Febuxostat-Minoxidil Salt Solvates: Crystal Structures, Characterization, Interconversion and Solubility Performance
Abstract
:1. Introduction
2. Materials and Methods
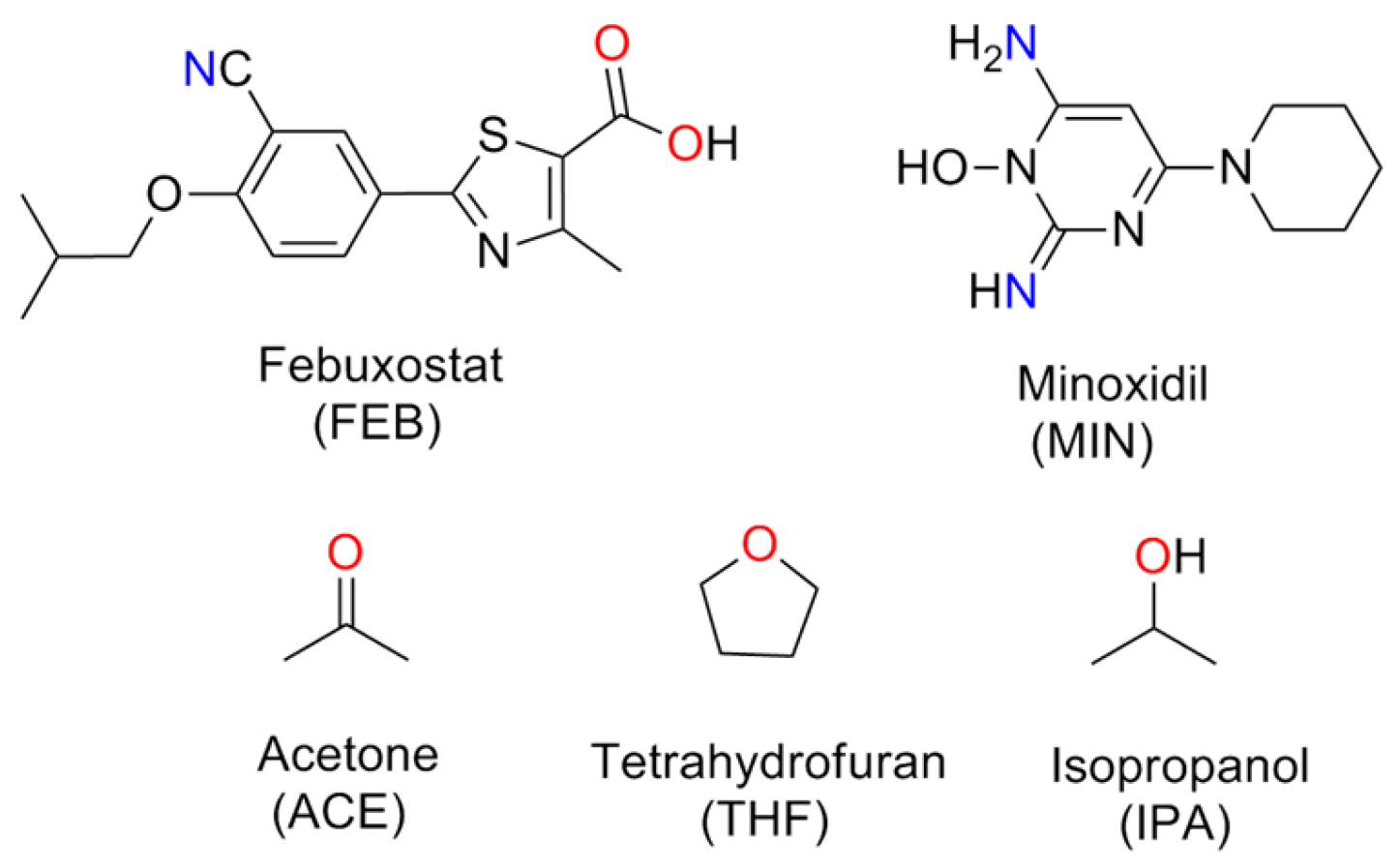
2.1. Materials and Equipment
2.2. Preparation of FEB-MIN·ACE
2.3. Preparation of FEB-MIN·THF
2.4. Preparation of FEB-MIN·IPA
2.5. Desolvation Experiments
2.6. X-ray Crystallographic Studies
2.7. Solvent-Exchange Experiments
2.8. Equilibrium Solubility Experiments
3. Results and Discussion
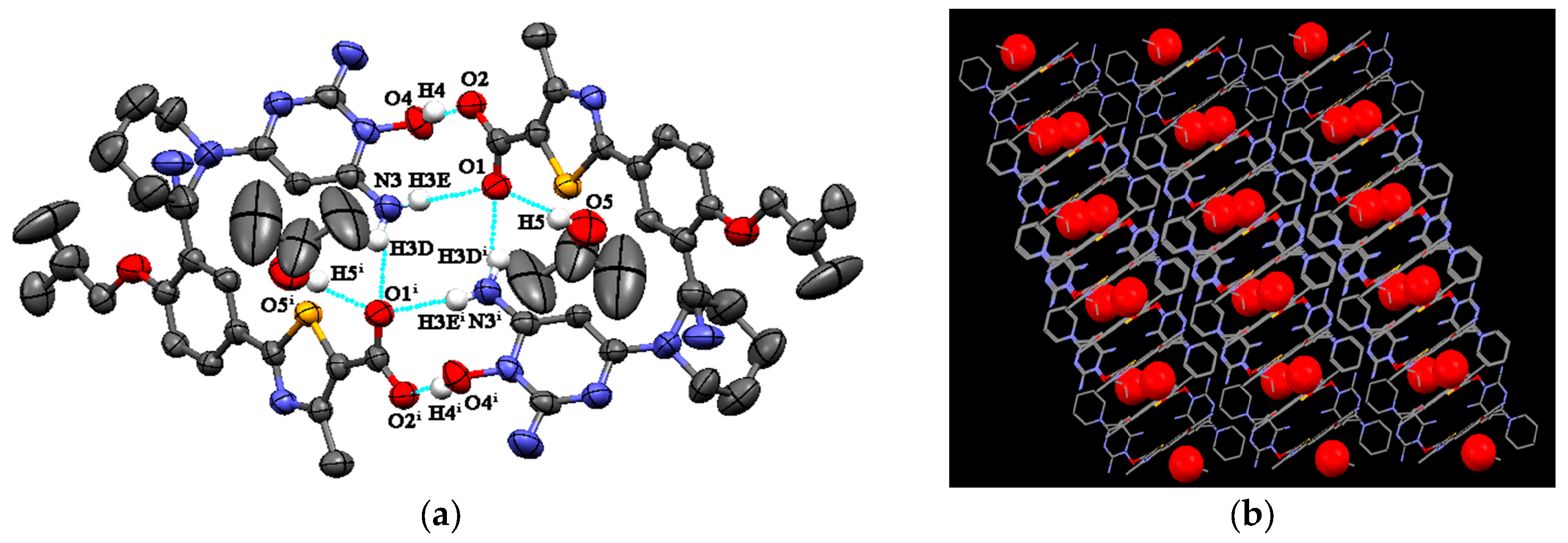
3.1. Crystal Structures
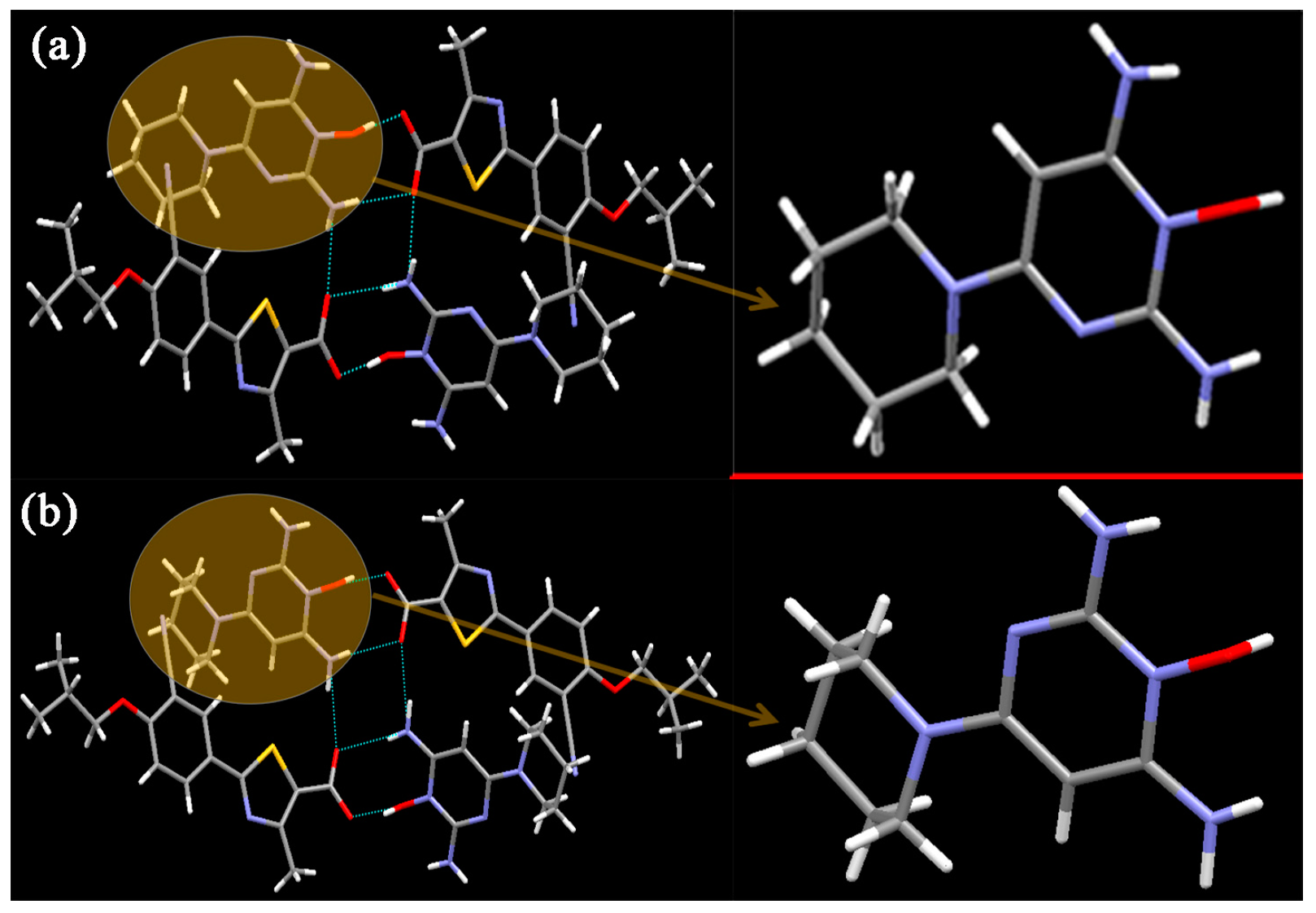
3.1.1. Crystal Structures of FEB-MIN·ACE and FEB-MIN·THF
3.1.2. Crystal Structure of FEB-MIN·IPA
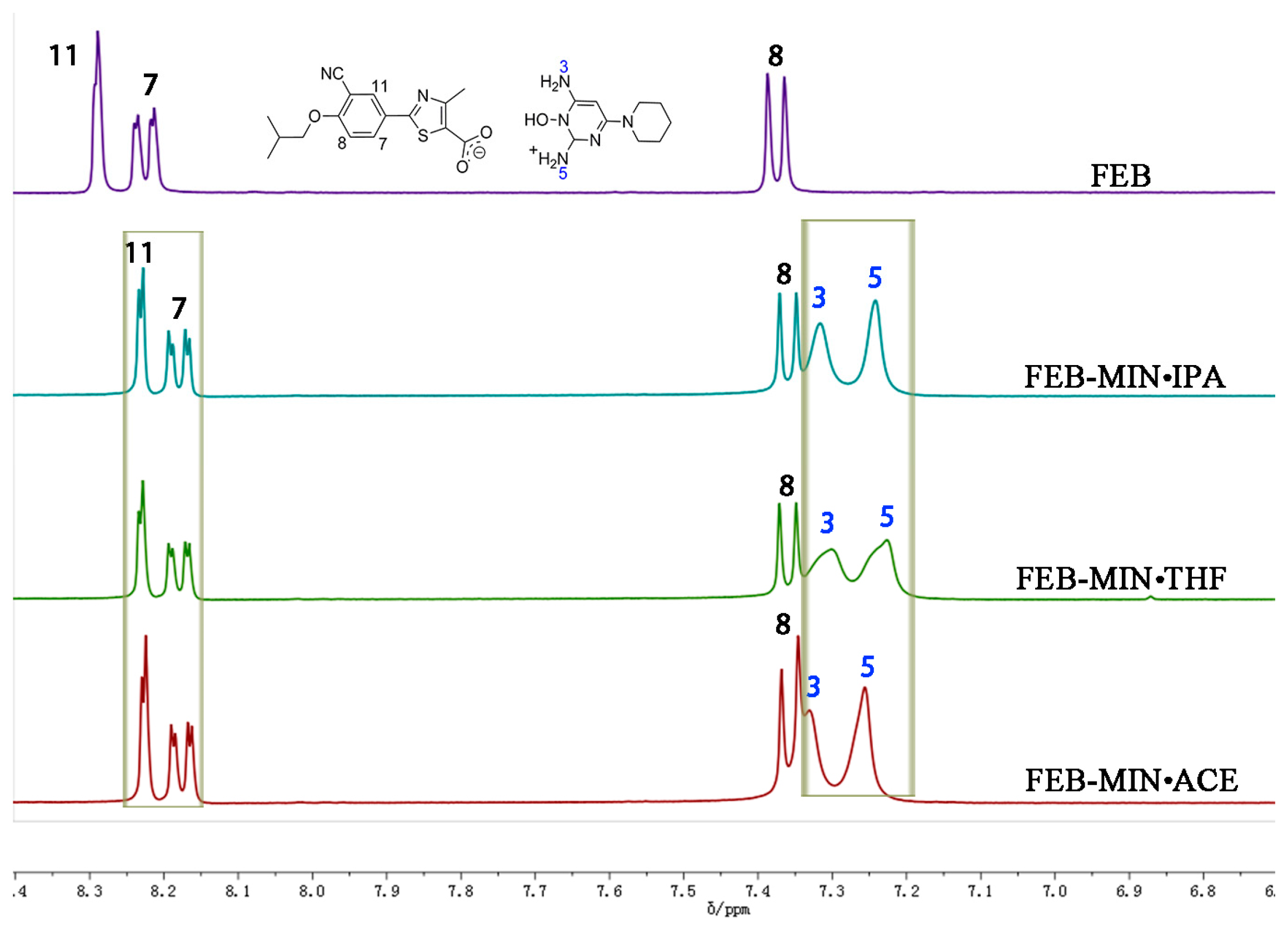
3.2. Nuclear Magnetic Resonance (1H-NMR) Spectral Analysis
3.3. IR Spectral Analysis
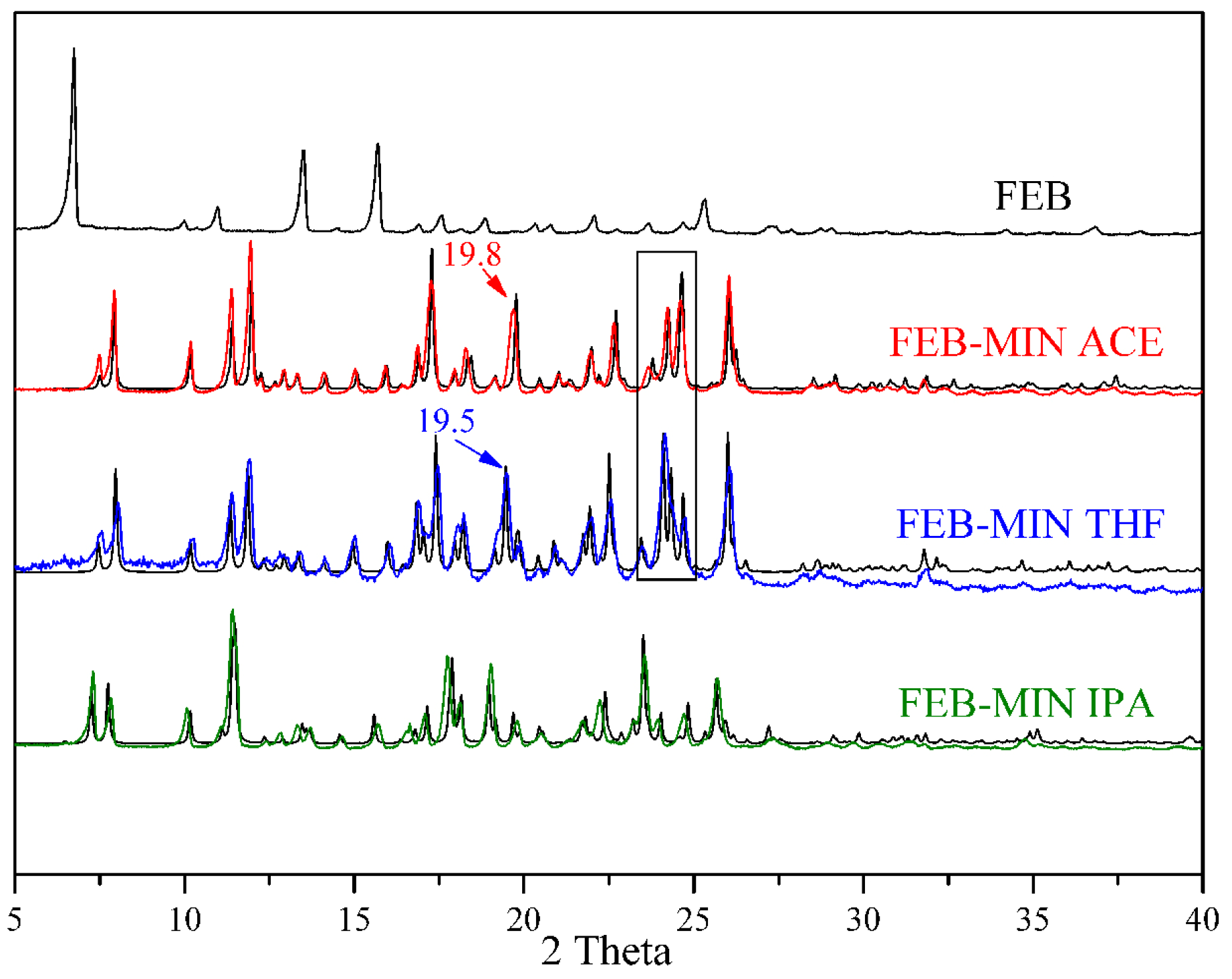
3.4. Powder X-ray Diffraction (PXRD) Analysis
3.5. Thermal Analysis
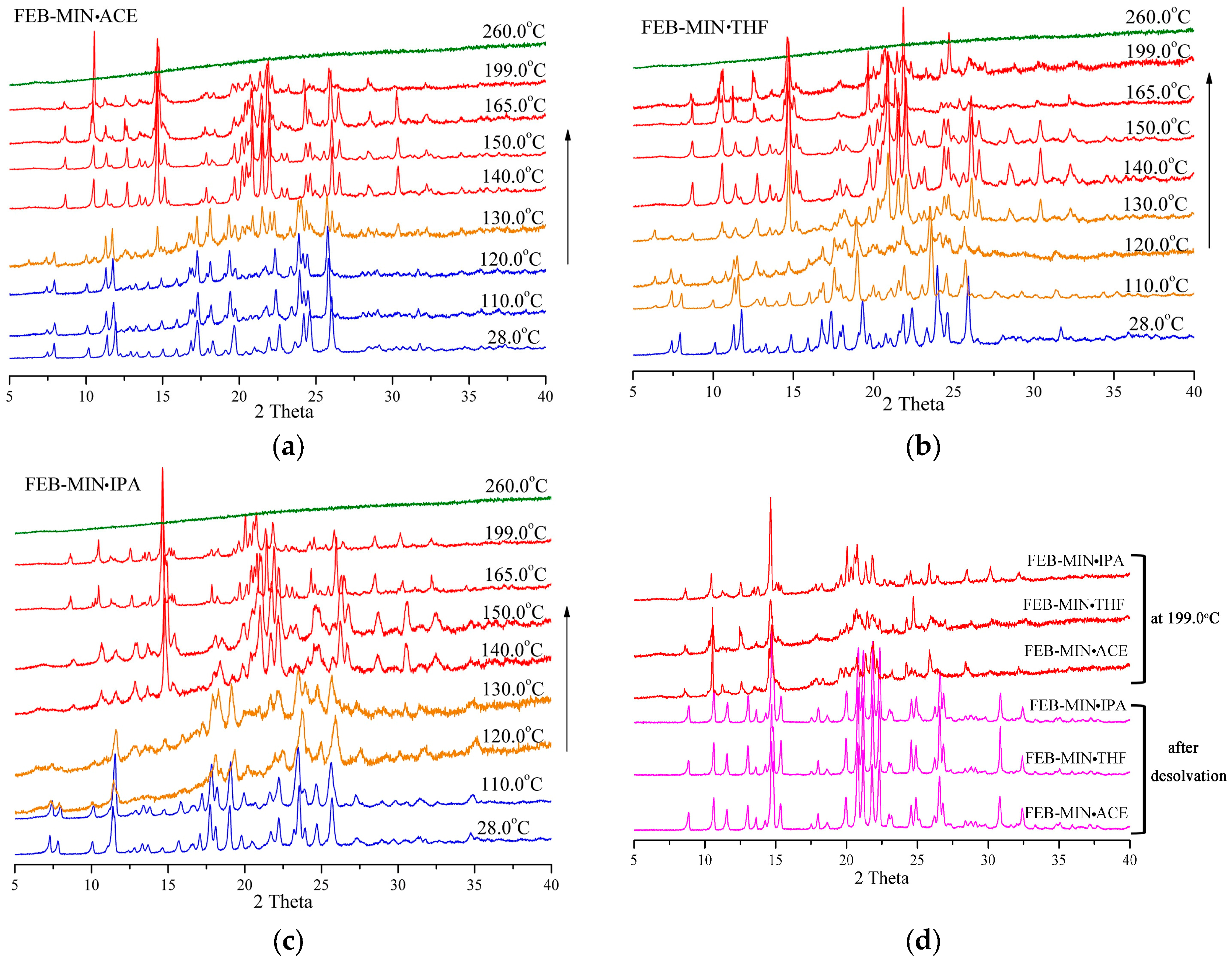
3.6. Variable Temperature Powder X-ray Diffraction (VT-PXRD) Analysis
3.7. Solvent-Exchange Experiments
3.8. Solubility Study
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Shan, N.; Perry, M.L.; Weyna, D.R.; Zaworotko, M.J. Impact of pharmaceutical cocrystals: The effects on drug pharmacokinetics. Expert Opin. Drug Metab. Toxicol. 2014, 10, 1255–1271. [Google Scholar] [CrossRef] [PubMed]
- Domingos, S.; Andre, V.; Quaresma, S.; Martins, I.C.B.; Piedade, M.D.; Duarte, M.T. New forms of old drugs: Improving without changing. J. Pharm. Pharmacol. 2015, 67, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Patel, V.; Mehta, T. Design of experiment approach in development of febuxostat nanocrystal: Application of soluplus as stabilizer. Powder Technol. 2016, 302, 396–405. [Google Scholar] [CrossRef]
- Pandya, R.B.; Mehta, T.A.; Gohel, M.C. Solid dispersion adsorbate-a novel technique for dissolution enhancement of febuxostat. Int. J. Pharm. Sci. Res. 2015, 6, 4236–4242. [Google Scholar]
- Ahuja, B.K.; Jena, S.K.; Paidi, S.K.; Bagri, S.; Suresh, S. Formulation, optimization and in vitro-in vivo evaluation of febuxostat nanosuspension. Int. J. Pharm. 2015, 478, 540–552. [Google Scholar] [CrossRef] [PubMed]
- Prohens, R.; Barbas, R.; Portell, A.; Font-Bardia, M.; Alcobe, X.; Puigjaner, C. Polymorphism of cocrystals: The promiscuous behavior of agomelatine. Cryst. Growth Des. 2016, 16, 1063–1070. [Google Scholar] [CrossRef]
- Sanphui, P.; Bolla, G.; Nangia, A. High solubility piperazine salts of the nonsteroidal anti-inflammatory drug (NSAID) meclofenamic acid. Cryst. Growth Des. 2012, 12, 2023–2036. [Google Scholar] [CrossRef]
- Aitipamula, S.; Chow, P.S.; Tan, R.B.H. Polymorphism in cocrystals. A review and assessment of its significance. CrystEngComm 2014, 16, 3451–3465. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, L. Solvent effect on the self-assembly of salt solvates of an antihypertensive drug azilsartan and 2-methylimidazole. J. Mol. Struct. 2017, 1137, 320–327. [Google Scholar] [CrossRef]
- Du, M.; Jiang, X.J.; Tan, X.; Zhang, Z.H.; Cai, H. Co-crystallization of a versatile building block 4-amino-3,5-bis (4-pyridyl)-1,2,4-triazole with R-isophthalic acids (R = –H, –NH2, –SO3H, and –COOH): Polymorphism and substituent effect on structural diversity. CrystEngComm 2009, 11, 454–462. [Google Scholar] [CrossRef]
- Okamoto, K.; Eger, B.T.; Nishino, T.; Kondo, S.; Pai, E.F.; Nishino, T. An extremely potent inhibitor of xanthine oxidoreductase. J. Biol. Chem. 2003, 278, 1848–1855. [Google Scholar] [CrossRef] [PubMed]
- Becker, M.A.; Schumacher, H.R., Jr.; Wortmann, R.L.; MacDonald, P.A.; Eustace, D.; Palo, W.A.; Streit, J.; Joseph-Ridge, N. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N. Engl. J. Med. 2005, 353, 2450–2461. [Google Scholar] [CrossRef] [PubMed]
- Ernst, M.E.; Fravel, M.A. Febuxostat: A selective xanthine-oxidase/xanthine-dehydrogenase inhibitor for the management of hyperuricemia in adults with gout. Clin. Ther. 2009, 31, 2503–2518. [Google Scholar] [CrossRef] [PubMed]
- Dass, R.; Jaiswal, S.; Gupta, G.D. Formulation and evaluation of febuxostat fast disintegrating tablet. Indo Am. J. Pharm. Res. 2014, 4, 2928–2936. [Google Scholar]
- Yadav, J.A.; Khomane, K.S.; Modi, S.R.; Ugale, B.; Yadav, R.N.; Nagaraja, C.M.; Kumar, N.; Bansal, A.K. Correlating single crystal structure, nanomechanical, and bulk compaction behavior of febuxostat polymorphs. Mol. Pharm. 2017, 14, 866–874. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.Y.; Qian, J.J.; Gu, J.M.; Tang, G.P.; Hu, X.R. Febuxostat methanol solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2011, 67, o1232. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Wang, Y.; Lu, T. 2-[3-cyano-4-(2-methylpropoxy) phenyl]-4-methylthiazole-5-carboxylic acid pyridine solvate. Acta Crystallogr. Sect. E Struct. Rep. Online 2009, 65, o2603. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Hu, X.R.; Gu, J.-M.; Tang, G.P. Crystal structure of febuxostat-acetic acid (1/1). Acta Crystallogr. Sect. E Struct. Rep. Online 2015, 71, o295–o296. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Gu, J.; Hu, X. Syntheses, structure characterization and dissolution of two novel cocrystals of febuxostat. J. Mol. Struct. 2017, 1130, 480–486. [Google Scholar] [CrossRef]
- Maddileti, D.; Jayabun, S.K.; Nangia, A. Soluble cocrystals of the xanthine oxidase inhibitor febuxostat. Cryst. Growth Des. 2013, 13, 3188–3196. [Google Scholar] [CrossRef]
- Zhang, X.R.; Zhang, L. Simultaneous enhancements of solubility and dissolution rate of poorly water-soluble febuxostat via salts. J. Mol. Struct. 2017, 1137, 328–334. [Google Scholar] [CrossRef]
- Han, X.M.; Qi, W.P.; Dong, W.X.; Guo, M.R.; Ma, P.Q.; Wang, J. Preparation, optimization and in vitro-in vivo investigation for capsules of the choline salt of febuxostat. Asian J. Pharm. Sci. 2016, 11, 715–721. [Google Scholar] [CrossRef]
- Zeng, Q.Z.; Ouyang, J.; Zhang, S.; Zhang, L. Structural characterization and dissolution profile of mycophenolic acid cocrystals. Eur. J. Pharm. Sci. 2017, 102, 140–146. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Rani, D.; Singh, C.; Kumar, A.; Sharma, V.K. Formulation Development and In-vitro Evaluation of Minoxidil Bearing Glycerosomes. Am. J. Biomed. Res. 2016, 4, 27–37. [Google Scholar]



| Formula | FEB-MIN·ACE | FEB-MIN·THF | FEB-MIN·IPA |
|---|---|---|---|
| (C16H15N2O3S) (C9H16N5O) (C3H6O) | (C16H15N2O3S) (C9H16N5O) (C4H8O) | (C16H15N2O3S) (C9H16N5O) (C3H8O) | |
| Formula Weight | 583.71 | 597.73 | 585.80 |
| Crystal System | triclinic | triclinic | triclinic |
| Space group | P-1 | P-1 | P-1 |
| Wavelength (Å) | 0.71073 | 0.71073 | 0.71073 |
| a(Å) | 8.7459(8) | 8.8090(8) | 8.8360(8) |
| b(Å) | 12.5214(10) | 12.6426(15) | 13.1049(13) |
| c(Å) | 14.9201(13) | 14.9052(13) | 14.6481(11) |
| α(°) | 109.959(8) | 109.349(10) | 110.509(8) |
| β(°) | 95.476(7) | 96.149(7) | 96.687(7) |
| γ(°) | 94.023(7) | 94.585(9) | 96.470(8) |
| V(Å3) | 1519.5(2) | 1545.4(3) | 1556.2(2) |
| Z | 2 | 2 | 2 |
| F(000) | 620 | 636 | 624 |
| D/g·cm−3 | 1.276 | 1.285 | 1.250 |
| T(K) | 293(2) | 293(2) | 293(2) |
| Reflections collected | 2869 | 2500 | 1932 |
| Independent reflections | 5359 | 5402 | 5328 |
| Reflections observed (I > 2σ(I)) | 3723 | 3522 | 3090 |
| θ range (°) | 2.93 to 25.03 | 2.84 to 25.02 | 2.86 to 25.02 |
| Data/restraints/parameters | 5359/10/395 | 5402/16/409 | 5328/3/383 |
| Goodness-of-fit on F2 | 1.054 | 1.049 | 1.010 |
| Final R indices [I > 2σ(I)] | R1 = 0.0847 | R1 = 0.0942 | R1 = 0.0699 |
| ωR2 = 0.2297 | ωR2 = 0.2654 | ωR2 = 0.1624 | |
| R indices (all data) | R1 = 0.1131, | R1 = 0.1282, | R1 = 0.1235, |
| ωR2 = 0.2599 | ωR2 = 0.3116 | ωR2 = 0.2039 | |
| Largest diff. peak and hole (eÅ−3) | 0.935, −0.613 | 0.644, −0.759 | 0.389, −0.242 |
| Salt Solvates | D-H···A | Symmetry | D–H (Å) | H···A (Å) | D···A (Å) | ∠D–H···A (◦) |
|---|---|---|---|---|---|---|
| FEB-MIN·ACE | O4-H4···O2 | −x + 2, −y + 2, −z | 0.97 | 1.50 | 2.465 | 174 |
| N5-H5B···O1 | −x + 2, −y + 2, −z | 0.80 | 2.05 | 2.864 | 162 | |
| N5-H5A···O1i | x, y, z | 0.78 | 2.28 | 3.034 | 164 | |
| FEB-MIN·THF | O4-H4···O2 | x + 1, y, z | 0.82 | 1.67 | 2.472 | 167 |
| N5-H5B···O1 | x + 1, y, z | 0.86 | 2.10 | 2.854 | 146 | |
| N5-H5A ···O1ii | −x + 1, −y + 1, −z + 1 | 0.86 | 2.17 | 3.003 | 164 | |
| FEB-MIN·IPA | O4-H4···O2 | −x + 1, −y + 1, −z + 1 | 0.82 | 1.67 | 2.484 | 172 |
| N3-H3D···O1i | x, y, z | 0.86 | 2.16 | 2.994 | 165 | |
| N3-H3E···O1 | −x + 1,−y + 1, −z + 1 | 0.86 | 2.11 | 2.878 | 148 |
| Number | 2θ (FEB-MIN·ACE) | 2θ (FEB-MIN·THF) | 2θ (FEB-MIN·IPA) | |||
|---|---|---|---|---|---|---|
| Experimental | Simulated | Experimental | Simulated | Experimental | Simulated | |
| 1 | 7.55 | 7.56 | 7.42 | 7.46 | 7.34 | 7.30 |
| 2 | 7.96 | 7.98 | 7.92 | 7.96 | 7.82 | 7.80 |
| 3 | 10.19 | 10.22 | 10.15 | 10.16 | 10.16 | 10.22 |
| 4 | 11.42 | 11.48 | 11.30 | 11.34 | 11.47 | 11.52 |
| 5 | 11.99 | 12.04 | 11.81 | 11.86 | 13.49 | 13.52 |
| 6 | 12.95 | 12.99 | 12.90 | 12.94 | 14.65 | 14.62 |
| 7 | 13.34 | 13.40 | 13.31 | 13.34 | 15.63 | 15.64 |
| 8 | 14.17 | 14.26 | 14.06 | 14.10 | 16.73 | 16.84 |
| 9 | 15.09 | 15.18 | 14.89 | 14.96 | 17.17 | 17.20 |
| 10 | 15.96 | 16.02 | 15.89 | 15.98 | 17.85 | 17.92 |
| 11 | 17.30 | 17.42 | 16.76 | 16.84 | 18.17 | 18.20 |
| 12 | 17.99 | 18.11 | 17.33 | 17.40 | 19.03 | 19.00 |
| 13 | 18.34 | 18.58 | 18.12 | 18.18 | 19.77 | 19.74 |
| 14 | 19.75 | 19.90 | 19.09 | 19.14 | 20.53 | 20.50 |
| 15 | 21.06 | 21.16 | 19.48 | 19.46 | 21.77 | 21.86 |
| 16 | 22.00 | 22.12 | 19.74 | 19.84 | 22.35 | 22.44 |
| 17 | 22.71 | 22.84 | 22.47 | 22.50 | 23.55 | 23.56 |
| 18 | 24.26 | 24.40 | 24.05 | 24.10 | 24.00 | 24.08 |
| 19 | 24.63 | 24.78 | 24.63 | 24.68 | 24.82 | 24.88 |
| 20 | 26.05 | 26.18 | 25.97 | 26.00 | 25.68 | 25.70 |
| Salt Solvates | 24 h | 48 h | |||||
|---|---|---|---|---|---|---|---|
| FEB-MIN·ACE (%) | FEB-MIN·THF (%) | FEB-MIN·IPA (%) | FEB-MIN·ACE (%) | FEB-MIN·THF (%) | FEB-MIN·IPA (%) | ||
| Entry 1 | FEB-MIN·ACE | 74.7 | 18.2 | 7.1 | 75.8 | 21.2 | 3.0 |
| Entry 2 | FEB-MIN·THF | 40.4 | 50.0 | 9.3 | 47.8 | 50.0 | 2.2 |
| Entry 3 | FEB-MIN·IPA | 45.6 | 34.0 | 20.4 | 50.5 | 38.4 | 11.1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.-Y.; Du, R.-K.; Du, Y.-L.; Zhang, C.-J.; Guan, S.; Dong, C.-Z.; Zhang, L. Febuxostat-Minoxidil Salt Solvates: Crystal Structures, Characterization, Interconversion and Solubility Performance. Crystals 2018, 8, 85. https://doi.org/10.3390/cryst8020085
Li L-Y, Du R-K, Du Y-L, Zhang C-J, Guan S, Dong C-Z, Zhang L. Febuxostat-Minoxidil Salt Solvates: Crystal Structures, Characterization, Interconversion and Solubility Performance. Crystals. 2018; 8(2):85. https://doi.org/10.3390/cryst8020085
Chicago/Turabian StyleLi, Li-Yang, Rong-Kai Du, You-Li Du, Chun-Jing Zhang, Su Guan, Chang-Zhi Dong, and Lei Zhang. 2018. "Febuxostat-Minoxidil Salt Solvates: Crystal Structures, Characterization, Interconversion and Solubility Performance" Crystals 8, no. 2: 85. https://doi.org/10.3390/cryst8020085
APA StyleLi, L.-Y., Du, R.-K., Du, Y.-L., Zhang, C.-J., Guan, S., Dong, C.-Z., & Zhang, L. (2018). Febuxostat-Minoxidil Salt Solvates: Crystal Structures, Characterization, Interconversion and Solubility Performance. Crystals, 8(2), 85. https://doi.org/10.3390/cryst8020085





