Structural and Magnetic Studies of Cr3+ Substituted Nickel Ferrite Nanomaterials Prepared by Sol-Gel Auto-Combustion
Abstract
:1. Introduction
2. Experimental
2.1. Sample Preparation
2.2. Characterization
3. Results and Discussion
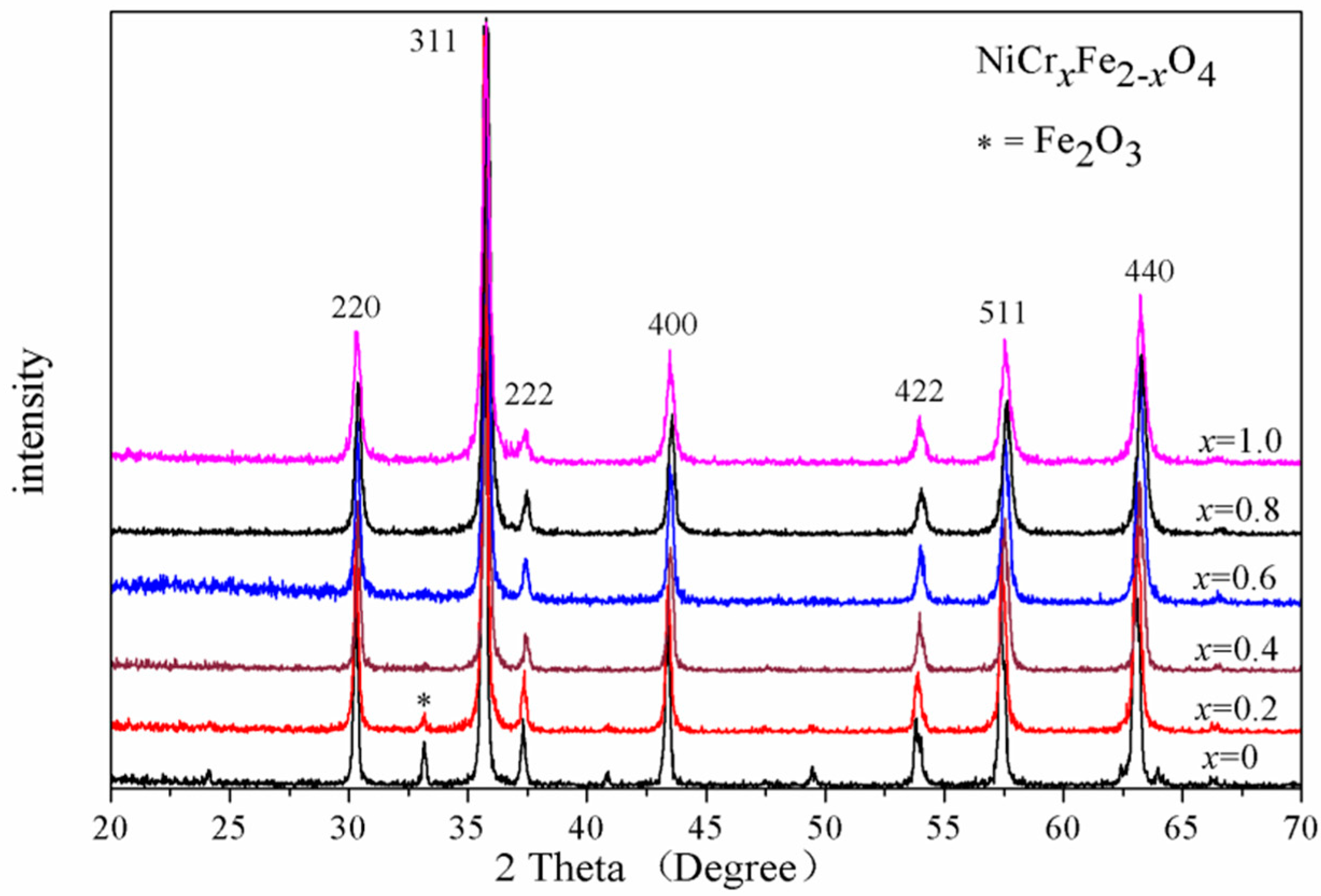
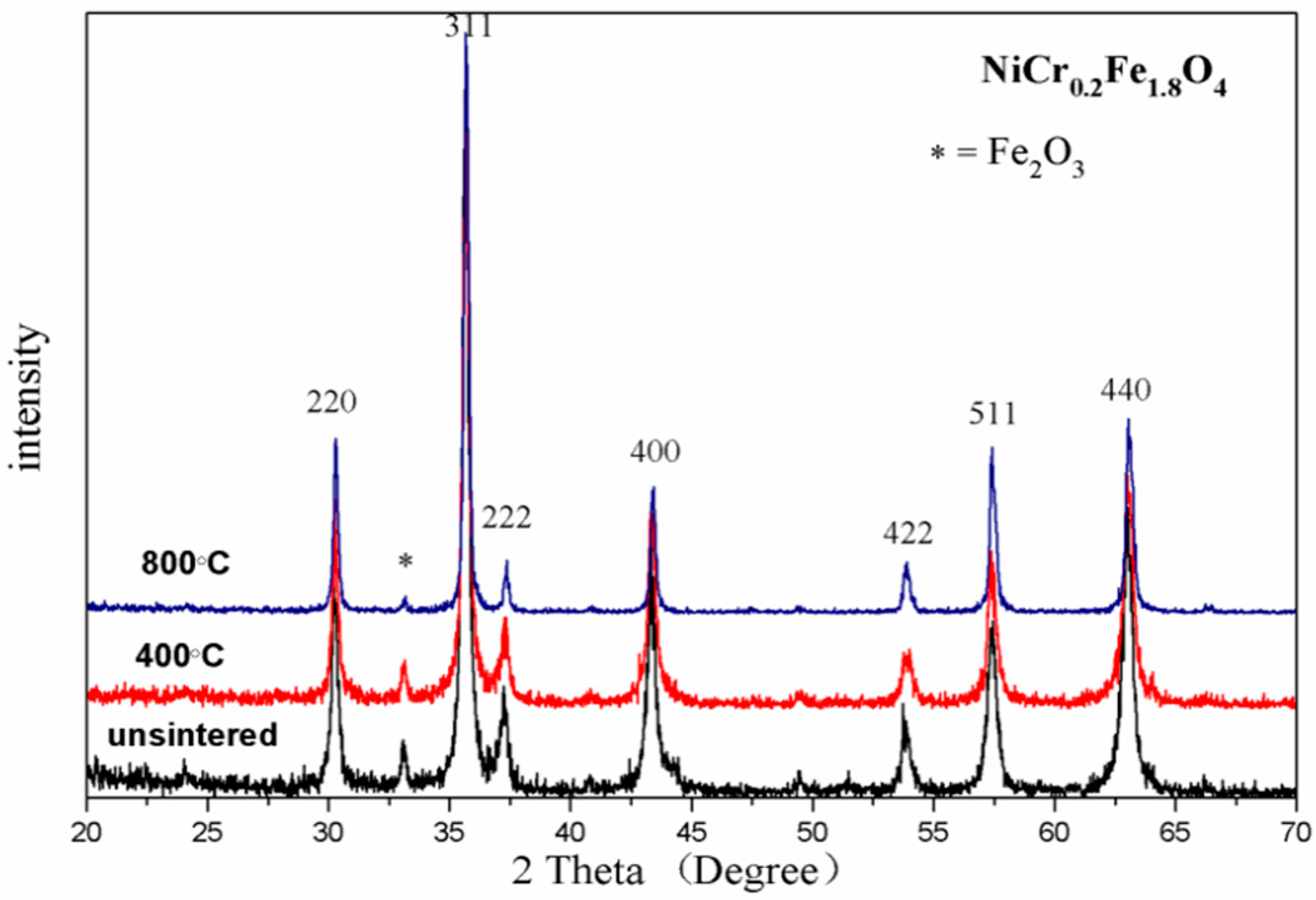
3.1. X-ray Diffraction (XRD) Analysis
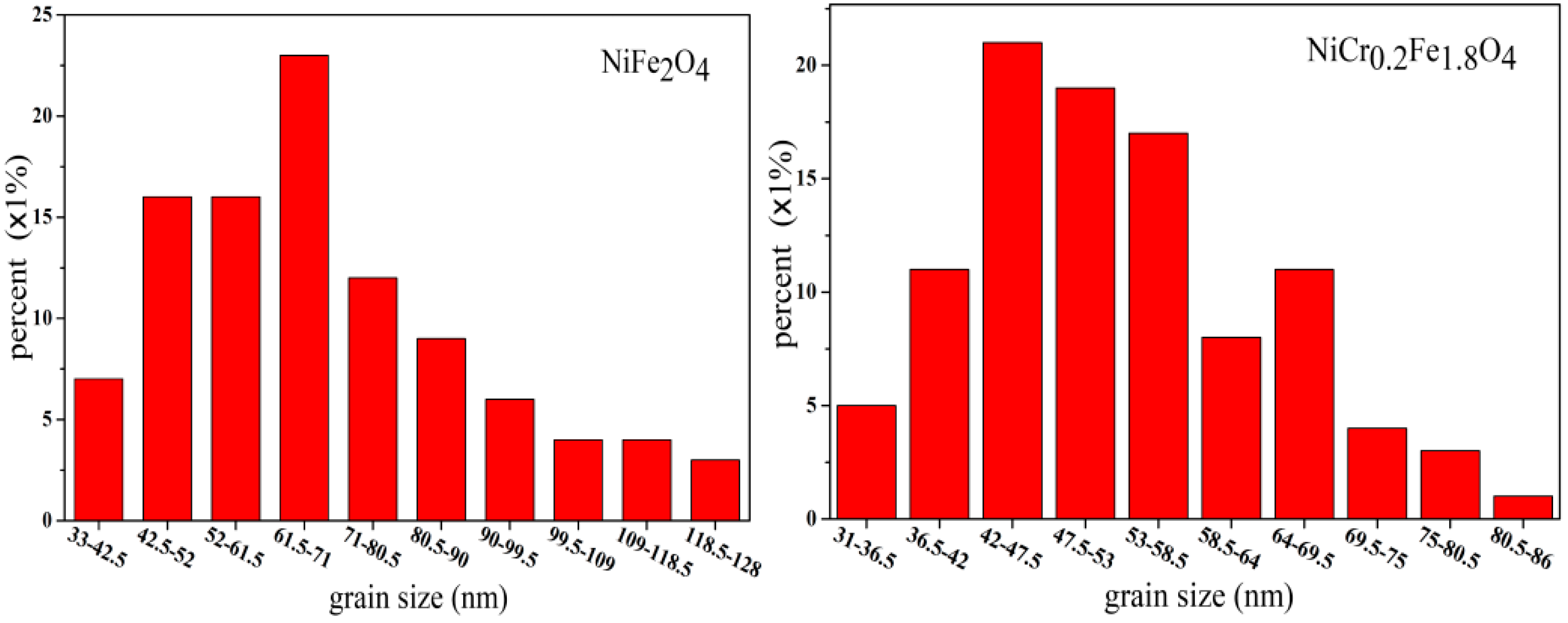
3.2. Structures and Grain Size
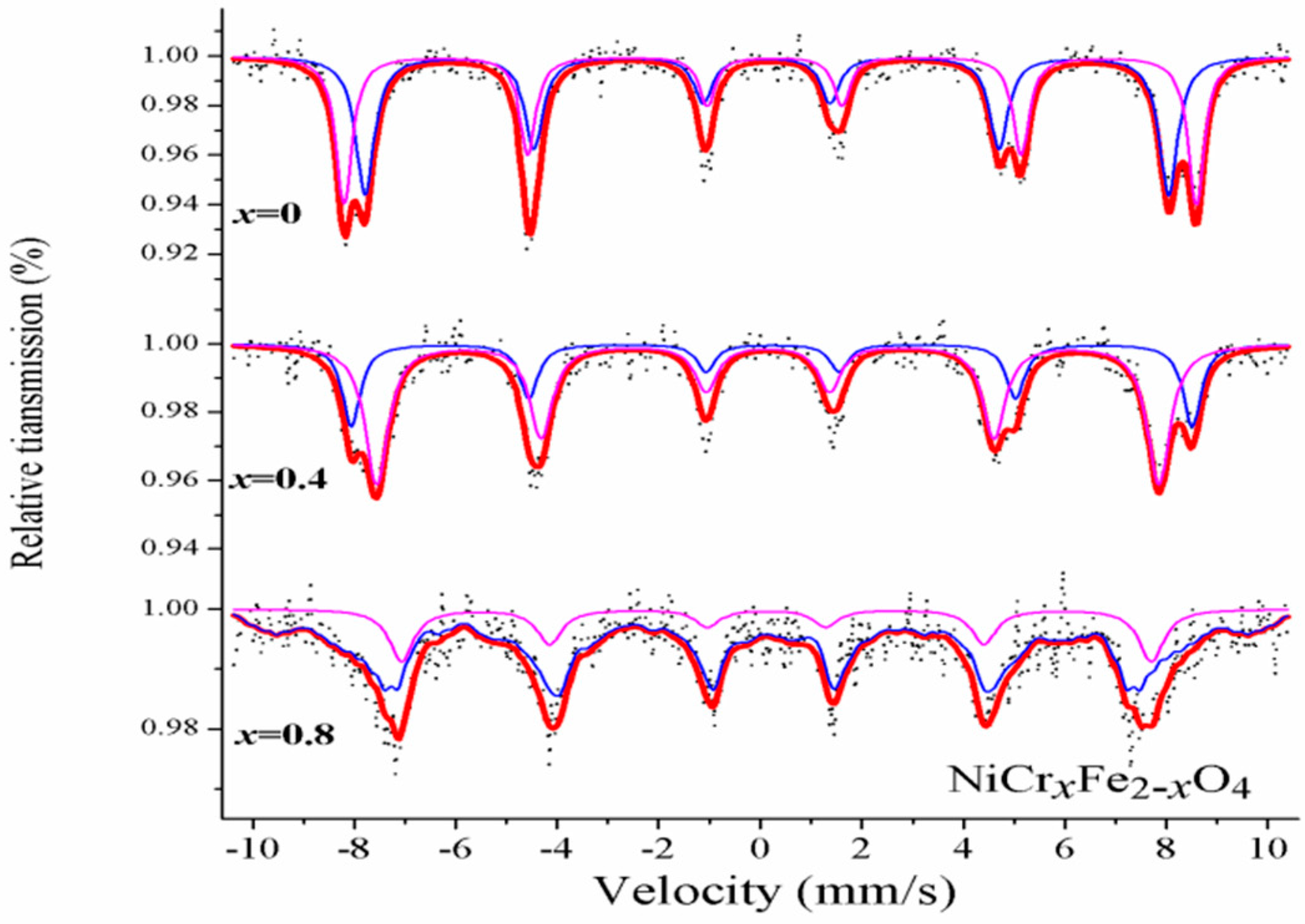
3.3. Mössbauer Spectroscopy
3.4. Magnetic Property of Particles
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Patange, S.M.; Shirsath, S.E.; Jadhav, S.S.; Lohar, K.S.; Mane, D.R.; Jadhav, K.M. Rietveld refinement and switching properties of Cr3+ substituted NiFe2O4 ferrites. Mater. Lett. 2010, 64, 722–724. [Google Scholar] [CrossRef]
- He, Y.; Lei, C.; Lin, Q.; Dong, J.; Yu, Y.; Wang, L. Mössbauer and Structural properties of La-substituted Ni0.4Cu0.2Zn0.4Fe2O4 nanocrystalline ferrite. Sci. Adv. Mater. 2015, 7, 1809–1815. [Google Scholar] [CrossRef]
- Anh, L.N.; Duong, N.P.; Loan, T.T.; Nguyet, D.T.T.; Hien, T.D. Synchrotron and Magnetic Study of Chromium-Substituted Nickel Ferrites Prepared by Using Sol-Gel Route. IEEE Trans. Magn. 2014, 50, 1–5. [Google Scholar]
- Lee, S.H.; Yoon, S.J.; Lee, G.J.; Kim, H.S.; Yo, C.H.; Ahn, K.; Lee, D.H.; Kim, K.H. Electrical and magnetic properties of NiCrxFe2−xO4 spinel (0 ≤ x ≤ 0.6). Mater. Chem. Phys. 1999, 61, 147–152. [Google Scholar] [CrossRef]
- Prasad, A.S.; Dolia, S.N.; Pareek, S.P.; Samariya, A.; Sharma, P.K.; Dhawan, M.S. Sol-gel synthesized high anisotropy magnetic nanoparticles of NiCrxFe2−xO4. J. Sol-Gel Sci. Technol. 2013, 66, 372–377. [Google Scholar] [CrossRef]
- Patange, S.M.; Shirsath, S.E.; Toksha, B.G.; Jadhav, S.S.; Shukla, S.J.; Jadhav, K.M. Cation distribution by Rietveld, spectral and magnetic studies of chromium-substituted nickel ferrites. Appl. Phys. A 2009, 95, 429–434. [Google Scholar] [CrossRef]
- Xia, A.; Liu, S.; Jin, C.; Chen, L.; Lv, Y. Hydrothermal Mg1−xZnxFe2O4 spinel ferrites: Phase formation and mechanism of saturation magnetization. Mater. Lett. 2013, 105, 199–201. [Google Scholar] [CrossRef]
- Iqbal, M.J.; Siddiquah, M.R. Electrical and magnetic properties of chromium-substituted cobalt ferrite nanomaterials. J. Alloys Compd. 2008, 453, 513–518. [Google Scholar] [CrossRef]
- Chae, K.P.; Lee, Y.B.; Lee, J.G.; Lee, S.H. Crystallographic and magnetic properties of CoCrxFe2−xO4 ferrite powders. J. Magn. Magn. Mater. 2000, 220, 59–64. [Google Scholar] [CrossRef]
- Singhal, S.; Jauhar, S.; Singh, J.; Chandra, K.; Bansal, S. Investigation of structural, magnetic, electrical and optical properties of chromium substituted cobalt ferrites (CoCrxFe2−xO4, 0 ≤ x ≤ 1) synthesized using sol gel auto combustion method. J. Mol. Struct. 2012, 1012, 182–188. [Google Scholar] [CrossRef]
- Bayoumy, W.A.; Gabal, M.A. Synthesis characterization and magnetic properties of Cr-substituted NiCuZn nanocrystalline ferrite. J. Alloys Compd. 2010, 506, 205–209. [Google Scholar] [CrossRef]
- Lin, Q.; Lei, C.; He, Y.; Xu, J.; Wang, R. Mössbauer and XRD studies of Ni0.6Cu0.2Zn0.2CexFe2−xO4 ferrites By Sol-Gel auto-combustion. J. Nanosci. Nanotechnol. 2015, 15, 2997–3003. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Farea, A.M.M.; Batoo, K.M.; Lee, C.G.; Koo, B.H.; Yousef, A.; Alimuddin. Mössbauer studies of Co0.5CdxFe2.5−xO4 (0.0 ≤ x ≤ 0.5) ferrite. Phys. B Condens. Matter. 2008, 403, 3604–3607. [Google Scholar] [CrossRef]
- Toksha, B.G.; Shirsath, S.E.; Mane, M.L.; Patange, S.M.; Jadhav, S.S.; Jadhav, K.M. Autocombustion High-Temperature Synthesis, Structural, and Magnetic Properties of CoCrxFe2−xO4 (0 ≤ x ≤ 1.0). J. Phys. Chem. C 2011, 115, 20905–20912. [Google Scholar] [CrossRef]
- Gabal, M.A.; Al Angari, Y.M.; Al-Agel, F.A. Synthesis, characterization and magnetic properties of Cr-substituted Co-Zn ferrites Nanopowders. J. Mol. Struct. 2013, 1035, 341–347. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, Y.M. Effect of Gd substitution on structural and magnetic properties of Zn-Cu-Cr ferrites prepared by novel rheological technique. Mater. Sci. Technol. 2009, 25, 415–418. [Google Scholar] [CrossRef]
- Singhal, S.; Barthwal, S.K.; Chandra, K. XRD, magnetic and Mössbauer spectral studies of nano size aluminum substituted cobalt ferrites (CoAlxFe2−xO4). J. Magn. Magn. Mater. 2006, 306, 233–240. [Google Scholar] [CrossRef]
- Harzali, H.; Marzouki, A.; Saida, F.; Megriche, A.; Mgaidi, A. Structural, magnetic and optical properties of nanosized Ni0.4Cu0.2Zn0.4R0.05Fe1.95O4 (R = Eu3+, Sm3+, Gd3+ and Pr3+) ferrites synthesized by co-precipitation method with ultrasound irradiation. J. Magn. Magn. Mater. 2018, 460, 89–94. [Google Scholar] [CrossRef]
- He, Y.; Yang, X.; Lin, J.; Lin, Q.; Dong, J. Mössbauer spectroscopy, Structural and magnetic studies of Zn2+ substituted magnesium ferrite nanomaterials prepared by Sol-Gel method. J. Nanomater. 2015, 2015, 854840. [Google Scholar] [CrossRef]
- Kumar, A.; Shen, J.; Yang, W.; Zhao, H.; Sharma, P.; Varshney, D.; Li, Q. Impact of Rare Earth Gd3+ Ions on Structural and Magnetic Properties of Ni0.5Zn0.5Fe2−xGdxO4 Spinel Ferrite: Useful for Advanced Spintronic Technologies. J. Supercond. Novel Magn. 2018, 31, 1173–1182. [Google Scholar] [CrossRef]
- Lin, L.Z.; Tu, X.Q.; Wang, R.; Peng, L. Structural and magnetic properties of Cr-substituted NiZnCo ferrite Nanopowders. J. Magn. Magn. Mater. 2015, 381, 328–331. [Google Scholar]
- Cao, C.; Xia, A.; Liu, S.; Tong, L. Synthesis and magnetic properties of hydrothermal magnesium–zinc spinel ferrite powders. J. Mater. Sci. Mater. Electron. 2013, 24, 4901–4905. [Google Scholar] [CrossRef]
- Li, L.Z.; Zhong, X.X.; Wang, R.; Tu, X.Q.; Peng, L. Structural and magnetic properties of Co-substituted NiCu ferrite nanopowders. J. Magn. Magn. Mater. 2017, 433, 98–103. [Google Scholar] [CrossRef]
- Lin, J.; He, Y.; Lin, Q.; Wang, R.; Chen, H. Microstructural and Mössbauer spectroscopy Studies of Mg1−xZnxFe2O4 (x = 0.5, 0.7) nanoparticles. J. Spectrosc. 2014, 2014, 540319. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, H.; Zhang, B.; Ji, G.; He, Y.; Lin, Q. A proposed electron transmission mechanism between Fe3+/Co2+ and Fe3+/Fe3+ in the spinel structure and its practical evidence in quaternary Fe0.5Ni0.5Co2S4. J. Mater. Chem. C 2016, 4, 5476–5482. [Google Scholar] [CrossRef]


| Content (x) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| 0 | 8.34062 | 575 | 5.3664 |
| 0.2 | 8.33600 | 446 | 5.3562 |
| 0.4 | 8.31862 | 453 | 5.3735 |
| 0.6 | 8.31741 | 375 | 5.3581 |
| 0.8 | 8.30992 | 308 | 5.3548 |
| 1.0 | 8.31979 | 255 | 5.3179 |
| Temperature (°C) | Lattice Parameter (Å) | Average Crystallite Size (Å) | Density (g/cm3) |
|---|---|---|---|
| unsintered | 8.34459 | 293 | 5.3411 |
| 400 °C | 8.34400 | 300 | 5.3424 |
| 800 °C | 8.36000 | 446 | 5.3562 |
| Content (x) | Component | Isomer Shift (I.S.) (mm/s) | Quadrupole Shift (Q.S.) (mm/s) | H(T) | Line Width (Γ) (mm/s) | Relative Area (A0) (%) |
|---|---|---|---|---|---|---|
| 0 | Sextet (A) | 0.124 | 0.003 | 48.089 | 0.439 | 51.7 |
| Sextet (B) | 0.235 | −0.087 | 52.051 | 0.384 | 48.3 | |
| 0.4 | Sextet (A) | 0.146 | −0.003 | 47.786 | 0.544 | 68.3 |
| Sextet (B) | 0.230 | −0.014 | 51.359 | 0.429 | 31.7 | |
| 0.8 | Sextet (A) | 0.147 | −0.219 | 43.373 | 0.352 | 80.2 |
| Sextet (B) | 0.224 | 0.191 | 45.820 | 0.587 | 19.8 |
| Content (x) | Ms (emu/g) | Hc (Oe) | Mr (emu/g) |
|---|---|---|---|
| 0 | 40.12 | 177.25 | 13.64 |
| 0.2 | 31.02 | 152.09 | 9.06 |
| 0.4 | 22.87 | 154.61 | 6.94 |
| 0.6 | 14.06 | 185.70 | 4.80 |
| 0.8 | 8.11 | 253.79 | 2.87 |
| 1.0 | 4.46 | 361.83 | 1.55 |
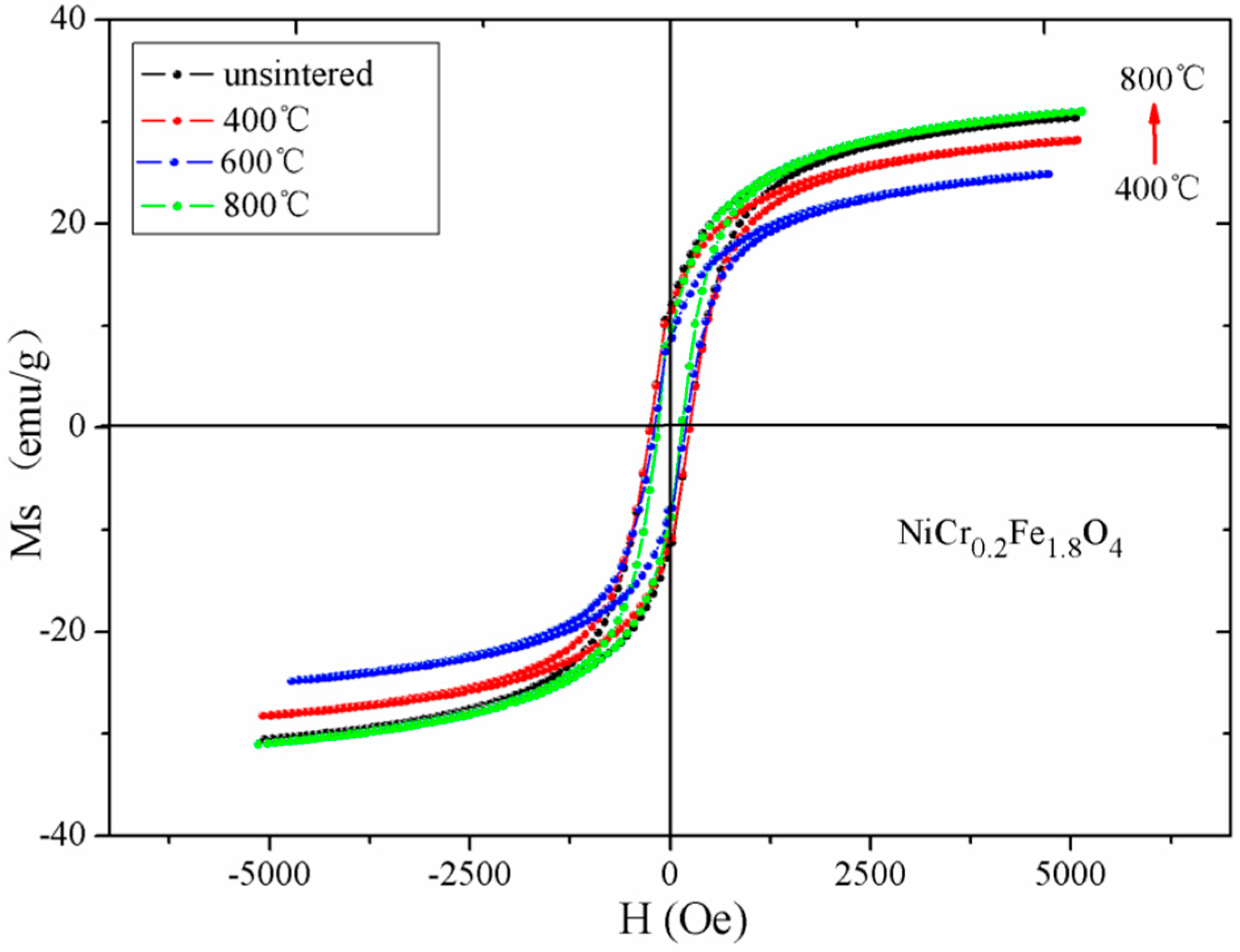
| Temperature (°C) | Ms (emu/g) | Hc (Oe) | Mr (emu/g) |
|---|---|---|---|
| unsintered | 30.47 | 247.03 | 11.62 |
| 400 °C | 28.19 | 247.45 | 11.10 |
| 600 °C | 24.87 | 207.85 | 6.25 |
| 800 °C | 31.02 | 152.09 | 9.06 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, J.; He, Y.; Du, X.; Lin, Q.; Yang, H.; Shen, H. Structural and Magnetic Studies of Cr3+ Substituted Nickel Ferrite Nanomaterials Prepared by Sol-Gel Auto-Combustion. Crystals 2018, 8, 384. https://doi.org/10.3390/cryst8100384
Lin J, He Y, Du X, Lin Q, Yang H, Shen H. Structural and Magnetic Studies of Cr3+ Substituted Nickel Ferrite Nanomaterials Prepared by Sol-Gel Auto-Combustion. Crystals. 2018; 8(10):384. https://doi.org/10.3390/cryst8100384
Chicago/Turabian StyleLin, Jinpei, Yun He, Xianglin Du, Qing Lin, Hu Yang, and Hongtao Shen. 2018. "Structural and Magnetic Studies of Cr3+ Substituted Nickel Ferrite Nanomaterials Prepared by Sol-Gel Auto-Combustion" Crystals 8, no. 10: 384. https://doi.org/10.3390/cryst8100384





