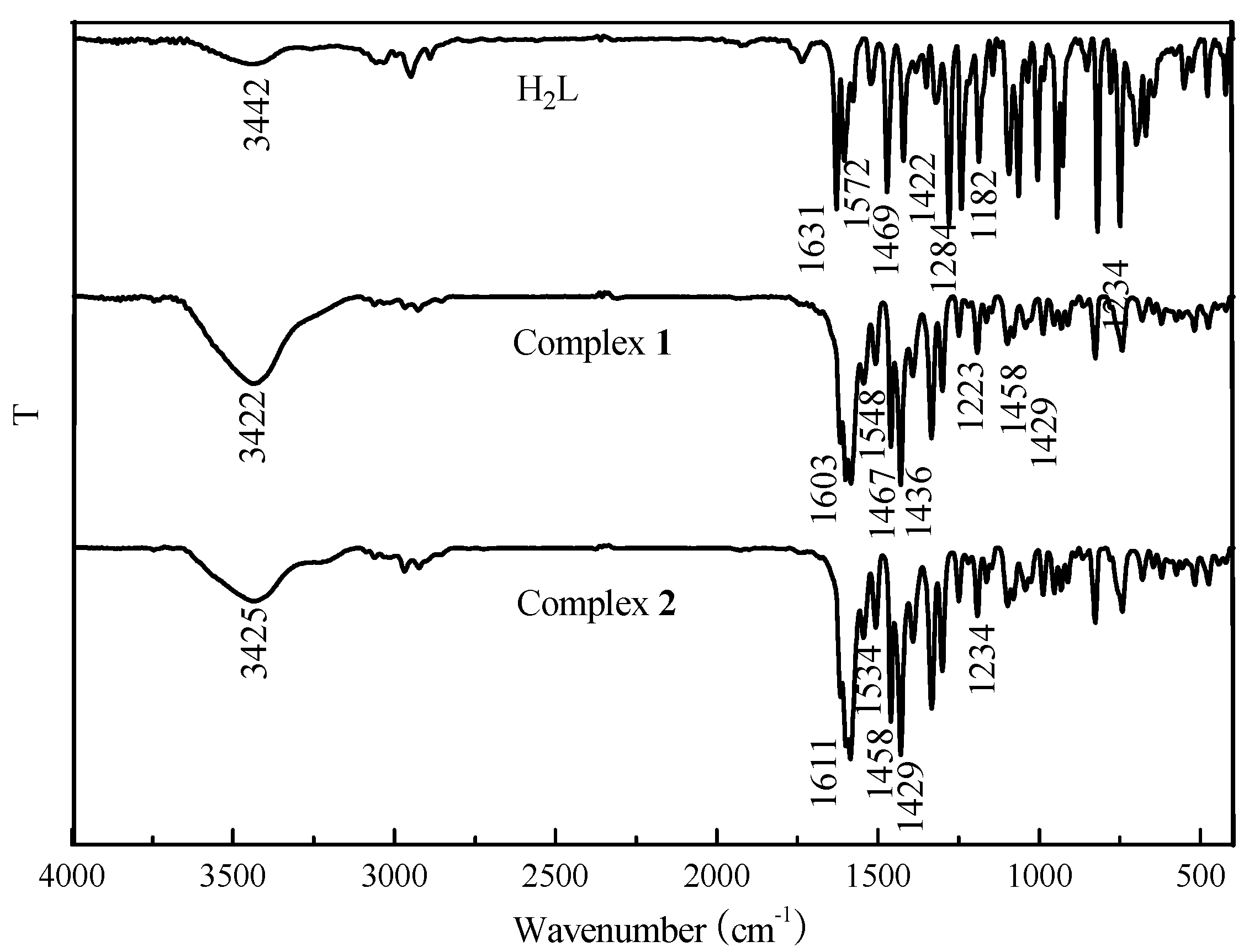
3.1. IR Spectra
The IR spectra of H
2L and its corresponding complexes
1 and
2 exhibit various bands in the 400–4000 cm
−1 region (
Figure 1). The most important IR bands are listed in
Table 2. In general, the O-H stretching frequency of most compounds is usually expected in the 3300–3800 cm
−1 region, but this frequency of the free ligand H
2L is displaced to 3437 cm
−1 because of the intramolecular hydrogen bond O-H···N=C interaction. The free ligand exhibits characteristic C=N stretching bands at 1631 cm
−1, while those of complexes
1 and
2 were observed at 1603 and 1611 cm
−1, respectively. The C=N stretching frequencies are all shifted to lower frequencies by 28 cm
−1 and 20 cm
−1 upon complexation, indicating a decrease in the C=N bond order due to the coordinated bond of the Co(II) atom with the imino nitrogen lone pair [
50]. The Ar–O stretching frequency appears as a strong band at 1284 cm
−1 for H
2L and at 1234 cm
−1 for complex
2. Meanwhile, a bending vibration of phenolic alcohol in H
2L at 1182 cm
−1, which disappears in the complexes, indicated the phenol hydroxy groups of H
2L were protonated and the oxygen atom coordinated to the Co(II) ions [
13,
15]. The Ar-O stretching frequency was shifted to a lower frequency, indicating that a Co-O bond had been formed between the Co(II) ion and phenolic oxygen atom of the ligand.
The far-infrared spectra of complexes
1 and
2 were also obtained in the region 500–100 cm
−1 in order to identify frequencies due to the Co–O and Co–N bonds. The FT-IR spectra of the complexes
1 and
2 showed ν(Co–N) and ν(Co–O) vibration absorption frequencies possibly at 452, 446 cm
−1 and 431, 422 cm
−1, respectively. But as pointed out by Percy and Thornton [
51], the metal–oxygen and metal–nitrogen frequency assignments are at times very difficult.
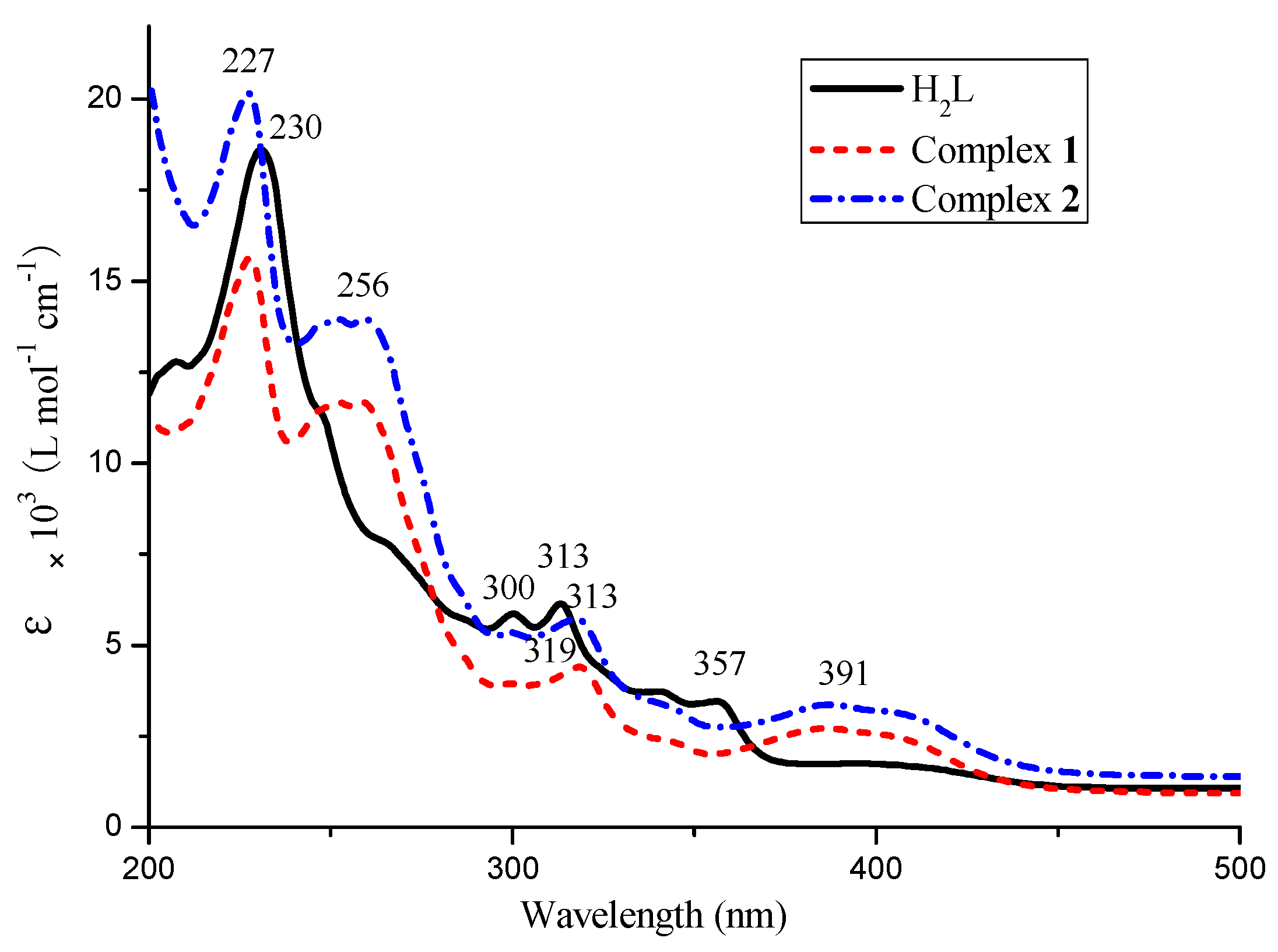
3.2. UV-Vis Spectra
The UV-Vis absorption spectra of H
2L and its corresponding complexes
1 and
2 in 5.0 × 10
−5 mol·L
−1 DMF solution are shown in
Figure 2. The electronic absorption spectrum of the free ligand H
2L consists of two relatively intense bands at 230 nm, 316 nm and one weak band at 357 nm, the first absorptions observed at 230 nm and 316 nm can be signed to the π-π* transition of the benzene rings, while the absorption peak at 357 nm was attributed to the intra-ligand π-π* transition of the oxime group [
52]. The complexes
1 and
2 show almost identical UV–Vis absorption spectra. The absorption bands around 313 nm are only marginally red-shifted (4–6 nm) in the spectra of the complexes. Upon coordination of the ligands, the absorption bands at about 357 nm disappeared and the new bands at 256 nm appeared in the UV-Vis spectra of the complexes
1 and
2, which indicates that the oxime nitrogen atoms are involved in coordination to Co(II) atoms [
53]. In addition, the other new absorption peak is observed at ca. 391 nm in Co(II) complexes, which is attributed to the M→L charge-transfer transition. This is characteristic of the transition metal complexes with Salen-type ligands [
54,
55].
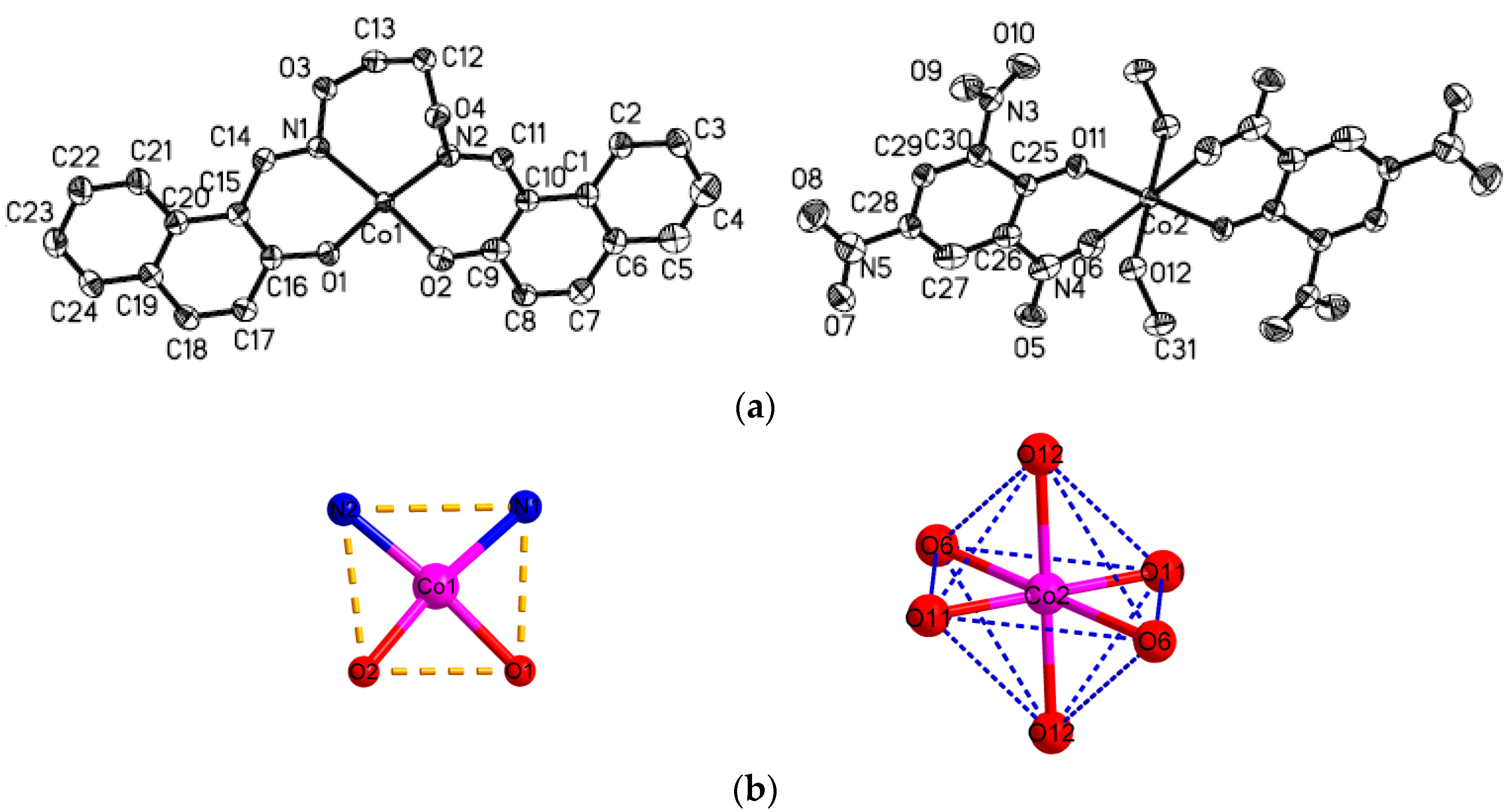
3.3. Crystal Structure of Complexes 1 and 2
The crystal structures of complexes
1 and
2 with an atom numbering scheme is exhibited in
Figure 3 and
Figure 4. The selected bond lengths and angles of complexes
1 and
2 are in
Table 3. The complex
1 crystallizes in the monoclinic system and
P2(1)/
n space group and Z = 1. The complex
1 consist of two [CoL] and one [Co(Pic)
2(CH
3OH)
2] molecules, and in [CoL] molecule, the Co1 atom is tetra-coordinated by two phenoxy O and two oxime N atoms from one ligand anion L
2−. The O1, O2, N1, N2 atoms from the same ligand anion consist of the square plane with the dihedral angle of N1-N2-O1and O1-O2-N1 of 2.30°, and the Co(II) atom deviates from the plane 0.069 Å. The Co–O/N bond distances in the square plane are in the range of 1.917(2) to 2.013(2) Å. Therefore, the local coordination geometry around the Co1 center can be described as a distorted square-planar as shown in
Figure 3b.
The [Co(Pic)
2(CH
3OH)
2] molecule is rigorously centrosymmetric, and contains one Co(II) center, two Pic
− and two coordinated methanol molecules. The Co(II) atom is hexa-coordinated by two phenoxy O and two nitro O atoms from two Pic
−, and the other two O atoms from two coordinated methanol molecules to form an octahedral geometry as shown in
Figure 3b. The O6, O11, O6
i, and O11
i atoms constitute the equatorial plane with the dihedral angle of O6-O6
i-O11 and O11-O11
i-O6 of 0.00°, and the Co(II) atom strays from the plane 0.00 Å. The Co-O/N bond distances in the equatorial plane are in the range of 1.904(2) to 2.335(2) Å. The axial positions are occupied by O12 and O12
i from methanol molecules with bond lengths of 1.993 Å. The
trans-coordination angles of O12-Co2-O12
i, O6
i-Co2-O6, O11
i-Co2-O11 are all 180.0°, whereas the
cis-coordination angles of O12-Co2-O6, O11-Co2-O6, O12-Co2-O11 are 82.92(6)°, 81.33(7)°, 89.82(7)°, respectively. Therefore, the local coordination geometry around the Co2 center can be described as a slightly distorted octahedron.
According to the above, this complex is very interesting from the structural point of view. First, complex
1 with the picrate anion contains in the crystal structurethe tetracoordinate molecule [Co(L)] with a distorted square planar coordination geometry. This geometry is rather rare for the Co(II) complexes, especially when the abundance of this topology is compared with the tetrahedral and distorted tetrahedral geometries. Second, in the CSD only five other examples of Co(II) complexes with coordinated picrate anions can be found. Two of them, the nitro oxygen atoms of the picrate anion were not involved in coordination with Co(II) ions and in the other three Co(II) complexes [
56,
57,
58], the coordination mode of picric anions is consistent with the coordination pattern reported in this paper. The Co-O(NO
2) bond lengths in complex
1 is 2.335 Ǻ, which is within the range of previously published Co(II) complexes involving coordinated picrate anion with that of 2.459 Å, 2.190 Å, 2.110 Å, and 2.149 Å [
57,
58,
59], respectively.
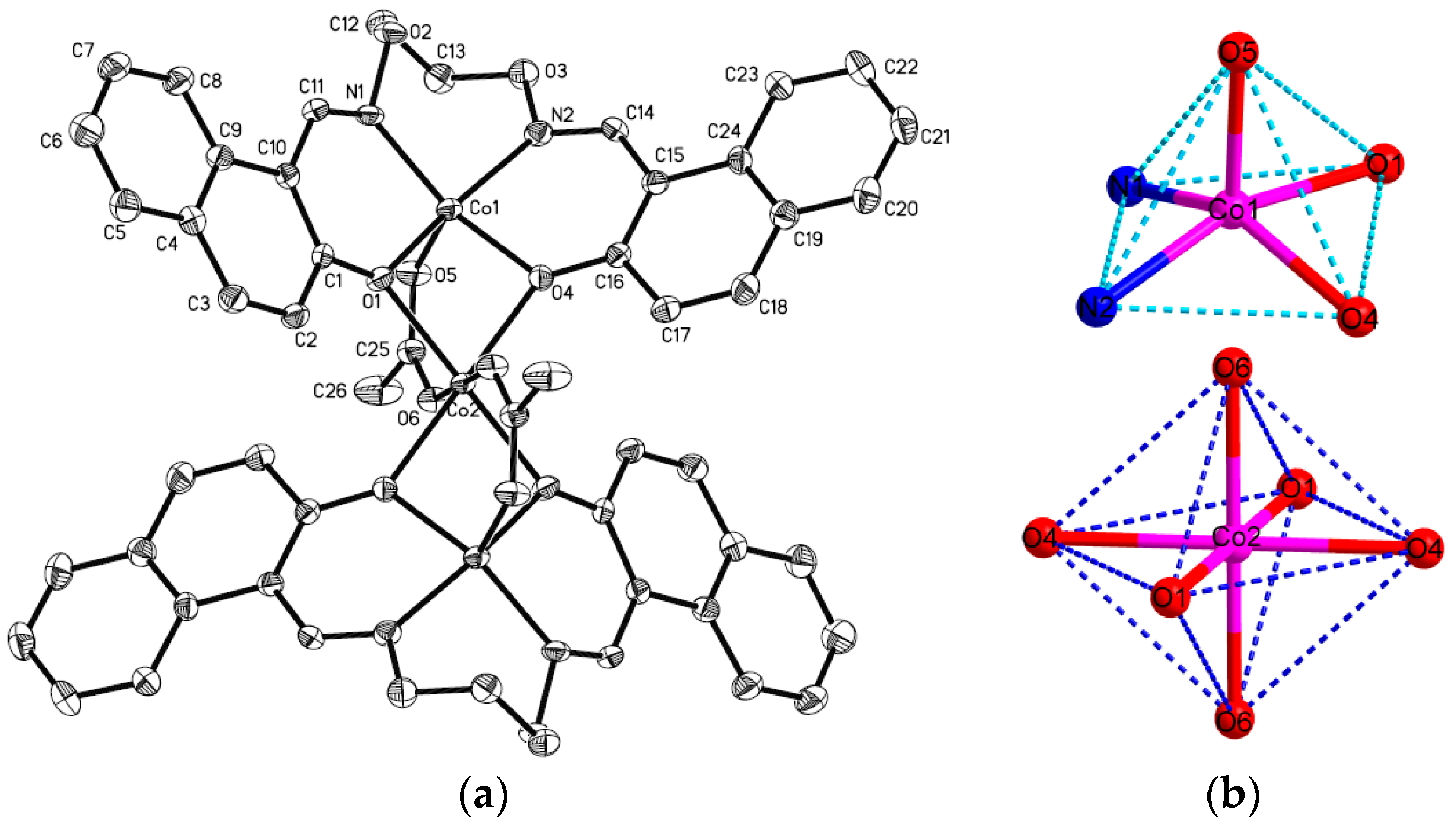
The complex
2 crystallizes in the triclinic system and
P-1 space group,
Z = 2. The symmetric [{CoL(
μ-OAc)}
2Co] unit consists of three Co(II) atoms, two ligand anion L
2− units, and two coordinated acetate ions. The terminal Co(II) atom (Co1 and Co1
i) is penta-coordinated by two nitrogen atoms (N1 and N2), two oxygen atoms (O1 and O4) in the N
2O
2 moiety of the L
2− unit, and one oxygen atom (O5) from the bridging acetate anion. Crystallographic data τ = 0.13 suggests a slightly distorted tetragonal pyramid coordination arrangement for the complex
2 [
59]. However, the coordination geometry of the hexa-coordinated central Co2 atom deviates slightly from an ideal octahedron. The Co2 atom has an O
2O
2 donor set from four
μ-phenoxo oxygen atoms (O1, O4, O1
i, O4
i) from two [CoL] chelates. Meanwhile, each of the two acetate anions bridges the terminal Co1 and central Co2 atoms in a
syn-
syn fashion. Hence the central Co2 atom finally has an O
2O
2O
2 donor set, in which the coordination sphere is completed by
μ-phenoxo oxygen atoms (O1, O4, O1
i, O4
i) from two [CoL] chelates, and both of oxygen atoms O6 and O6
i from the ligating acetate ions which adopt a familiar
μ-O-C-O fashion, and constitute a slightly distorted octahedral geometry. The Co2-O bond distances are in the range of 2.037(2) to 2.170(2) Å, the coordinated angles of O1-Co2-O1
i, O4-Co2-O4
i, and O6-Co2-O6
i are all 180°. Therefore, the local coordination geometry around the central Co2 atom can be described as deviating slightly from the ideal octahedron as shown in
Figure 4b. The trinuclear structure is stabilized by the two
μ-acetato ligands bridging Co2-Co1 and Co2-Co1
i with shorter separations of Co···Co (3.065 Å), which neutralize the whole charge of complex
2.
3.4. Supramolecular Interaction of Complexes 1 and 2
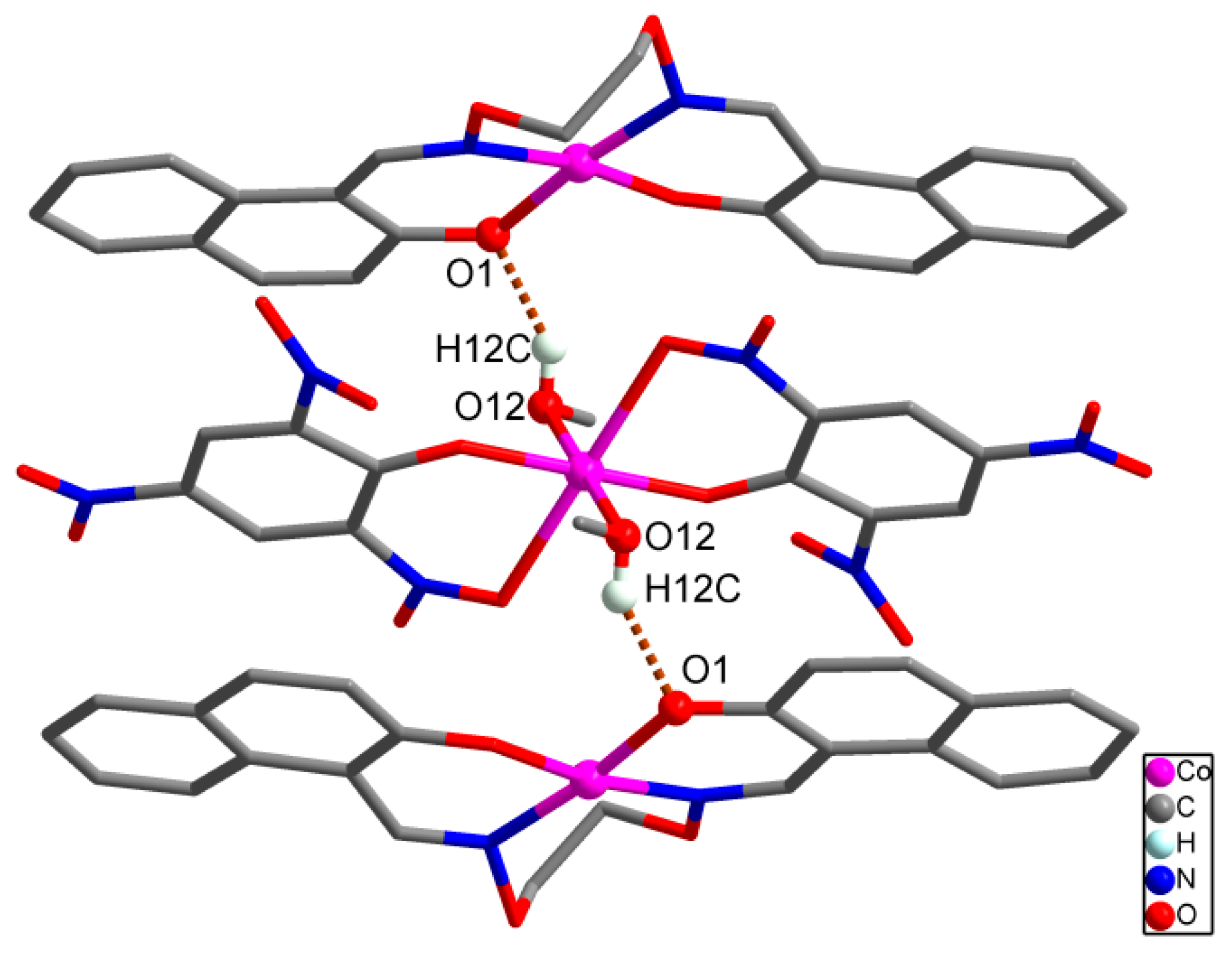
It should be noted that important intermolecular hydrogen bonding interactions exist between the molecules in complex
1 as listed in
Table 4. Firstly, intermolecular hydrogen bonds between the coordinated methanol molecules of [Co(Pic)
2(CH
3OH)
2] unit and oxygen atoms of the
μ-naphthoxo group in the ligand anion L
2− of [CoL] unit, O12-H12C···O1, are formed between the [CoL] and [Co(Pic)
2(CH
3OH)
2] units which link the neighboring one [Co(Pic)
2(CH
3OH)
2] unit and two [CoL] units into a tri-polymer as shown in
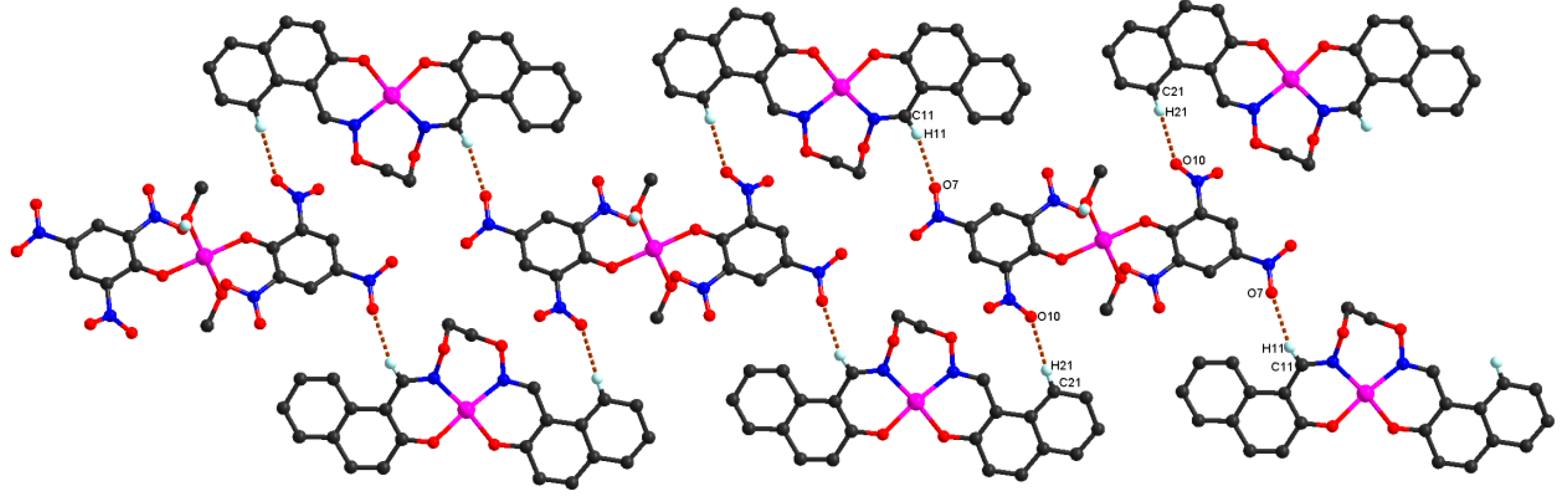
Figure 5. In addition, every tri-polymer further links eight other adjacent tri-polymer units into an infinite 3D–network supramolecular structure by four pairs of intermolecular C11-H11···O7 and C21-H21···O10 hydrogen bonds (
Figure 6) between the two -CH groups of the ligand anion L
2− in [CoL] units and the oxygen atoms of the picric acid anion Pic
− in [Co(Pic)
2(CH
3OH)
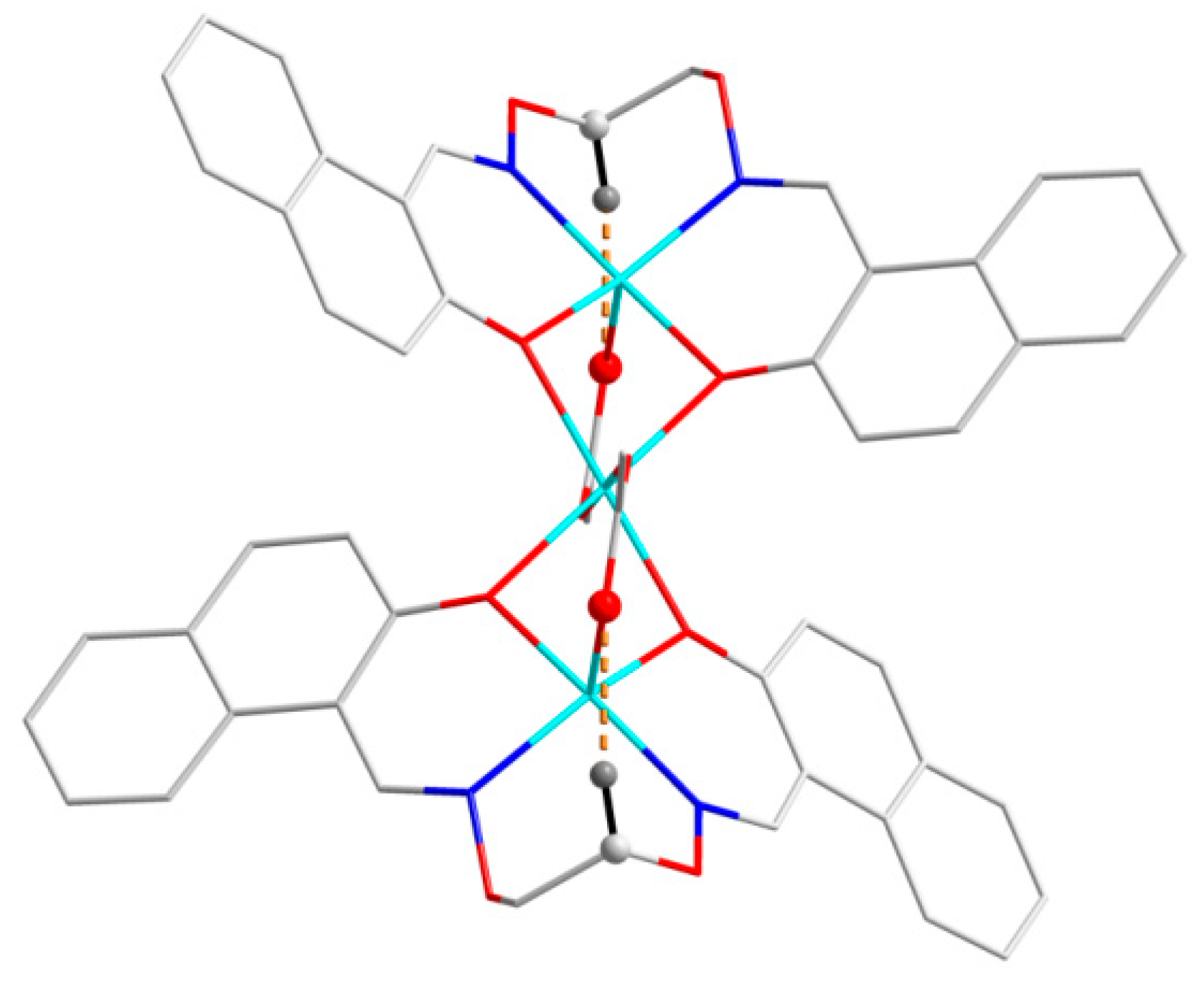
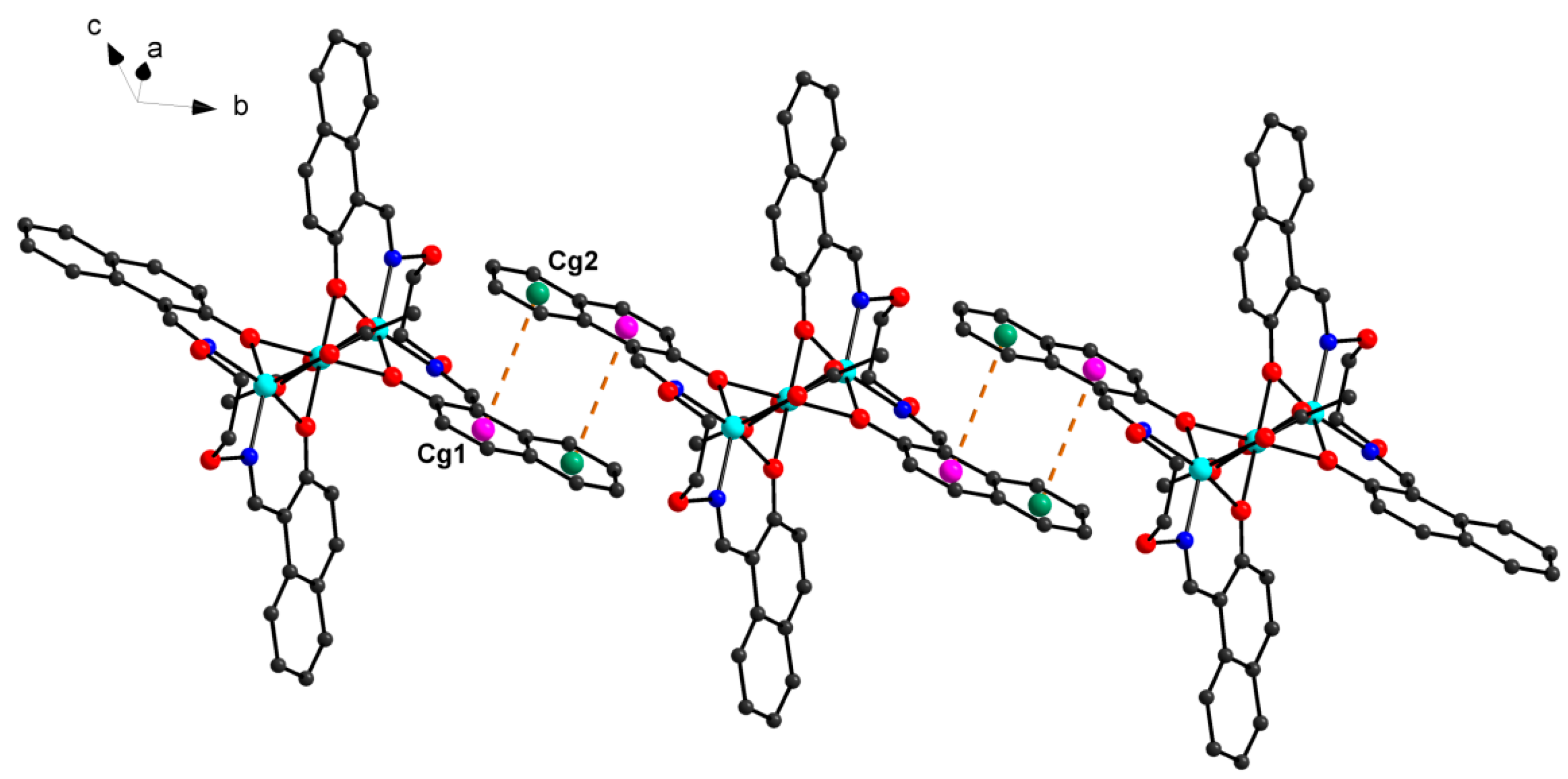
2] units, respectively. Furthermore, this linkage is further stabilized by the π···π stacking between the benzene ring (
Table 5 and
Figure 7) of the adjacent [CoL] and [Co(Pic)
2(CH
3OH)
2] units. Consequently, with the help of O-H···O and C-H···O hydrogen bonds, π···π stacking interactions, the crystal structure of
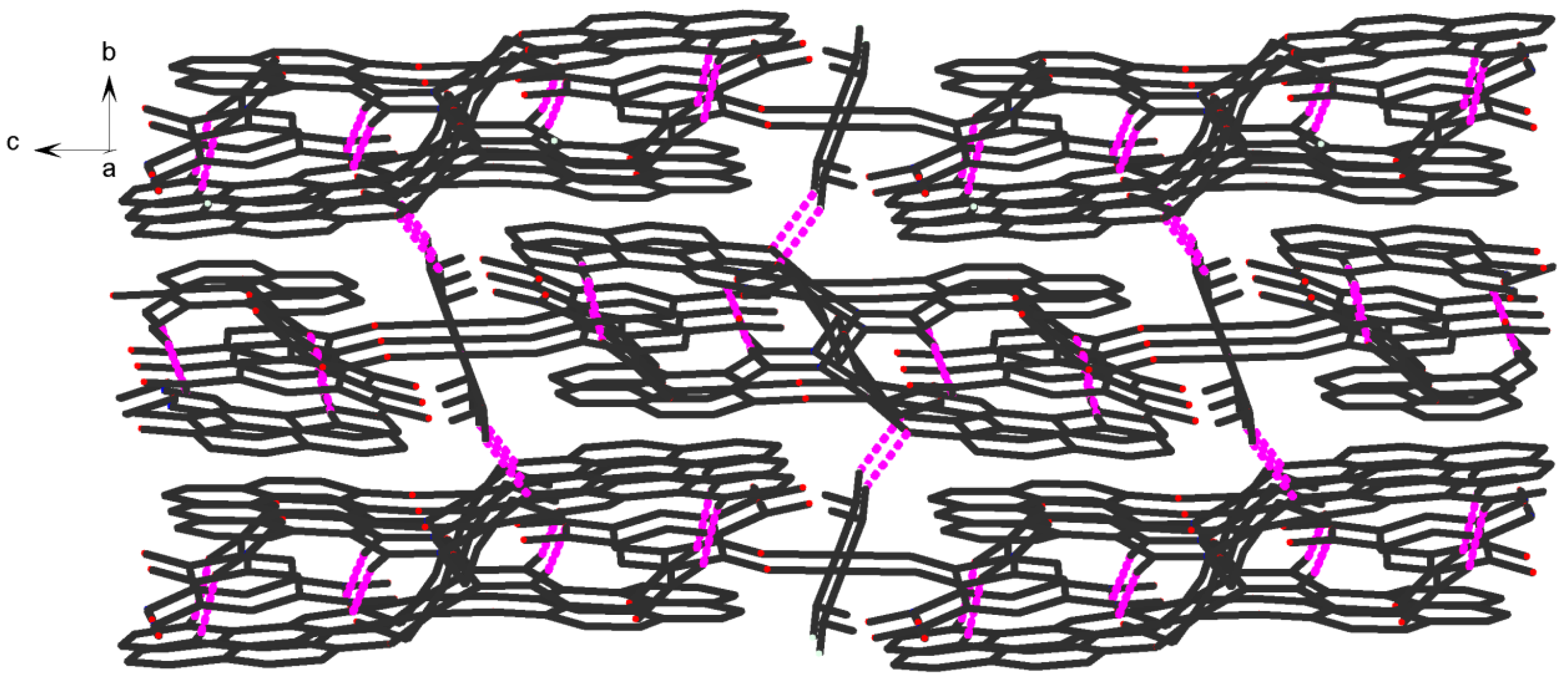
1 shows an assembly 3D supramolecular network structure (
Figure 8) introduced by a coordinated picrate.
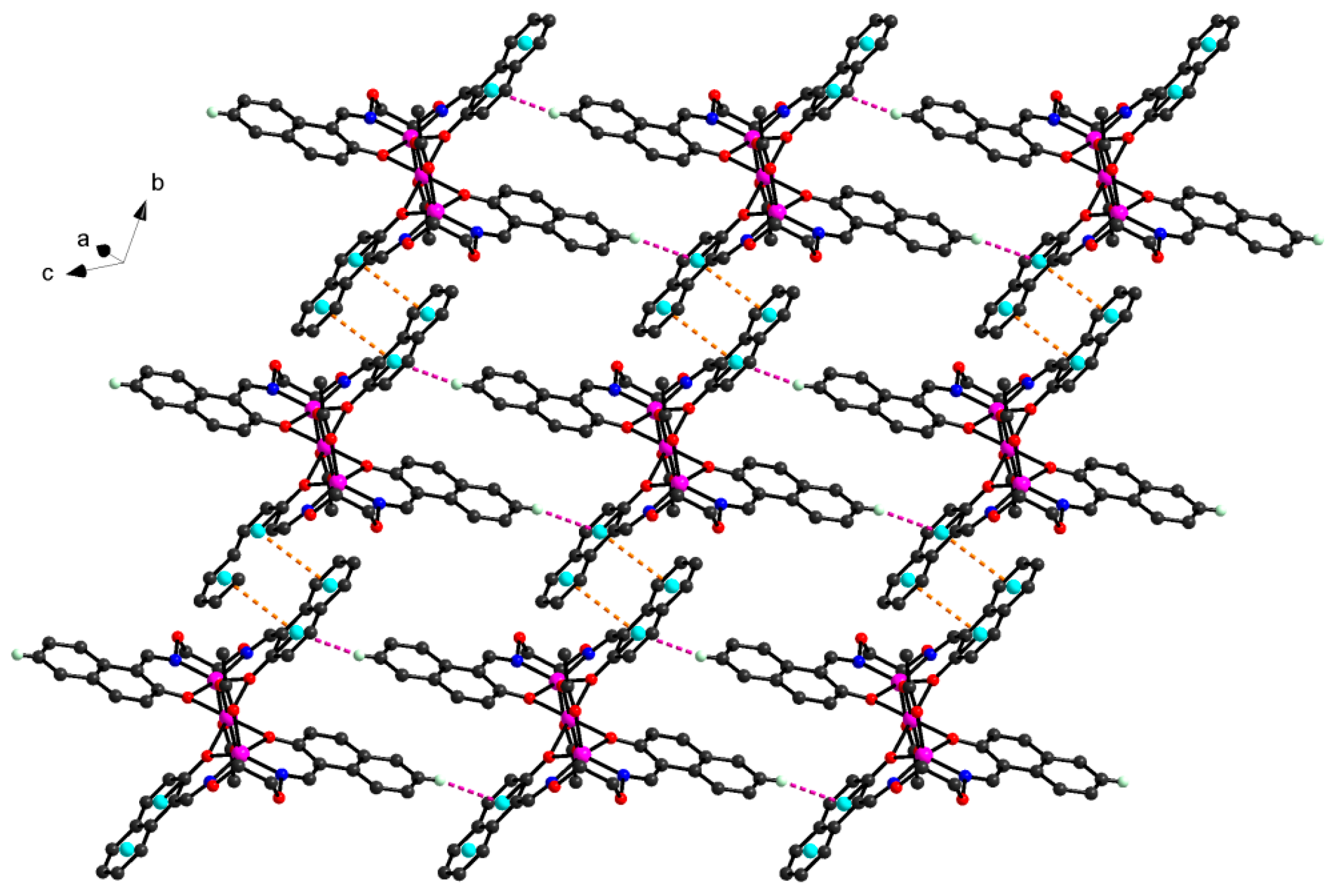
In the crystal structure of
2, a pair of intramolecular C13-H13A···O5 hydrogen bonds are formed between the oxygen (O5) atom of the coordinated acetate anion and the –C13H13A group of the O-alkyl chain of L
2− unit as shown in
Figure 9. The hydrogen bond data are given in
Table 6. Complex
2 is stabilized by intermolecular C6-H6···π
centroid(C19-C24) interactions between the -CH group of the benzene ring and the aromatic rings of L
2− unit linking the neighboring molecules into a 1D infinite chain parallel to the
c axis (
Figure 10). Synchronously, this linkage is further stabilized by a pair of intermolecular π
centroid(C15-C19,C24)-π
centroid(C19-C24) stacking interactions between the aromatic rings to form the other 1D infinite chain along
b axis (
Figure 11 and
Table 7). Then these two 1D chains interlink with each other resulting in the crystal packing of
2 showing a 2D-layer supramolecular structure parallel to the
bc-planes (
Figure 12).
3.5. Anion Effects
Obviously, coordination anions play an important part in the formation of the above different structures of Co(II) complexes. From the structure description, the free ligand H2L presents very different coordination modes when different anions are involved in the coordination with Co(II) atoms. Complexes 1 and 2 were prepared in exactly the same way from a mixture of ligand H2L with Co(Pic)2·6H2O and Co(OAc)2·4H2O in methanol solution, respectively, but the big structural differences in 1 and 2 suggest that the coordination anions indeed affect the ultimate structures of the assemblies: when the Pic− is involved in the coordination with Co(II) atoms, the mononuclear [CoL] unit, and the [Co(Pic)2(CH3OH)2] unit in the Co(II) complex 1 with distorted square-planar and slightly distorted octahedron coordination polyhedron formed, respectively. Meanwhile, the OAc− involved in the coordination with Co(II) atoms resulted in a trinuclear structure with the acetate ions coordinated to three Co(II) atoms through Co-O-C-O-Co bridges, possessing two distorted square pyramidal and an octahedral geometry around the central Co(II) atom. As a unique structure of the tetradentate coordination environment of N2O2, Salamo-type ligands have very good coordination ability. However, for some of the transition metals, the coordination still has some limitations, thus counter anions can easily coordinate with metal ions. In this article, the Pic− in complex 1 has a larger volume which has a hard coordination with metal ions. While, the OAc− in 2 has less steric hindrance than 1, it can easily coordinate to metal ions acting as a second ligand.