Effect of Transition Metal Substitution on the Charge-Transfer Phase Transition and Ferromagnetism of Dithiooxalato-Bridged Hetero Metal Complexes, (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Characterization
2.3. Measurements of Physical Properties
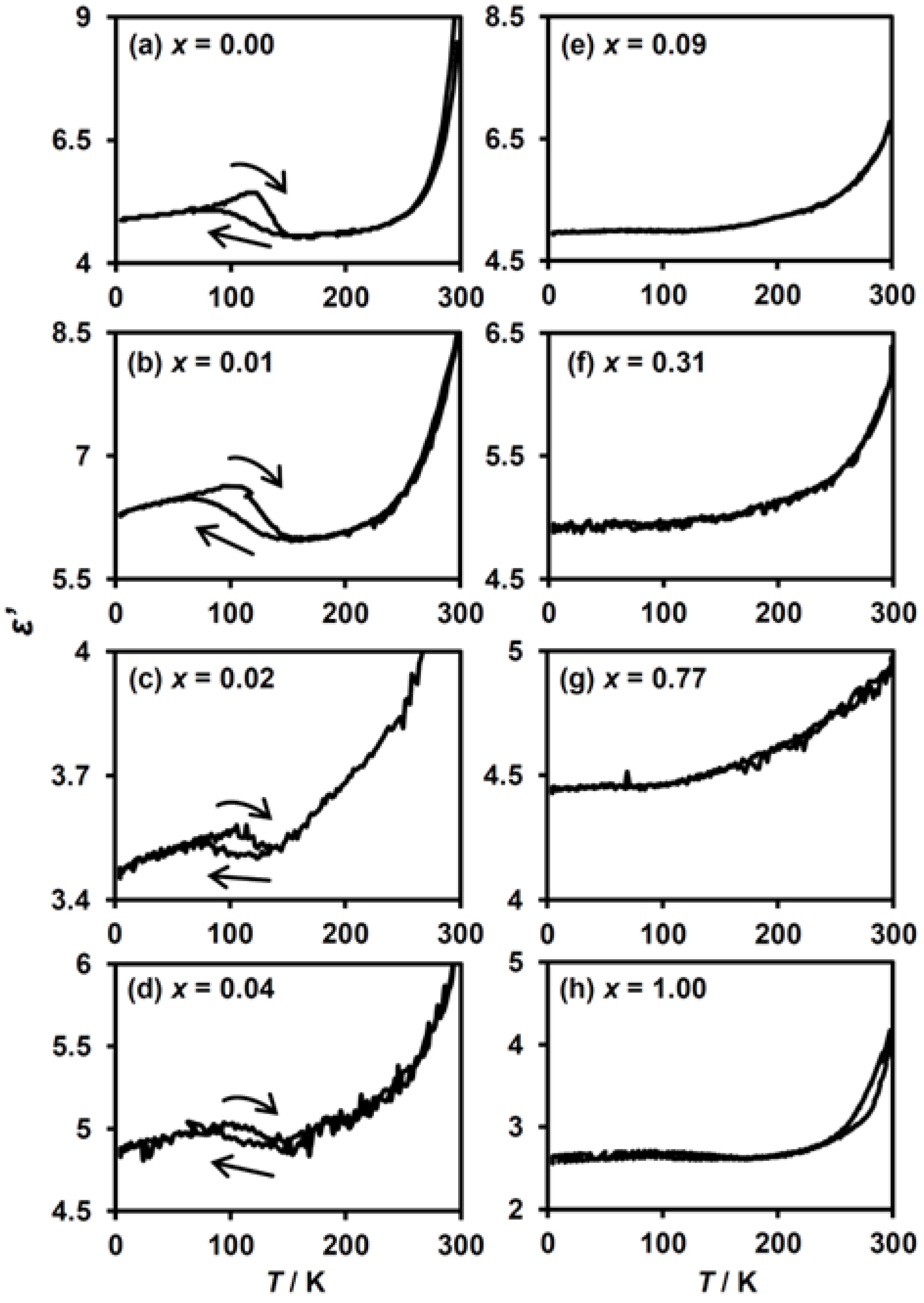
3. Results
3.1. Characterization
3.1.1. The Composition of Metallic Ions
3.1.2. Powder X-Ray Analysis
3.2. Physical Properties for (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]
3.2.1. Magnetism of (n-C3H7)4N[FeIIFeIII(dto)3] and a Series of Nonmagnetic Substituted Complexes
3.2.2. Magnetism of (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]
3.2.3. Dielectric Constant Measurements of (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A


References
- Castillo, O.; Luque, A.; Sertucha, J.; Román, P.; Lloret, F. Synthesis, Crystal Structure, and Magnetic Properties of a One-Dimensional Polymeric Copper(II) Complex Containing an Unusual 1,1′-Bicoordinated Oxalato Bridge. Inorg. Chem. 2000, 39, 6142–6144. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.C.; Cho, J.; Lough, A. Syntheses, isolation, and structures of nickel(II) and copper(II) coordination polymers with a tetraaza macrocyclic ligand. J. Inorg. Chim. Acta 2001, 317, 252–258. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.C.; Lough, A.J.; Lee, B.M. One-dimensional macrocyclic zinc(II) coordination polymer containing an unusual bis-monodentate oxalate bridge. Inorg. Chem. Commun. 2007, 10, 303–306. [Google Scholar] [CrossRef]
- Kim, J.; Park, A.H.; Kim, J.C.; Lough, A.J.; Pyun, S.Y.; Roh, J.; Lee, B.M. 1D copper(II) and zinc(II) coordination polymers containing an unusual twisted oxalate bridge. Inorg. Chim. Acta 2008, 361, 2087–2093. [Google Scholar] [CrossRef]
- Pei, Y.; Journaux, Y.; Kahn, O. Ferromagnetic interactions between t2g3 and eg2 magnetic orbitals in a CrIIINiII3 tetranuclear compound. Inorg. Chem. 1989, 28, 100–103. [Google Scholar] [CrossRef]
- Ohba, M.; Tamaki, H.; Matsumoto, N.; Ōkawa, H. Oxalate-bridged dinuclear chromium(III)-M(II) (M = copper, nickel, cobalt, iron, manganese) complexes: Synthesis, structure, and magnetism. Inorg. Chem. 1993, 32, 5385–5390. [Google Scholar] [CrossRef]
- Glerup, J.; Goodson, P.A.; Hodgson, D.J.; Michelsen, K. Magnetic Exchange through Oxalate Bridges: Synthesis and Characterization of (μ-Oxalato)dimetal(II) Complexes of Manganese, Iron, Cobalt, Nickel, Copper, and Zinc. Inorg. Chem. 1995, 34, 6255–6264. [Google Scholar] [CrossRef]
- Vivas, C.Y.; Delgado, F.S.; Ruiz-Pérez, C.; Julve, M. Preparation and crystal structure of the oxalato-bridged CrIII–AgI two-dimensional compound {Ag3(H2O)[Cr(dpa)(ox)2]3}n·2nH2O (dpa = 2,2′-dipyridylamine). CrystEngComm 2004, 6, 11–18. [Google Scholar] [CrossRef]
- Armentano, D.; De Munno, G.; Lloret, F.; Julve, M. Bis and tris(oxalato)ferrate(III) complexes as precursors of polynuclear compounds. CrystEngComm 2005, 7, 57–66. [Google Scholar] [CrossRef]
- Ballester, G.; Coronado, E.; Giménez-Saiz, C.; Romero, F.M. Nitroxide Radicals as Templating Agents in the Synthesis of Magnets Based on Three-Dimensional Oxalato-Bridged Heterodimetallic Networks. Angew. Chem. Int. Ed. 2001, 40, 792–795. [Google Scholar] [CrossRef]
- Kahn, O. Dinuclear Complexes with Predictable Magnetic Properties. Angew. Chem. Int. Ed. 1985, 24, 834–850. [Google Scholar] [CrossRef]
- Rao, C.N.R.; Natarajan, S.; Vaidhyanathan, R. Metal Carboxylates with Open Architectures. Angew. Chem. Int. Ed. 2004, 43, 1466–1496. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; Martí-Gastaldo, C.; Romero, F.M. Multifunctionality in hybrid magnetic materials based on bimetallic oxalate complexes. Chem. Soc. Rev. 2011, 40, 473–497. [Google Scholar] [CrossRef] [PubMed]
- Tamaki, H.; Zhong, Z.J.; Matsumoto, N.; Kida, S.; Koikawa, M.; Achiwa, N.; Hashimoto, Y.; Ōkawa, H. Design of metal-complex magnets. Syntheses and magnetic properties of mixed-metal assemblies {NBu4[MCr(ox)3]}x (NBu4+ = tetra(n-butyl)ammonium ion; ox2− = oxalate ion; M = Mn2+, Fe2+, Co2+, Ni2+, Cu2+, Zn2+). J. Am. Chem. Soc. 1992, 114, 6974–6979. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J. Intercalation of decamethylferrocenium cations in bimetallic oxalate-bridged two-dimensional magnets. Chem. Commun. 1997, 0, 1727–1728. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Ensling, J.; Gütlich, P. Hybrid Molecular Magnets Obtained by Insertion of Decamethylmetallocenium Cations into Layered, Bimetallic Oxalate Complexes: [ZIIICp*2][MIIMIII(ox)3] (ZIII=Co, Fe; MIII=Cr, Fe; MII=Mn, Fe, Co, Cu, Zn; ox=oxalate; Cp*=pentamethylcyclopentadienyl). Chem. Eur. J. 2000, 6, 552–563. [Google Scholar] [CrossRef]
- Coronado, E.; Giménez-Saiz, C.; Gómez-García, C.J.; Romero, F.M.; Tarazón, A. A bottom-up approach from molecular nanographenes to unconventional carbon materials. J. Mater. Chem. 2008, 18, 929–934. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Waerenborgh, J.C. Multifunctional Magnetic Materials Obtained by Insertion of Spin-Crossover FeIII Complexes into Chiral 3D Bimetallic Oxalate-Based Ferromagnets. Inorg. Chem. 2011, 50, 9122–9130. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Mínguez, E.; Soriano-Portillo, A.; Waerenborgh, J.C. Multifunctional Magnetic Materials Obtained by Insertion of a Spin-Crossover FeIII Complex into Bimetallic Oxalate-Based Ferromagnets. Chem. Eur. J. 2010, 16, 2207–2219. [Google Scholar] [CrossRef] [PubMed]
- Clemente-León, M.; Coronado, E.; López-Jordà, M.; Desplanches, C.; Asthana, S.; Wang, H.; Létard, J.-F. A hybrid magnet with coexistence of ferromagnetism and photoinduced Fe(III) spin-crossover. Chem. Sci. 2011, 2, 1121–1127. [Google Scholar] [CrossRef]
- Clemente-León, M.; Coronado, E.; López-Jordà, M. 2D and 3D bimetallic oxalate-based ferromagnets prepared by insertion of different FeIII spin crossover complexes. Dalton Trans. 2010, 39, 4903–4910. [Google Scholar] [CrossRef] [PubMed]
- Bénard, S.; Rivière, E.; Yu, P.; Nakatani, K.; Delouis, J.F. A Photochromic Molecule-Based Magnet. Chem. Mater. 2001, 13, 159–162. [Google Scholar] [CrossRef]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Laukhin, V. Coexistence of ferromagnetism and metallic conductivity in a molecule-based layered compound. Nature 2000, 408, 447–449. [Google Scholar] [CrossRef] [PubMed]
- Alberola, A.; Coronado, E.; Galán-Mascarós, J.R.; Giménez-Saiz, C.; Gómez-García, C.J. A Molecular Metal Ferromagnet from the Organic Donor Bis(ethylenedithio)tetraselenafulvalene and Bimetallic Oxalate Complexes. J. Am. Chem. Soc. 2003, 125, 10774–10775. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R.; Gómez-García, C.J.; Martínez-Ferrero, E.; van Smaalen, S. Incommensurate Nature of the Multilayered Molecular Ferromagnetic Metals Based on Bis(ethylenedithio)tetrathiafulvalene and Bimetallic Oxalate Complexes. Inorg. Chem. 2004, 43, 4808–4810. [Google Scholar] [CrossRef] [PubMed]
- Coronado, E.; Galán-Mascarós, J.R. Hybrid molecular conductors. J. Mater. Chem. 2005, 15, 66–74. [Google Scholar] [CrossRef]
- Coronado, E.; Curreli, S.; Giménez-Saiz, C.; Gómez-García, C.J. The Series of Molecular Conductors and Superconductors ET4[AFe(C2O4)3]·PhX (ET = bis(ethylenedithio)tetrathiafulvalene; (C2O4)2– = oxalate; A+ = H3O+, K+; X = F, Cl, Br, and I): Influence of the Halobenzene Guest Molecules on the Crystal Structure and Superconducting Properties. Inorg. Chem. 2012, 51, 1111–1126. [Google Scholar] [CrossRef] [PubMed]
- Ōkawa, H.; Shigematsu, A.; Sadakiyo, M.; Miyagawa, T.; Yoneda, K.; Ohba, M.; Kitagawa, H. Oxalate-Bridged Bimetallic Complexes {NH(prol)3}[MCr(ox)3] (M = MnII, FeII, CoII; NH(prol)3+ = Tri(3-hydroxypropyl)ammonium) Exhibiting Coexistent Ferromagnetism and Proton Conduction. J. Am. Chem. Soc. 2009, 131, 13516–13522. [Google Scholar] [CrossRef] [PubMed]
- Pardo, E.; Train, C.; Liu, H.; Chamoreau, L.-M.; Dkhil, B.; Boubekeur, K.; Lloret, F.; Nakatani, K.; Tokoro, H.; Ohkoshi, S.; et al. Multiferroics by Rational Design: Implementing Ferroelectricity in Molecule-Based Magnets. Angew. Chem. Int. Ed. 2012, 51, 8356–8360. [Google Scholar] [CrossRef] [PubMed]
- Bénard, S.; Yu, P.; Coradin, T.; Rivière, E.; Nakatani, K.; Clément, R. Design of strongly NLO-active molecularly-based ferromagnets. Adv. Mater. 1997, 9, 981–984. [Google Scholar] [CrossRef]
- Bénard, S.; Yu, P.; Audière, J.P.; Rivière, E.; Clément, R.; Guilhem, J.; Tchertanov, L.; Nakatani, K. Structure and NLO Properties of Layered Bimetallic Oxalato-Bridged Ferromagnetic Networks Containing Stilbazolium-Shaped Chromophores. J. Am. Chem. Soc. 2000, 122, 9444–9454. [Google Scholar] [CrossRef]
- Lacroix, P.G.; Malfant, I.; Bénard, S.; Yu, P.; Rivière, E.; Nakatani, K. Hybrid Molecular-Based Magnets Containing Organic NLO Chromophores: A Search toward an Interplay between Magnetic and NLO Behavior. Chem. Mater. 2001, 13, 441–449. [Google Scholar] [CrossRef]
- Ōkawa, H.; Mitsumi, M.; Ohba, M.; Kodera, M.; Matsumoto, N. Dithiooxalato(dto)-Bridged Bimetallic Assemblies {NPr4[MCr(dto)3]}x (M = Fe, Co, Ni, Zn; NPr4 = Tetrapropylammonium Ion): New Complex-Based Ferromagnets. Bull. Chem. Soc. Jpn. 1994, 67, 2139–2144. [Google Scholar] [CrossRef]
- Kojima, N.; Aoki, W.; Seto, M.; Kobayashi, Y.; Maeda, Y. Reversible charge-transfer phase transition in [(n-C3H7)4N][FeIIFeIII(dto)3] (dto = C2O2S2). Synth. Met. 2001, 121, 1796–1797. [Google Scholar] [CrossRef]
- Kojima, N.; Aoki, W.; Itoi, M.; Ono, Y.; Seto, M.; Kobayashi, Y.; Maeda, Y. Charge transfer phase transition and ferromagnetism in a mixed-valence iron complex, (n-C3H7)4N[FeIIFeIII(dto)3] (dto=C2O2S2). Solid State Commun. 2001, 120, 165–170. [Google Scholar] [CrossRef]
- Nakamoto, T.; Miyazaki, Y.; Itoi, M.; Ono, Y.; Kojima, N.; Sorai, M. Heat Capacity of the Mixed-Valence Complex {[(n-C3H7)4N][FeIIFeIII(dto)3]}∞, Phase Transition because of Electron Transfer, and a Change in Spin-State of the Whole System. Angew. Chem. Int. Ed. 2001, 40, 4716–4719. [Google Scholar] [CrossRef]
- Itoi, M.; Taira, A.; Enomoto, M.; Matsushita, N.; Kojima, N.; Kobayashi, Y.; Asai, K.; Koyama, K.; Nakano, T.; Uwatoko, Y. Crystal structure and structural transition caused by charge-transfer phase transition for iron mixed-valence complex (n-C3H7)4N[FeIIFeIII(dto)3] (dto = C2O2S2). J. Solid State Commun. 2004, 130, 415–420. [Google Scholar] [CrossRef]
- Itoi, M.; Ono, Y.; Kojima, N.; Kato, K.; Osaka, K.; Takata, M. Charge-Transfer Phase Transition and Ferromagnetism of Iron Mixed-Valence Complexes (n-CnH2n+1)4N[FeIIFeIII(dto)3] (n = 3–6; dto = C2O2S2). Eur. J. Inorg. Chem. 2006, 1198–1207. [Google Scholar] [CrossRef]
- Kida, N.; Hikita, M.; Kashima, I.; Okubo, M.; Itoi, M.; Enomoto, M.; Kato, K.; Tanaka, M.; Kojima, N. Control of Charge Transfer Phase Transition and Ferromagnetism by Photoisomerization of Spiropyran for an Organic−Inorganic Hybrid System, (SP)[FeIIFeIII(dto)3] (SP = spiropyran, dto = C2O2S2). J. Am. Chem. Soc. 2009, 131, 212–220. [Google Scholar] [CrossRef] [PubMed]
- Ida, H.; Okazawa, A.; Kojima, N.; Shimizu, R.; Yamada, Y.; Enomoto, M. Effect of Nonmagnetic Substitution on the Magnetic Properties and Charge-Transfer Phase Transition of an Iron Mixed-Valence Complex, (n-C3H7)4N[FeIIFeIII(dto)3] (dto = C2O2S2). Inorg. Chem. 2012, 51, 8989–8996. [Google Scholar] [CrossRef] [PubMed]
- Enomoto, M.; Kojima, N. Magnetic dilution effect on the charge transfer phase transition and the ferromagnetic transition for an iron mixed-valence complex, (n-C3H7)4N[FeII1−xZnIIxFeIII(dto)3] (dto = C2O2S2). Polyhedron 2009, 28, 1826–1829. [Google Scholar] [CrossRef]
- Jones, H.O.; Tasker, H.S. CCXII.—The action of mercaptans on acid chlorides. Part II. The acid chlorides of phosphorus, sulphur, and nitrogen. J. Chem. Soc. 1909, 95, 1910–1918. [Google Scholar] [CrossRef]
- Robinson, C.S.; Jones, H.O. VII.—Complex thio-oxalates. J. Chem. Soc. 1912, 101, 62–76. [Google Scholar] [CrossRef]
- Dwyer, F.P.; Sargeson, A.M. The Resolution of the Tris-(thio-oxalato)1 Complexes of Co(III), Cr(III) and Rh(III). J. Am. Chem. Soc. 1959, 81, 2335–2336. [Google Scholar] [CrossRef]
- Irving, H.; Williams, R.J.P. The Stability of Transition-metal Complexes. J. Phys. Chem. 1953, 0, 3192–3210. [Google Scholar] [CrossRef]
- Sigel, H.; McCormick, D.B. Discriminating Behavior of Metal Ions and Ligands with Regard to Their Biological Significance. Acc. Chem. Res. 1970, 3, 201–208. [Google Scholar] [CrossRef]
- Atovymann, O.L.; Shilov, G.V.; Lyubovskaya, R.N.; Zhilyaeva, E.I.; Ovaneseyan, N.S.; Pirumova, S.I.; Gusakovskaya, I.G.; Morozov, Y.G. Crystal structure of the molecular ferromagnet NBu4[MnCr(C2O4)3] (Bu=n-C4H9). JETP Lett. 1993, 58, 766–769. [Google Scholar]
- Decurtins, S.; Schmale, H.W.; Oswald, H.R.; Linden, A.; Ensling, J.; Giitlich, P.; Hauser, A. A polymeric two-dimensional mixed-metal network. Crystal structure and magnetic properties of {[P(Ph)4][MnCr(ox)3]}. Inorg. Chim. Acta 1994, 216, 65–73. [Google Scholar] [CrossRef]
- Decurtins, S.; Schmalle, H.W.; Pellaux, R.; Schneuwly, P.; Hauser, A. Chiral, Three-Dimensional Supramolecular Compounds: Homo- and Bimetallic Oxalate- and 1,2-Dithiooxalate-Bridged Networks. A Structural and Photophysical Study. Inorg. Chem. 1996, 35, 1451–1460. [Google Scholar] [CrossRef] [PubMed]
- Ovanesyan, N.S.; Shilov, G.V.; Sanina, N.A.; Pyalling, A.A.; Atovmyan, L.O.; Bottyán, L. Structural and Magnetic Properties of Two-Dimensional Oxalate-Bridged Bimetallic Compounds. Mol. Cryst. Liq. Cryst. 1999, 335, 91–104. [Google Scholar] [CrossRef]
- Ovanesyan, N.S.; Makhaev, V.D.; Aldoshin, S.M.; Gredin, P.; Boubekeur, K.; Train, C.; Gruselle, M. Structure, magnetism and optical properties of achiral and chiral two-dimensional oxalate-bridged anionic networks with symmetric and symmetric ammonium cations. Dalton Trans. 2005, 0, 3101–3107. [Google Scholar] [CrossRef] [PubMed]
- Nuttall, C.J.; Day, P. Modeling Stacking Faults in the Layered Molecular-Based Magnets AMIIFe(C2O4)3 {MII = Mn, Fe; A = Organic Cation}. J. Solid State Chem. 1999, 147, 3–10. [Google Scholar] [CrossRef]
- Kotani, M. On the Magnetic Moment of Complex Ions. (I). J. Phys. Soc. Jpn. 1949, 4, 293–297. [Google Scholar] [CrossRef]
- Kotani, M. Properties of d-Electrons in Complex Salts. Part I: Paramagnetism of Complex Salts. Prog. Theor. Phys. Suppl. 1960, 14, 1–16. [Google Scholar] [CrossRef]
- Ono, Y.; Okubo, M.; Kojima, N. Crystal Structure and Ferromagnetism of (n-C3H7)4N[CoIIFeIII(dto)3] (dto = C2O2S2). Solid State Commun. 2003, 126, 291–296. [Google Scholar] [CrossRef]
- Mayoh, B.; Day, P. Charge transfer in mixed-valence solids. Part VIII. Contribution of valence delocalisation to the ferromagnetism of Prussian Blue. J. Chem. Soc. Dalton Trans. 1976, 0, 1483–1486. [Google Scholar] [CrossRef]
- Carling, S.G.; Bradley, J.M.; Visser, D.; Day, P. Magnetic and structural characterization of the layered materials AMnFe(C2S2O2)3. Polyhedron 2003, 22, 2317–2324. [Google Scholar] [CrossRef]
- Kida, N.; Enomoto, M.; Watanabe, I.; Suzuki, T.; Kojima, N. Spin dynamics of the charge-transfer phase transition of an iron mixed-valence complex observed using muon spin relaxation spectroscopy. Phys. Rev. B 2008, 77, 144427. [Google Scholar] [CrossRef]
- Enomoto, M.; Kida, N.; Watanabe, I.; Suzuki, T.; Kojima, N. Spin dynamics of the ferromagnetic transition in iron mixed-valence complexes, (n-CnH2n+1)4N[FeIIFeIII(dto)3] (dto = C2O2S2, n = 3-5) by μSR. Physica B 2009, 404, 642–644. [Google Scholar] [CrossRef]









| Ratio of raw materials | 0.00 | 0.01 | 0.07 | 0.05 | 0.28 | 0.50 | 0.80 | 1.00 |
| Resulting values of x | 0.00 | 0.01 | 0.02 | 0.04 | 0.09 | 0.31 | 0.77 | 1.00 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Enomoto, M.; Ida, H.; Okazawa, A.; Kojima, N. Effect of Transition Metal Substitution on the Charge-Transfer Phase Transition and Ferromagnetism of Dithiooxalato-Bridged Hetero Metal Complexes, (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]. Crystals 2018, 8, 446. https://doi.org/10.3390/cryst8120446
Enomoto M, Ida H, Okazawa A, Kojima N. Effect of Transition Metal Substitution on the Charge-Transfer Phase Transition and Ferromagnetism of Dithiooxalato-Bridged Hetero Metal Complexes, (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]. Crystals. 2018; 8(12):446. https://doi.org/10.3390/cryst8120446
Chicago/Turabian StyleEnomoto, Masaya, Hiromichi Ida, Atsushi Okazawa, and Norimichi Kojima. 2018. "Effect of Transition Metal Substitution on the Charge-Transfer Phase Transition and Ferromagnetism of Dithiooxalato-Bridged Hetero Metal Complexes, (n-C3H7)4N[FeII1−xMnIIxFeIII(dto)3]" Crystals 8, no. 12: 446. https://doi.org/10.3390/cryst8120446





