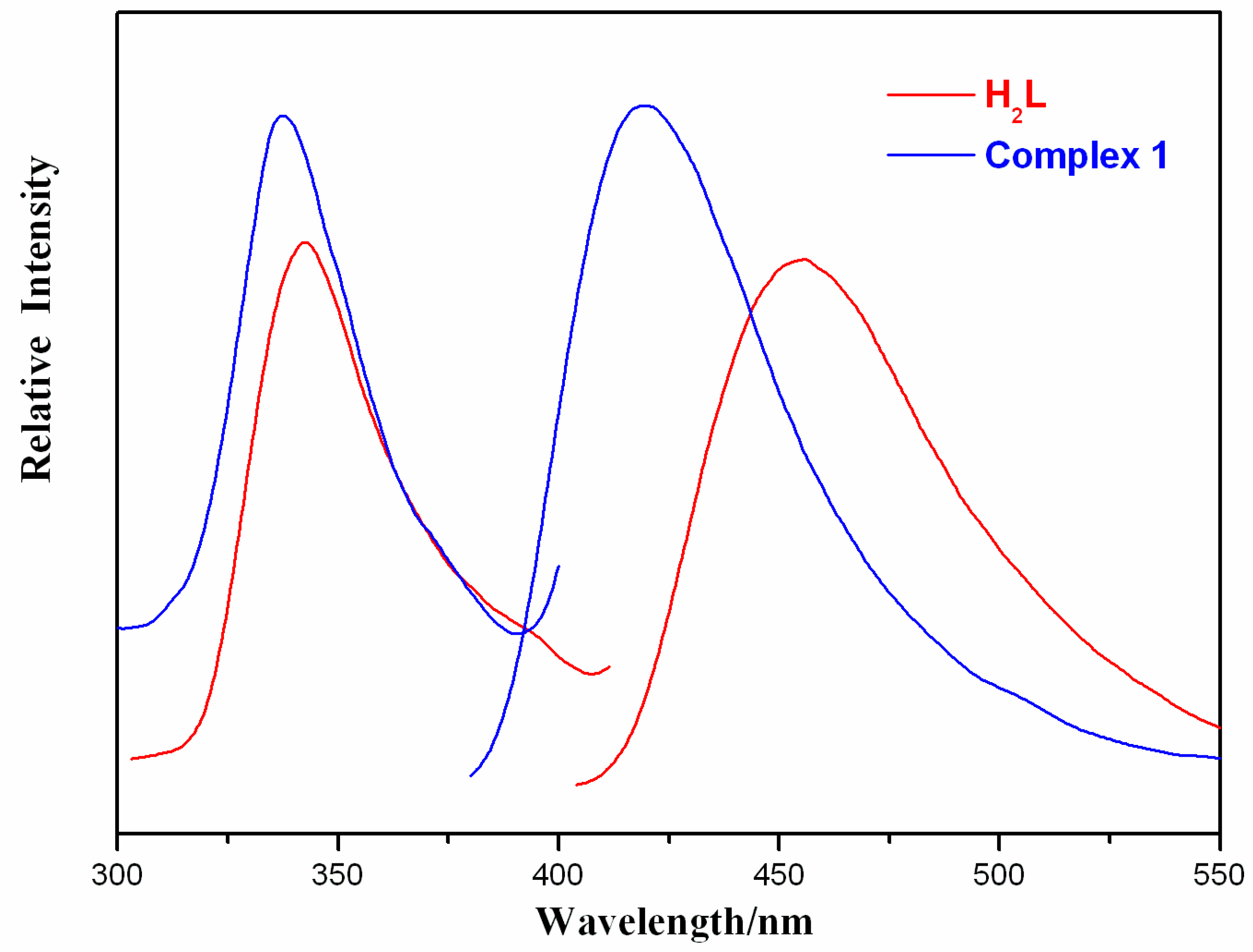
2.3. Photoluminescent Property
3. Experimental Section
3.1. Materials and Instrumentation
3.2. Synthesis of [Cd3(H2L)3(Pza)2(H2O)2]n (1)
3.3. Crystal Structure Determination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Furukawa, H.; Cordova, K.E.; O’Keeffe, M.; Yaghi, O.M. The chemistry and applications of metal-organic frameworks. Science 2013, 341, 974. [Google Scholar] [CrossRef] [PubMed]
- Brenner, W.; Ronson, T.K.; Nitschke, J.R. Separation and selective formation of fullerene adducts within an MII8L6 cage. J. Am. Chem. Soc. 2017, 139, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Yang, J.; Wang, R.; Zhang, L.; Yu, J.; Sun, D. Luminescent terbium-organic framework exhibiting selective sensing of nitroaromatic compounds (NACs). Cryst. Growth Des. 2015, 15, 2589–2592. [Google Scholar] [CrossRef]
- Li, Y.W.; Yan, H.; Hu, T.L.; Ma, H.Y.; Li, D.C.; Wang, S.N.; Yao, Q.X.; Dou, J.M.; Xu, J.; Bu, X.H. Two microporous Fe-based MOFs with multiple active sites for selective gas adsorption. Chem. Commun. 2017, 53, 2394–2397. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.M.; Zhou, X.; Li, H.X.; Yang, X.X.; Lang, J.P. Luminescent two-dimensional coordination polymer for selective and recyclable sensing of nitroaromatic compounds with high sensitivity in water. Cryst. Growth Des. 2015, 15, 2753–2760. [Google Scholar] [CrossRef]
- Li, D.S.; Wu, Y.P.; Zhao, J.; Zhang, J.; Lu, J.Y. Metal-organic frameworks based upon non-zeotype 4-connected topology. Coord. Chem. Rev. 2014, 261, 1–27. [Google Scholar] [CrossRef]
- Zhou, D.D.; He, C.T.; Liao, P.Q.; Xue, W.; Zhang, W.X.; Zhou, H.L.; Zhang, J.P.; Chen, X.M. A flexible porous Cu(II) bis-imidazolate framework with ultrahigh concentration of active sites for efficient and recyclable CO2 capture. Chem. Commun. 2013, 49, 11728–11730. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Deiberta, B.J.; Li, J. Luminescent metal–organic frameworks for chemical sensing and explosive detection. Chem. Soc. Rev. 2014, 43, 5815–5840. [Google Scholar] [CrossRef] [PubMed]
- Férey, G. Hybrid porous solids: Past, present, future. Chem. Soc. Rev. 2008, 37, 191–214. [Google Scholar] [CrossRef] [PubMed]
- Long, J.R.; Yaghi, O.M. The pervasive chemistry of metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1213–1214. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.Q.; Zhang, W.Y.; Wang, R.D.; Ren, C.Y.; Li, Q.Q.; Fan, Y.P.; Liu, B.; Liu, P.; Wang, Y.Y. Effect of coordinated solvent molecules on metal coordination sphere and solvent-induced transformations. Cryst. Growth Des. 2017, 17, 517–526. [Google Scholar] [CrossRef]
- Zhao, J.P.; Han, S.D.; Zhao, R.; Yang, Q.; Chang, Z.; Bu, X.H. Tuning the structure and magnetism of heterometallic sodium(1+)–cobalt(2+) formate coordination polymers by varying the metal ratio and solvents. Inorg. Chem. 2013, 52, 2862–2869. [Google Scholar] [CrossRef] [PubMed]
- Zang, S.Q.; Dong, M.M.; Fan, Y.J.; Hou, H.W.; Mak, T.C.W. Four cobaltic coordination polymers based on 5-iodo-isophthalic acid: Halogen-related interaction and solvent effect. Cryst. Growth Des. 2012, 12, 1239–1246. [Google Scholar] [CrossRef]
- Sapianik, A.A.; Zorina-Tikhonova, E.N.; Kiskin, M.A.; Samsonenko, D.G.; Kovalenko, K.A.; Sidorov, A.A.; Eremenko, I.L.; Dybtsev, D.N.; Blake, A.J.; Argent, S.P.; et al. Rational synthesis and investigation of porous metal-organic framework materials from a preorganized heterometallic carboxylate building block. Inorg. Chem. 2017, 56, 1599–1608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Jiang, J. Synthesis and crystal structures of two novel O, N-containing spiro compounds. Crystals 2016, 6, 69. [Google Scholar] [CrossRef]
- Li, Y.W.; Ma, H.; Chen, Y.Q.; He, K.H.; Li, Z.X.; Bu, X.H. Structure modulation in Zn(II)−1,4-Bis(imidazol-1-yl)benzene frameworks by varying dicarboxylate anions. Cryst. Growth Des. 2012, 12, 189–196. [Google Scholar] [CrossRef]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Chen, M.S.; Su, Z.; Sun, W.Y. Temperature dependent selective gas sorption of the microporous metal-imidazolate framework [Cu(L)] [H2L = 1,4-di(1H-imidazol-4-yl) benzene]. Chem. Commun. 2011, 47, 752–754. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Chen, M.; Takamizawa, S.; Wang, P.; Lv, G.C.; Sun, W.Y. Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property. Chem. Commun. 2011, 47, 4902–4904. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.S.; Sheng, L.Q.; Zhao, Y.; Liu, Z.D.; Qiao, R.; Yang, S. Syntheses, structures, and properties of a series of polyazaheteroaromatic core-based Zn(II) coordination polymers together with carboxylate auxiliary ligands. Cryst. Growth Des. 2016, 16, 229–241. [Google Scholar] [CrossRef]
- Chen, S.S.; Qiao, R.; Sheng, L.Q.; Zhao, Y.; Yang, S.; Chen, M.M.; Liu, Z.D.; Wang, D.H. Cadmium(II) and zinc(II) complexes with rigid 1-(1H-imidazol-4-yl)-3-(4H-tetrazol-5-yl)benzene and varied carboxylate ligands. CrystEngComm 2013, 15, 5713–5725. [Google Scholar] [CrossRef]
- Chen, S.S.; Liu, Q.; Zhao, Y.; Qiao, R.; Sheng, L.Q.; Liu, Z.D.; Yang, S.; Song, C.F. New metal-organic frameworks constructed from the 4-imidazole-carboxylate ligand: Structural diversities, luminescence, and gas adsorption properties. Cryst. Growth Des. 2014, 14, 3727–3741. [Google Scholar] [CrossRef]
- Kreno, L.E.; Leong, K.; Farha, O.K.; Allendorf, M.; Van Duyne, R.P.; Hupp, J.T. Metal–organic framework materials as chemical sensors. Chem. Rev. 2012, 112, 1105–1125. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Z.; Zhang, Z.Y.; Li, Z.L.; Shi, S.S.; Chen, S.S. Crystals Synthesis, crystal structures, and properties of two coordination polymers built from imidazolyl and carboxylate ligands. Crystals 2017, 7, 73. [Google Scholar] [CrossRef]
- Qiu, Y.C.; Li, Y.H.; Peng, G.; Cai, J.B.; Jin, L.M.; Ma, L.; Deng, H.; Zeller, M.; Batten, S.R. Cadmium metal-directed three-dimensional coordination polymers: In situ tetrazole ligand synthesis, structures, and luminescent properties. Cryst. Growth Des. 2010, 10, 1332–1340. [Google Scholar] [CrossRef]
- Meng, F.; Zhang, M.; Shen, K.; Li, Y.; Zheng, H. A series of MOFs based on a triangular tri(4-pyridylphenyl)amine ligand combined with carboxylate or nitrate auxiliary ligands. Dalton Trans. 2015, 44, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.A.; Zhao, Y.; Liu, Q.; Zhao, D.; Chen, K.; Sun, W.Y. Zinc(II) coordination polymers with substituted benzenedicarboxylate and tripodal imidazole ligands: Syntheses, structures and properties. CrystEngComm 2014, 16, 7536–7546. [Google Scholar] [CrossRef]
- Li, L.N.; Wang, S.Y.; Chen, T.L.; Sun, Z.H.; Luo, J.H.; Hong, M.C. Solvent-dependent formation of Cd(II) coordination polymers based on a C2-symmetric tricarboxylate linker. Cryst. Growth Des. 2012, 12, 4109–4115. [Google Scholar] [CrossRef]
- Song, S.Y.; Song, X.Z.; Zhao, S.N.; Qin, C.; Su, S.Q.; Zhu, M.; Hao, Z.M.; Zhang, H.J. Syntheses, structures and physical properties of transition metal–organic frameworks assembled from trigonal heterofunctional ligands. Dalton Trans. 2012, 41, 10412–10421. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Bai, Z.S.; Chen, M.S.; Su, Z.; Chen, S.S.; Sun, W.Y. Metal–organic frameworks with six- and four-fold interpenetration and their photoluminescence and adsorption property. CrystEngComm 2009, 11, 2728–2733. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
| Bond | d | Bond | d |
|---|---|---|---|
| Cd(1)–N(5) | 2.299(2) | Cd(1)–O(4) | 2.3324(18) |
| Cd(1)–N(7) | 2.3449(19) | Cd(2)–N(3) | 2.258(2) |
| Cd(2)–N(1) ii | 2.245(2) | Cd(2)–N(8) iii | 2.3473(19) |
| Cd(2)–O(2) | 2.4204(17) | Cd(2)–O(5) | 2.481(2) |
| Angle | ω | Angle | ω |
| N(5) i–Cd(1)–N(5) | 180.000(1) | N(5) i–Cd(1)–O(4) | 86.21(8) |
| N(5)–Cd(1)–O(4) | 93.79(8) | O(4)–Cd(1)–O(4) i | 180.0 |
| N(5) i–Cd(1)–N(7) | 93.10(7) | N(5)–Cd(1)–N(7) | 86.90(7) |
| O(4)–Cd(1)–N(7) | 73.27(6) | O(4) i–Cd(1)–N(7) | 106.73(6) |
| O(4)–Cd(1)–N(7) i | 106.73(6) | N(7)–Cd(1)–N(7) i | 180.00(6) |
| N(1) ii–Cd(2)–N(3) | 146.85(8) | N(1) ii–Cd(2)–N(8) iii | 116.08(8) |
| N(3)–Cd(2)–N(8) iii | 92.92(7) | N(1) ii–Cd(2)–O(2) iii | 86.93(7) |
| N(3)–Cd(2)–O(2) iii | 119.34(8) | N(8) iii–Cd(2)–O(2) iii | 70.78(6) |
| N(1) ii–Cd(2)–O(2) | 87.58(7) | N(3)–Cd(2)–O(2) | 86.32(7) |
| N(8) iii–Cd(2)–O(2) | 128.20(6) | O(2) iii–Cd(2)–O(2) | 65.03(7) |
| N(1) ii–Cd(2)–O(5) | 80.02(7) | N(3)–Cd(2)–O(5) | 81.14(8) |
| N(8) iii–Cd(2)–O(5) | 96.39(7) | O(2) iii–Cd(2)–O(5) | 155.45(8) |
| O(2)–Cd(2)–O(5) | 134.25(7) |
| D–H···A | d(D–H) | d(H···A) | d(D···A) | ∠DHA |
|---|---|---|---|---|
| O(5)–H(5W1)···O(4) a | 0.83 | 1.90 | 2.722(3) | 173(3) |
| N(2)–H(2A)···O(1) b | 0.8600 | 1.9100 | 2.738(3) | 160.00 |
| N(4)–H(4A)···O(3) c | 0.8600 | 1.8900 | 2.744(3) | 176.00 |
| N(6)–H(6)···O(3) d | 0.8600 | 1.9200 | 2.745(3) | 161.00 |
| C(4)–H(4)···O(1) b | 0.9300 | 2.5300 | 3.071(3) | 117.00 |
| C(12)–H(12)···O(5) e | 0.9300 | 2.4900 | 3.033(3) | 117.00 |
| Empirical Formula | C46H36N16O10Cd3 |
|---|---|
| Formula weight | 1310.14 |
| Temperature/K | 296(2) |
| Crystal system | Triclinic |
| Space group | P-1 |
| a/Å | 9.5416(10) |
| b/Å | 11.3868(12) |
| c/Å | 12.8411(14) |
| α/° | 65.8260(10) |
| β/° | 77.4100(10) |
| γ/° | 80.1150(10) |
| Volume/Å3 | 1236.9(2) |
| Z | 1 |
| ρcalcmg/mm3 | 1.759 |
| μ/mm−1 | 1.353 |
| S | 1.090 |
| F(000) | 648 |
| Index ranges | −11 ≤ h ≤ 11, −13 ≤ k ≤ 14, −16 ≤ l ≤ 15 |
| Reflections collected | 9658 |
| Independent reflections | 5038 |
| Data/restraints/parameters | 5038/2/348 |
| Goodness-of-fit on F2 | 1.090 |
| Final R indexes [I ≥ 2σ(I)] | R1 = 0.0225, wR2 = 0.0619 |
| Final R indexes [all data] | R1 = 0.0259, wR2 = 0.0639 |
| Largest diff. peak/hole / e Å−3 | 1.298/−0.327 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.-A.; Shi, S.-S.; Han, S.-S.; Mei, J.; Chen, S.-S. Synthesis, Crystal Structures, and Properties of a New Supramolecular Polymer Based on Mixed Imidazole and Carboxylate Ligands. Crystals 2017, 7, 210. https://doi.org/10.3390/cryst7070210
Zhu M-A, Shi S-S, Han S-S, Mei J, Chen S-S. Synthesis, Crystal Structures, and Properties of a New Supramolecular Polymer Based on Mixed Imidazole and Carboxylate Ligands. Crystals. 2017; 7(7):210. https://doi.org/10.3390/cryst7070210
Chicago/Turabian StyleZhu, Mei-An, Shan-Shan Shi, Shuai-Shuai Han, Jin Mei, and Shui-Sheng Chen. 2017. "Synthesis, Crystal Structures, and Properties of a New Supramolecular Polymer Based on Mixed Imidazole and Carboxylate Ligands" Crystals 7, no. 7: 210. https://doi.org/10.3390/cryst7070210





