Prussian Blue Analogue Mesoframes for Enhanced Aqueous Sodium-ion Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Cubic Crystals of 1
2.3. Synthesis of Mesoframes of 1
2.4. Characterization
2.5. Electrochemical Measurement
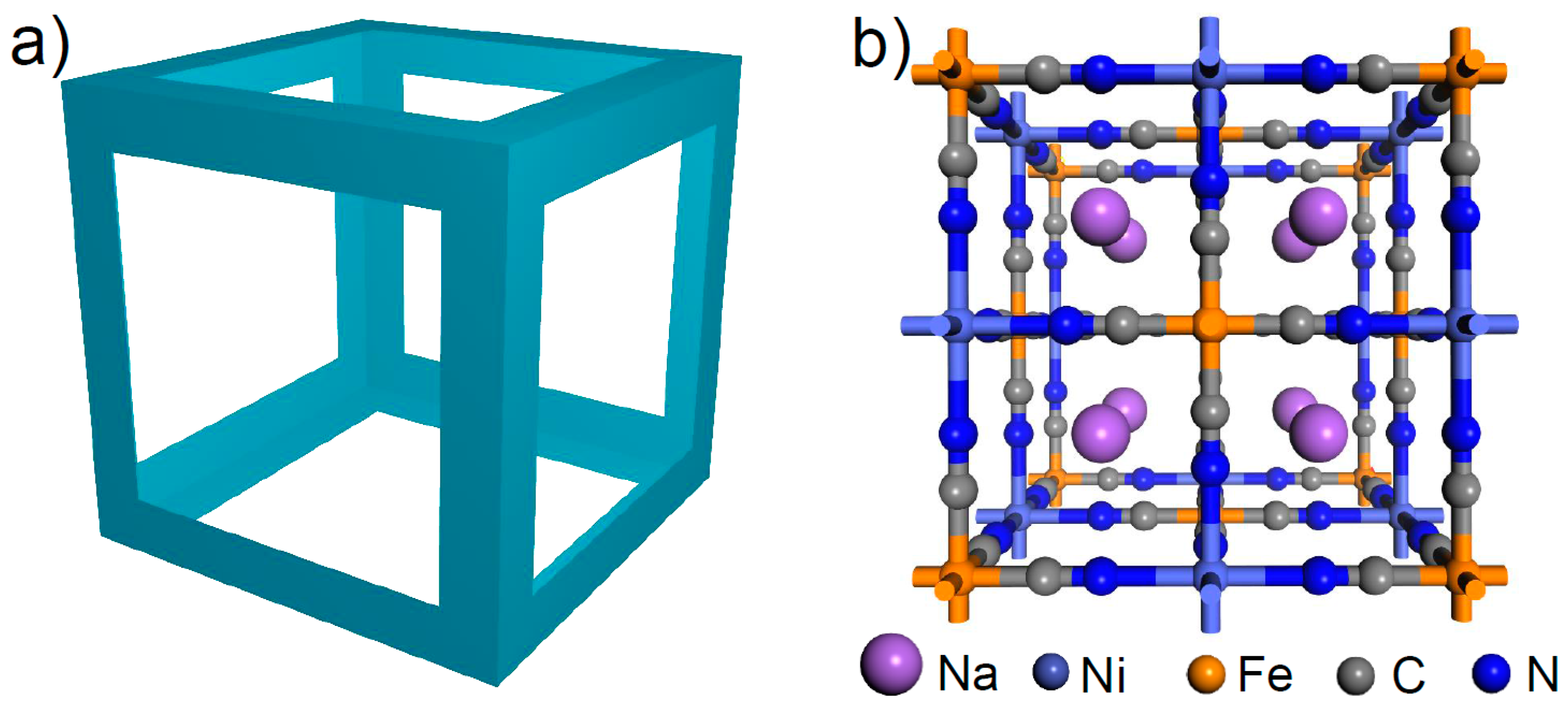
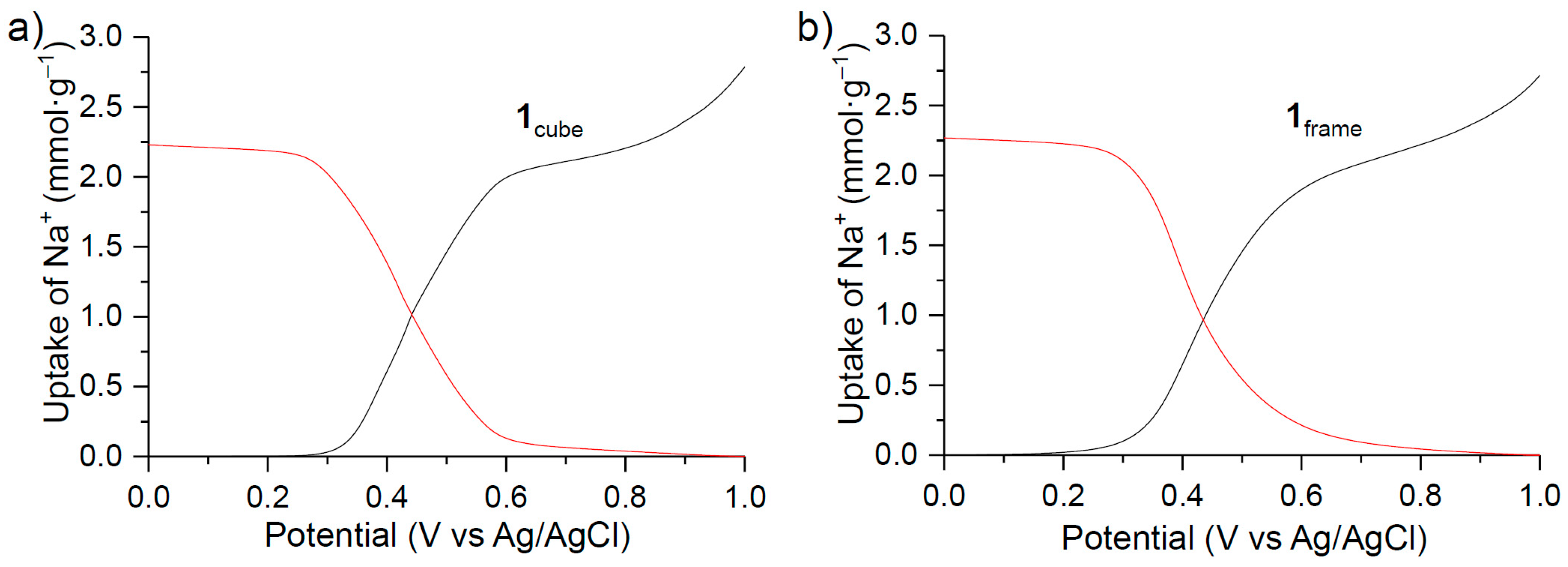
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- James, S.L. Metal-organic frameworks. Chem. Soc. Rev. 2003, 32, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Rowsell, J.L.C.; Yaghi, O.M. Metal-organic frameworks: A new class of porous materials. Micropor. Mesopor. Mater. 2004, 73, 3–14. [Google Scholar] [CrossRef]
- Eddaoudi, M.; Kim, J.; Rosi, N.; Vodak, D.; Wachter, J.; O’Keeffe, M.; Yaghi, O.M. Systematic design of pore size and functionality in isoreticular MOFs and their application in methane storage. Science 2002, 295, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Kitagawa, S.; Kitaura, R.; Noro, S. Functional porous coordination polymers. Angew. Chem. Int. Ed. 2004, 43, 2334–2375. [Google Scholar] [CrossRef] [PubMed]
- Chui, S.S.Y.; Lo, S.M.F.; Charmant, J.P.H.; Orpen, A.G.; Williams, I.D. A chemically functionalizable nanoporous material [Cu3(TMA)2(H2O)3]n. Science 1999, 283, 1148–1150. [Google Scholar] [CrossRef] [PubMed]
- Hwang, Y.K.; Hong, D.-Y.; Chang, J.-S.; Jhung, S.H.; Seo, Y.-K.; Kim, J.; Vimont, A.; Daturi, M.; Serre, C.; Ferey, G. Amine grafting on coordinatively unsaturated metal centers of MOFs: Consequences for catalysis and metal encapsulation. Angew. Chem. Int. Ed. 2008, 47, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Dan-Hardi, M.; Serre, C.; Frot, T.; Rozes, L.; Maurin, G.; Sanchez, C.; Ferey, G. A new photoactive crystalline highly porous titanium(iv) dicarboxylate. J. Am. Chem. Soc. 2009, 131, 10857–10859. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Hirai, K.; Nakagawa, K.; Takashima, Y.; Matsuda, R.; Tsuruoka, T.; Kondo, M.; Haruki, R.; Tanaka, D.; Sakamoto, H.; et al. Heterogeneously hybridized porous coordination polymer crystals: fabrication of heterometallic core-shell single crystals with an in-plane rotational epitaxial relationship. Angew. Chem. Int. Ed. 2009, 48, 1766–1770. [Google Scholar] [CrossRef] [PubMed]
- Salles, F.; Maurin, G.; Serre, C.; Llewellyn, P.L.; Knoefel, C.; Choi, H.J.; Filinchuk, Y.; Oliviero, L.; Vimont, A.; Long, J.R.; et al. Multistep n-2 breathing in the metal-organic framework co(1,4-benzenedipyrazolate). J. Am. Chem. Soc. 2010, 132, 13782–13788. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Reboul, J.; Diring, S.; Sumida, K.; Kitagawa, S. Structuring of metal-organic frameworks at the mesoscopic/macroscopic scale. Chem. Soc. Rev. 2014, 43, 5700–5734. [Google Scholar] [CrossRef] [PubMed]
- Rungtaweevoranit, B.; Zhao, Y.; Choi, K.M.; Yaghi, O.M. Cooperative effects at the interface of nanocrystalline metal-organic frameworks. Nano Res. 2016, 9, 47–58. [Google Scholar] [CrossRef]
- Hu, M.; Belik, A.A.; Imura, M.; Yamauchi, Y. Tailored design of multiple nanoarchitectures in metal-cyanide hybrid coordination polymers. J. Am. Chem. Soc. 2013, 135, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Wang, L.; Irran, E.; Yu, H.; Gao, J.; Fan, D.; Li, B.; Wang, J.; Ding, W.; Amin, A.M.; et al. Hollow ferrocenyl coordination polymer microspheres with micropores in shells prepared by ostwald ripening. Angew. Chem. Int. Ed. 2010, 49, 9237–9241. [Google Scholar] [CrossRef] [PubMed]
- Ameloot, R.; Vermoortele, F.; Vanhove, W.; Roeffaers, M.B.J.; Sels, B.F.; De Vos, D.E. Interfacial synthesis of hollow metal-organic framework capsules demonstrating selective permeability. Nat. Chem. 2011, 3, 382–387. [Google Scholar] [CrossRef] [PubMed]
- Brinzei, D.; Catala, L.; Mathoniere, C.; Wernsdorfer, W.; Gloter, A.; Stephan, O.; Mallah, T. Photoinduced superparamagnetism in trimetallic coordination nanoparticles. J. Am. Chem. Soc. 2007, 129, 3778–3779. [Google Scholar] [CrossRef] [PubMed]
- Volatron, F.; Catala, L.; Riviere, E.; Gloter, A.; Stephan, O.; Mallah, T. Spin-crossover coordination nanoparticles. Inorg. Chem. 2008, 47, 6584–6586. [Google Scholar] [CrossRef] [PubMed]
- Catala, L.; Brinzei, D.; Prado, Y.; Gloter, A.; Stephan, O.; Rogez, G.; Mallah, T. Core-multishell magnetic coordination nanoparticles: Toward multifunctionality on the nanoscale. Angew. Chem. Int. Ed. 2009, 48, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Diring, S.; Furukawa, S.; Takashima, Y.; Tsuruoka, T.; Kitagawa, S. Controlled multiscale synthesis of porous coordination polymer in nano/micro regimes. Chem. Mater. 2010, 22, 4531–4538. [Google Scholar] [CrossRef]
- Doherty, C.M.; Buso, D.; Hill, A.J.; Furukawa, S.; Kitagawa, S.; Falcaro, P. Using functional nano- and microparticles for the preparation of metal-organic framework composites with novel properties. Accounts Chem. Res. 2014, 47, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Torad, N.L.K.; Chiang, Y.-D.; Wu, K.C.W.; Yamauchi, Y. Size- and shape-controlled synthesis of Prussian Blue nanoparticles by a polyvinylpyrrolidone-assisted crystallization process. CrystEngComm 2012, 14, 3387–3396. [Google Scholar]
- Fan, L.; Chen, H.; Xiao, D.; Wang, E. Synthesis, structure, and characterization of a new metal-organic framework containing meso-helices. Z. Anorg. Allg. Chem. 2013, 639, 558–562. [Google Scholar] [CrossRef]
- Zhang, W.; Zhao, Y.; Malgras, V.; Ariga, K.; Yamauchi, Y.; Liu, J.; Jiang, J.S.; Hu, M. Synthesis of monocrystalline nanoframes of prussian blue analogues by controlled preferential etching. Angew. Chem. Int. Ed. 2016, 55, 8228–8234. [Google Scholar] [CrossRef] [PubMed]
- Ariga, K.; Malgras, V.; Ji, Q.; Zakaria, M.B.; Yamauchi, Y. Coordination nanoarchitectonics at interfaces between supramolecular and materials chemistry. Coordin. Chem. Rev. 2016, 320, 139–152. [Google Scholar] [CrossRef]
- Felts, A.C.; Andrus, M.J.; Knowles, E.S.; Quintero, P.K.; Ahir, A.R.; Risset, O.N.; Li, C.H.; Maurin, I.; Halder, G.J.; Abboud, K.A.; et al. Evidence for interface-induced strain and its influence on photomagnetism in prussian blue analogue core-shell heterostructures, RbaCob Fe(CN)(6) (c)center dot mH(2)O@KjNik Cr(CN)(6) (l)center dot nH(2)O. J. Phys. Chem. C 2016, 120, 5420–5429. [Google Scholar] [CrossRef]
- Yue, Y.; Zhang, Z.; Binder, A.J.; Chen, J.; Jin, X.; Overbury, S.H.; Dai, S. Hierarchically superstructured prussian blue analogues: Spontaneous assembly synthesis and applications as pseudocapacitive materials. Chem. Sus. Chem. 2015, 8, 177–183. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.S.; Zakaria, M.B.; Park, M.-S.; Alowasheeir, A.; Alshehri, S.M.; Yamauchi, Y.; Kim, H. Dual-textured Prussian Blue nanocubes as sodium ion storage materials. Electrochim. Acta 2017, 240, 300–306. [Google Scholar] [CrossRef]
- Yue, Y.; Binder, A.J.; Guo, B.; Zhang, Z.; Qiao, Z.A.; Tian, C.; Dai, S. Mesoporous Prussian blue analogues: Template-free synthesis and sodium-ion battery applications. Angew. Chem. Int. Ed. 2014, 53, 3134–3137. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Au, L.; McLellan, J.; Li, Z.-Y.; Marquez, M.; Xia, Y. Fabrication of cubic nanocages and nanoframes by dealloying Au/Ag alloy nanoboxes with an aqueous etchant based on Fe(NO3)(3) or NH4OH. Nano lett. 2007, 7, 1764–1769. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; El-Sayed, M.A. Aggregation of gold nanoframes reduces, rather than enhances, SERS efficiency due to the trade-off of the inter- and intraparticle plasmonic fields. Nano lett. 2009, 9, 3025–3031. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, M.A.; El-Sayed, M.A. Gold nanoframes: Very high surface plasmon fields and excellent near-infrared sensors. J. Am. Chem. Soc. 2010, 132, 12704–12710. [Google Scholar] [CrossRef] [PubMed]
- Sui, Y.; Fu, W.; Zeng, Y.; Yang, H.; Zhang, Y.; Chen, H.; Li, Y.; Li, M.; Zou, G. Synthesis of Cu2O nanoframes and nanocages by selective oxidative etching at room temperature. Angew. Chem. Int. Ed. 2010, 49, 4282–4285. [Google Scholar] [CrossRef] [PubMed]
- McEachran, M.; Keogh, D.; Pietrobon, B.; Cathcart, N.; Gourevich, I.; Coombs, N.; Kitaev, V. Ultrathin gold nanoframes through surfactant-free templating of faceted pentagonal silver nanoparticles. J. Am. Chem. Soc. 2011, 133, 8066–8069. [Google Scholar] [CrossRef] [PubMed]
- Hong, X.; Wang, D.; Cai, S.; Rong, H.; Li, Y. Single-crystalline octahedral au-ag nanoframes. J. Am. Chem. Soc. 2012, 134, 18165–18168. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.; Lu, N.; Xie, Z.; Wang, J.; Kim, M.J.; Xia, Y. Synthesis of Pd-Rh core-frame concave nanocubes and their conversion to rh cubic nanoframes by selective etching of the Pd cores. Angew. Chem. Int. Ed. 2012, 51, 10266–10270. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Kang, Y.; Huo, Z.; Zhu, Z.; Huang, W.; Xin, H.L.; Snyder, J.D.; Li, D.; Herron, J.A.; Mavrikakis, M.; et al. Highly crystalline multimetallic nanoframes with three-dimensional electrocatalytic surfaces. Science 2014, 343, 1339–1343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Roling, L.T.; Wang, X.; Vara, M.; Chi, M.; Liu, J.; Choi, S.-I.; Park, J.; Herron, J.A.; Xie, Z.; et al. Platinum-based nanocages with subnanometer-thick walls and well-defined, controllable facets. Science 2015, 349, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Wessells, C.D.; McDowell, M.T.; Peddada, S.V.; Pasta, M.; Huggins, R.A.; Cui, Y. Tunable reaction potentials in open framework nanoparticle battery electrodes for grid-scale energy storage. Acs Nano 2012, 6, 1688–1694. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Liu, N.; Nelson, J.; McDowell, M.T.; Huggins, R.A.; Toney, M.F.; Cui, Y. Full open-framework batteries for stationary energy storage. Nat. Commun. 2014, 5, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.Y.; Wessells, C.D.; Huggins, R.A.; Cui, Y. Highly reversible open framework nanoscale electrodes for divalent ion batteries. Nano lett. 2013, 13, 5748–5752. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-W.; Wang, R.Y.; Pasta, M.; Lee, S.W.; Liu, N.; Cui, Y. Manganese hexacyanomanganate open framework as a high-capacity positive electrode material for sodium-ion batteries. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed]
- Chae, M.S.; Hyoung, J.; Jang, M.; Lee, H.; Hong, S.-T. Potassium nickel hexacyanoferrate as a high-voltage cathode material for nonaqueous magnesium-ion batteries. J. Power Sources 2017, 363, 269–276. [Google Scholar] [CrossRef]
- Okubo, M.; Li, C.H.; Talham, D.R. High rate sodium ion insertion into core-shell nanoparticles of Prussian blue analogues. Chem. Commun. 2014, 50, 1353–1355. [Google Scholar] [CrossRef] [PubMed]
- Tang, W.; Zhu, Y.; Hou, Y.; Liu, L.; Wu, Y.; Loh, K.P.; Zhang, H.; Zhu, K. Aqueous rechargeable lithium batteries as an energy storage system of superfast charging. Energ. Environ. Sci. 2013, 6, 2093–2104. [Google Scholar] [CrossRef]
- Zhao, F.; Wang, Y.; Xu, X.; Liu, Y.; Song, R.; Lu, G.; Li, Y. Cobalt hexacyanoferrate nanoparticles as a high-rate and ultra-stable supercapacitor electrode material. ACS Appl. Mater. Inter. 2014, 6, 11007–11012. [Google Scholar] [CrossRef] [PubMed]
- Pasta, M.; Wessells, C.D.; Huggins, R.A.; Cui, Y. A high-rate and long cycle life aqueous electrolyte battery for grid-scale energy storage. Nat. Commun. 2012, 3, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Wessells, C.D.; Huggins, R.A.; Cui, Y. Copper hexacyanoferrate battery electrodes with long cycle life and high power. Nat. Commun. 2011, 2, 550–554. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Huang, Y.; Xie, M.; Zhang, Q.; Zhang, X.; Li, L.; Wu, F. Preparation of Prussian Blue submicron particles with a pore structure by two-step optimization for Na-ion battery cathodes. ACS Appl. Mater. Inter. 2016, 8, 16078–16086. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Lu, Y.; Liu, J.; Xu, M.; Cheng, J.; Zhang, D.; Goodenough, J.B. A superior low-cost cathode for a Na-ion battery. Angew. Chem. Int. Ed. 2013, 52, 1964–1967. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. High-quality Prussian blue crystals as superior cathode materials for room-temperature sodium-ion batteries. Energ. Environ. Sci. 2014, 7, 1643–1647. [Google Scholar] [CrossRef]
- Liu, Y.; Qiao, Y.; Zhang, W.; Li, Z.; Ji, X.; Miao, L.; Yuan, L.; Hu, X.; Huang, Y. Sodium storage in Na-rich NaxFeFe (CN)(6) nanocubes. Nano Energy 2015, 12, 386–393. [Google Scholar] [CrossRef]
- You, Y.; Yu, X.; Yin, Y.; Nam, K.-W.; Guo, Y.-G. Sodium iron hexacyanoferrate with high Na content as a Na-rich cathode material for Na-ion batteries. Nano Res. 2014, 8, 117–128. [Google Scholar] [CrossRef]
- Wessells, C.D.; Peddada, S.V.; Huggins, R.A.; Cui, Y. Nickel hexacyanoferrate nanoparticle electrodes for aqueous sodium and potassium ion batteries. Nano Lett. 2011, 11, 5421–5425. [Google Scholar] [CrossRef] [PubMed]
- Targholi, E.; Mousavi-Khoshdel, S.M.; Rahmanifara, M.; Yahya, M.Z.A. Cu- and Fe-hexacyanoferrate as cathode materials for Potassium ion battery: A First-principles study. Chem. Phys. Lett. 2017, 687, 244–249. [Google Scholar] [CrossRef]
- Lu, Y.; Wang, L.; Cheng, J.; Goodenough, J.B. Prussian blue: A new framework of electrode materials for sodium batteries. Chem. Commun. 2012, 48, 6544–6546. [Google Scholar] [CrossRef] [PubMed]
- Wessells, C.D.; Peddada, S.V.; McDowell, M.T.; Huggins, R.A.; Cui, Y. The effect of insertion species on nanostructured open framework hexacyanoferrate battery electrodes. J. Electrochem. Soc. 2012, 159, 98–103. [Google Scholar] [CrossRef]
- Kim, D.; Hwang, T.; Lim, J.-M.; Park, M.-S.; Cho, M.; Cho, K. Hexacyanometallates for sodium-ion batteries: Insights into higher redox potentials using d electronic spin configurations. Phys. Chem. Chem.l Phys. 2017, 19, 10443–10452. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, D.; Han, R.; Wei, G.; Qiao, Y. Nanostructured potassium and sodium ion incorporated Prussian blue frameworks as cathode materials for sodium-ion batteries. Chem. Commun. 2017, 53, 5569–5572. [Google Scholar] [CrossRef] [PubMed]
- Liang, G.; Xu, J.; Wang, X. Synthesis and characterization of organometallic coordination polymer nanoshells of Prussian blue using miniemulsion periphery polymerization (MEPP). J. Am. Chem. Soc. 2009, 131, 5378–5379. [Google Scholar] [CrossRef] [PubMed]
- McHale, R.; Ghasdian, N.; Liu, Y.; Ward, M.B.; Hondow, N.S.; Wang, H.; Miao, Y.; Brydson, R.; Wang, X. Prussian blue coordination polymer nanobox synthesis using miniemulsion periphery polymerization (MEPP). Chem. Commun. 2010, 46, 4574–4576. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Jiang, J.S. Facile synthesis of air-stable Prussian white microcubes via a hydrothermal method. Mater. Res. Bull. 2011, 46, 702–707. [Google Scholar] [CrossRef]
- McHale, R.; Liu, Y.; Ghasdian, N.; Hondow, N.S.; Ye, S.; Lu, Y.; Brydson, R.; Wang, X. Dual lanthanide role in the designed synthesis of hollow metal coordination (Prussian Blue analogue) nanocages with large internal cavity and mesoporous cage. Nanoscale 2011, 3, 3685–3694. [Google Scholar] [CrossRef] [PubMed]
- Roy, X.; Hui, J.K.H.; Rabnawaz, M.; Liu, G.; MacLachlan, M.J. Prussian blue nanocontainers: selectively permeable hollow metal-organic capsules from block lonomer emulsion-induced assembly. J. Am. Chem. Soc. 2011, 133, 8420–8423. [Google Scholar] [CrossRef] [PubMed]
- Risset, O.N.; Knowles, E.S.; Ma, S.; Meisel, M.W.; Talham, D.R. RbjMk Fe(CN)(6) (l) (M = Co, Ni) Prussian blue analogue hollow nanocubes: A new example of a multilevel pore system. Chem. Mater. 2013, 25, 42–47. [Google Scholar] [CrossRef]
- Shen, Q.; Jiang, J.; Fan, M.; Liu, S.; Wang, L.; Fan, Q.; Huang, W. Prussian blue hollow nanostructures: Sacrificial template synthesis and application in hydrogen peroxide sensing. J. Electroanal. Chem. 2014, 712, 132–138. [Google Scholar] [CrossRef]
- Hu, M.; Jiang, J.-S.; Zeng, Y. Prussian blue microcrystals prepared by selective etching and their conversion to mesoporous magnetic iron(III) oxides. Chem. Commun. 2010, 46, 1133–1135. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Torad, N.L.; Yamauchi, Y. Preparation of Various Prussian Blue analogue hollow nanocubes with single crystalline shells. Eur. J. Inorg. Chem. 2012, 30, 4795–4799. [Google Scholar] [CrossRef]
- Lee, S.-H.; Huh, Y.-D. Preferential evolution of Prussian blue’s morphology from cube to hexapod. B. Kor. Chem. Soc. 2012, 33, 1078–1080. [Google Scholar] [CrossRef]
- Song, Y.; He, J.; Wu, H.; Li, X.; Yu, J.; Zhang, Y.; Wang, L. Preparation of porous hollow CoOx nanocubes via chemical etching prussian blue analogue for glucose sensing. Electrochim. Acta 2015, 182, 165–172. [Google Scholar] [CrossRef]
- Han, L.; Yu, T.; Lei, W.; Liu, W.; Feng, K.; Ding, Y.; Jiang, G.; Xu, P.; Chen, Z. Nitrogen-doped carbon nanocones encapsulating with nickel-cobalt mixed phosphides for enhanced hydrogen evolution reaction. J. Mater. Chem. A 2017, 5, 16568–16572. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, G.; Ma, M.; Qiao, Y. Role of acid in tailoring Prussian blue as cathode for high-performance sodium-ion battery. Chem. Eur. J. 2017, 23, 15991–15996. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Xu, X.; Ji, Q.; Liu, R.; Liu, H.; Qiu, J.; Li, J. Porous nanobimetallic Fe-Mn cubes with high valent mn and highly efficient removal of arsenic(III). ACS Appl. Mater. Inter. 2017, 9, 14868–14877. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Furukawa, S.; Ohtani, R.; Sukegawa, H.; Nemoto, Y.; Reboul, J.; Kitagawa, S.; Yamauchi, Y. Synthesis of Prussian blue nanoparticles with a hollow interior by controlled chemical etching. Angew. Chem. Int. Ed. 2012, 51, 984–988. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhang, F.; Lu, H.; Hong, X.; Jiang, H.; Wu, Y.; Li, Y. Hollow Zn/Co ZIF particles derived from core-shell zif-67@zif-8 as selective catalyst for the semi-hydrogenation of acetylene. Angew. Chem. Int. Ed. 2015, 54, 10889–10893. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Qin, M.; Zhu, Z.; Yan, M.; Li, Q.; Zhang, L.; Liu, D.; Mai, L. Activation of sodium storage sites in prussian blue analogues via surface etching. Nano lett. 2017, 17, 4713–4718. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Yu, X.-Y.; Lou, X.W. Formation of Prussian-blue-analog nanocages via a direct etching method and their conversion into Ni-Co-Mixed oxide for enhanced oxygen evolution. Adv. Mater. 2016, 28, 4601–4605. [Google Scholar] [CrossRef] [PubMed]
- Ahirwar, P.; Clark, S.P.R.; Patel, V.; Rotter, T.J.; Hains, C.; Albrecht, A.; Dawson, L.R.; Balakrishnan, G. Perforated (In)GaSb quantum wells on GaSb substrates through the use of As(2) based in situ etches. J. Vac. Sci. Technol. B 2011, 29. [Google Scholar] [CrossRef]
- Li, G.; Li, Y.; Li, Y.; Peng, H.; Chen, K. Polyaniline nanorings and flat hollow capsules synthesized by in situ sacrificial oxidative templates. Macromolecules 2011, 44, 9319–9323. [Google Scholar] [CrossRef]
- Yang, K.C.; Jeon, M.H.; Yeom, G.Y. A study on the etching characteristics of magnetic tunneling junction materials using DC pulse-biased inductively coupled plasmas. Jpn. J. Appl. Phys. 2015, 54. [Google Scholar] [CrossRef]
- Zhang, C.; Zhou, Y.; Zhang, Y.; Zhao, S.; Fang, J.; Sheng, X.; Zhang, H. A novel hierarchical TiO2@Pt@mSiO(2) hollow nanocatalyst with enhanced thermal stability. J. Alloy. Compd. 2017, 701, 780–787. [Google Scholar] [CrossRef]
- Chen, L.; Bao, J.L.; Dong, X.; Truhlar, D.G.; Wang, Y.; Wang, C.; Xia, Y. Aqueous Mg-Ion battery based on polyimide anode and Prussian blue cathode. ACS Energy Lett. 2017, 2, 1115–1121. [Google Scholar] [CrossRef]
- Wu, X.; Sun, M.; Guo, S.; Qian, J.; Liu, Y.; Cao, Y.; Ai, X.; Yang, H. Vacancy-free Prussian blue nanocrystals with high capacity and superior cyclability for aqueous sodium-ion batteries. Chem. Nano Mat. 2015, 1, 188–193. [Google Scholar] [CrossRef]
- Whitacre, J.F.; Tevar, A.; Sharma, S. Na4Mn9O18 as a positive electrode material for an aqueous electrolyte sodium-ion energy storage device. Electrochem. Commun. 2010, 12, 463–466. [Google Scholar] [CrossRef]
- Wu, X.; Cao, Y.; Ai, X.; Qian, J.; Yang, H. A low-cost and environmentally benign aqueous rechargeable sodium-ion battery based on NaTi2(PO4)3–Na2NiFe(CN)6 intercalation chemistry. Electrochem. Commun. 2013, 31, 145–148. [Google Scholar] [CrossRef]
- Luo, J.-Y.; Cui, W.-J.; He, P.; Xia, Y.-Y. Raising the cycling stability of aqueous lithium-ion batteries by eliminating oxygen in the electrolyte. Nat. Chem. 2010, 2, 760–765. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ishihara, S.; Ariga, K.; Imura, M.; Yamauchi, Y. Kinetically controlled crystallization for synthesis of monodispersed coordination polymer nanocubes and their self-assembly to periodic arrangements. Chem. Eur. J. 2013, 19, 1882–1885. [Google Scholar] [CrossRef] [PubMed]
- Widmann, A.; Kahlert, H.; Wulff, H.; Scholz, F. Electrochemical and mechanochemical formation of solid solutions of potassium copper(II)/zinc(II) hexacyanocobaltate(III)/hexacyanoferrate(III) KCuxZn1-x hcc (x) hcf (1-x). J. Solid State Electr. 2005, 9, 380–389. [Google Scholar] [CrossRef]
- Kulesza, P.J.; Malik, M.A.; Denca, A.; Strojek, J. In situ FT-IR/ATR spectroelectrochemistry of Prussian blue in the solid state. Anal. Chem. 1996, 68, 2442–2446. [Google Scholar] [CrossRef]
- Hung, T.-F.; Chou, H.-L.; Yeh, Y.-W.; Chang, W.-S.; Yang, C.-C. Combined experimental and computational studies of a na2ni1-xcuxfe(cn)(6) cathode with tunable potential for aqueous rechargeable sodium-ion batteries. Chem. Europe. J. 2015, 21, 15686–15691. [Google Scholar] [CrossRef] [PubMed]
- Hung, T.-F.; Cheng, W.-J.; Chang, W.-S.; Yang, C.-C.; Shen, C.-C.; Kuo, Y.-L. Ascorbic acid-assisted synthesis of mesoporous sodium vanadium phosphate nanoparticles with highly sp(2)-coordinated carbon coatings as efficient cathode materials for rechargeable sodium-ion batteries. Chem. Eur. J. 2016, 22, 10620–10626. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Wu, X.-L.; Yin, Y.-X.; Guo, Y.-G. A zero-strain insertion cathode material of nickel ferricyanide for sodium-ion batteries. J. Mater. Chem. A 2013, 1, 14061–14065. [Google Scholar] [CrossRef]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, H.; Zhang, W.; Hu, M. Prussian Blue Analogue Mesoframes for Enhanced Aqueous Sodium-ion Storage. Crystals 2018, 8, 23. https://doi.org/10.3390/cryst8010023
Sun H, Zhang W, Hu M. Prussian Blue Analogue Mesoframes for Enhanced Aqueous Sodium-ion Storage. Crystals. 2018; 8(1):23. https://doi.org/10.3390/cryst8010023
Chicago/Turabian StyleSun, Huiyun, Wei Zhang, and Ming Hu. 2018. "Prussian Blue Analogue Mesoframes for Enhanced Aqueous Sodium-ion Storage" Crystals 8, no. 1: 23. https://doi.org/10.3390/cryst8010023




