The Electrical Properties of Tb-Doped CaF2 Nanoparticles under High Pressure
Abstract
:1. Introduction
2. Materials and Methods
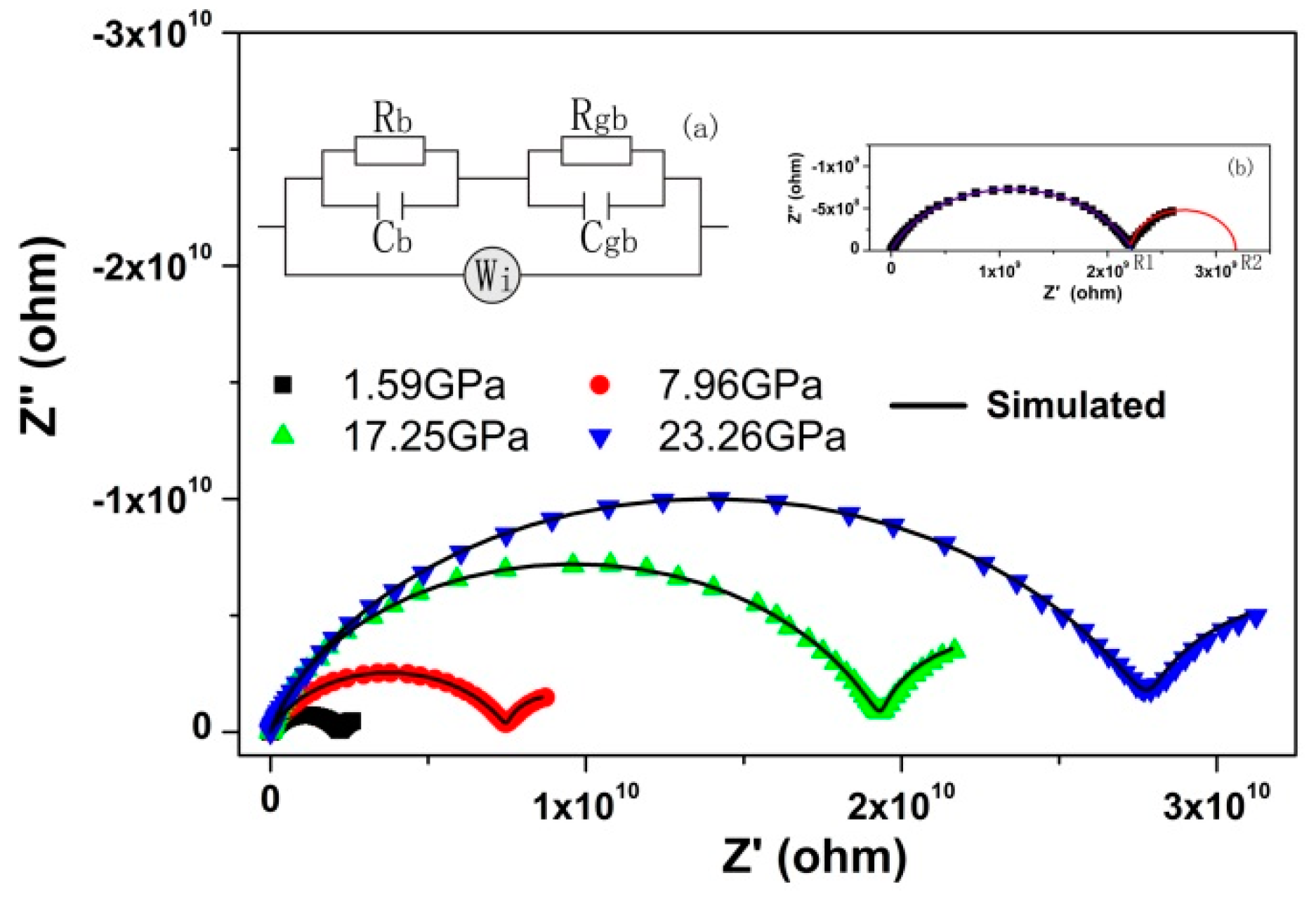
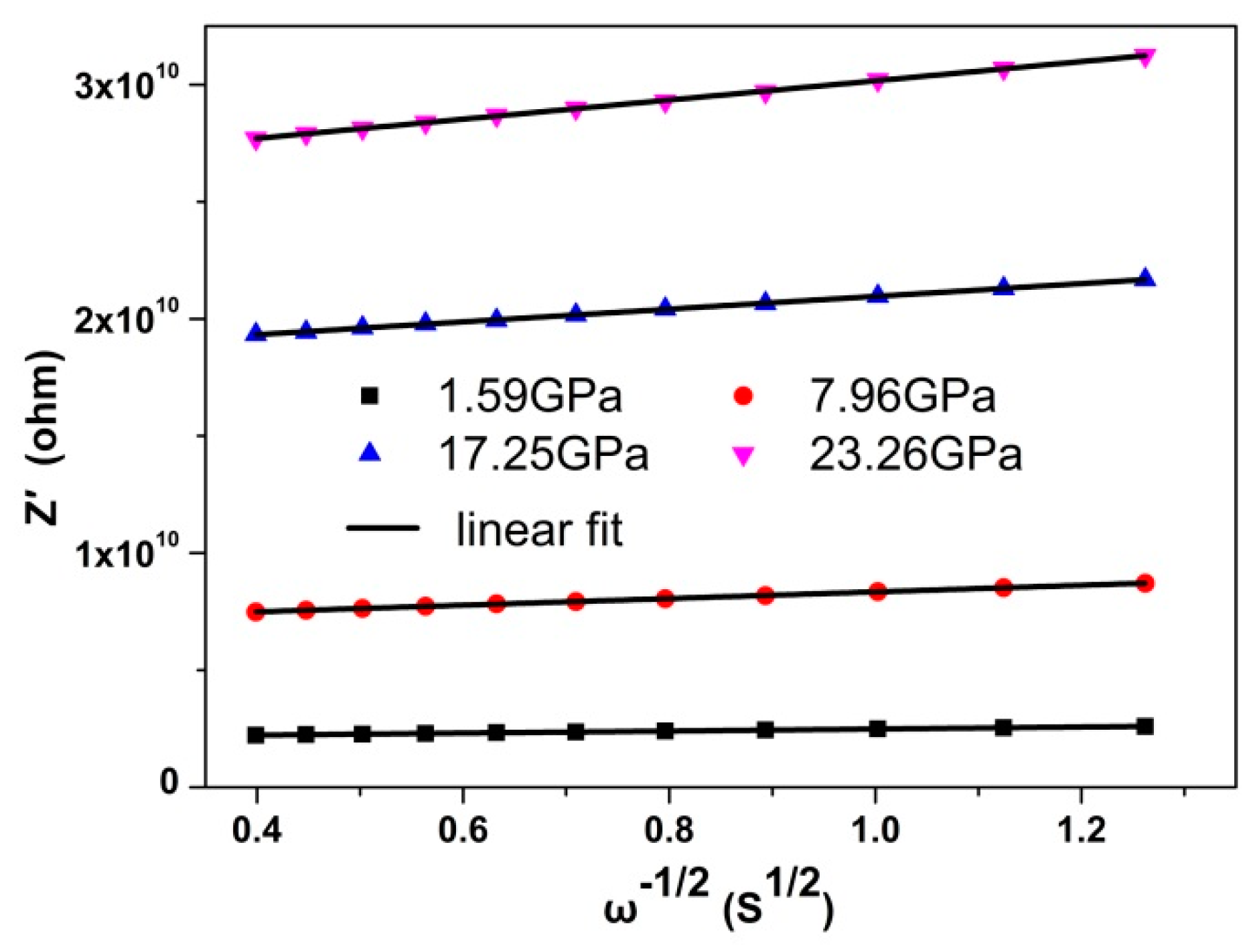
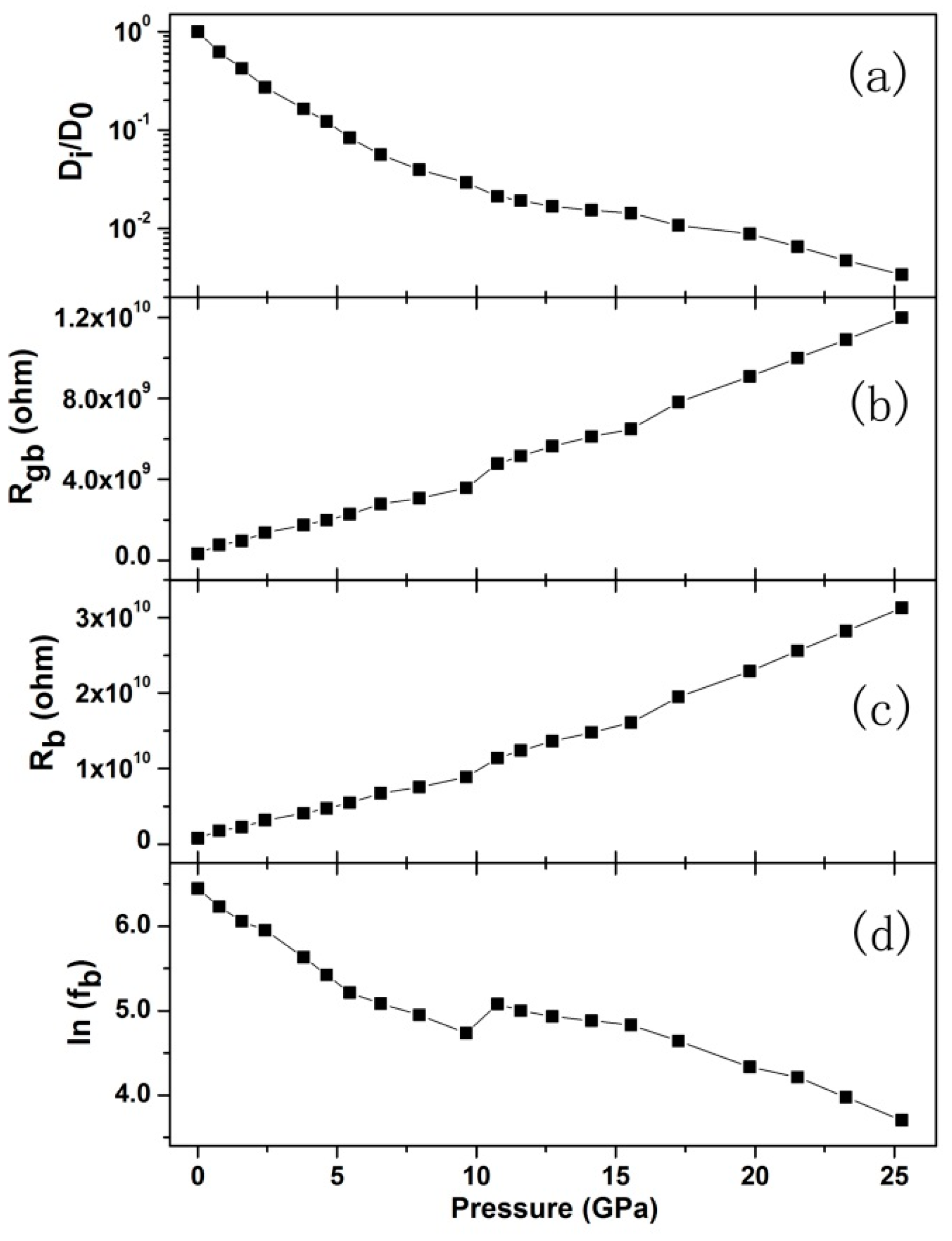
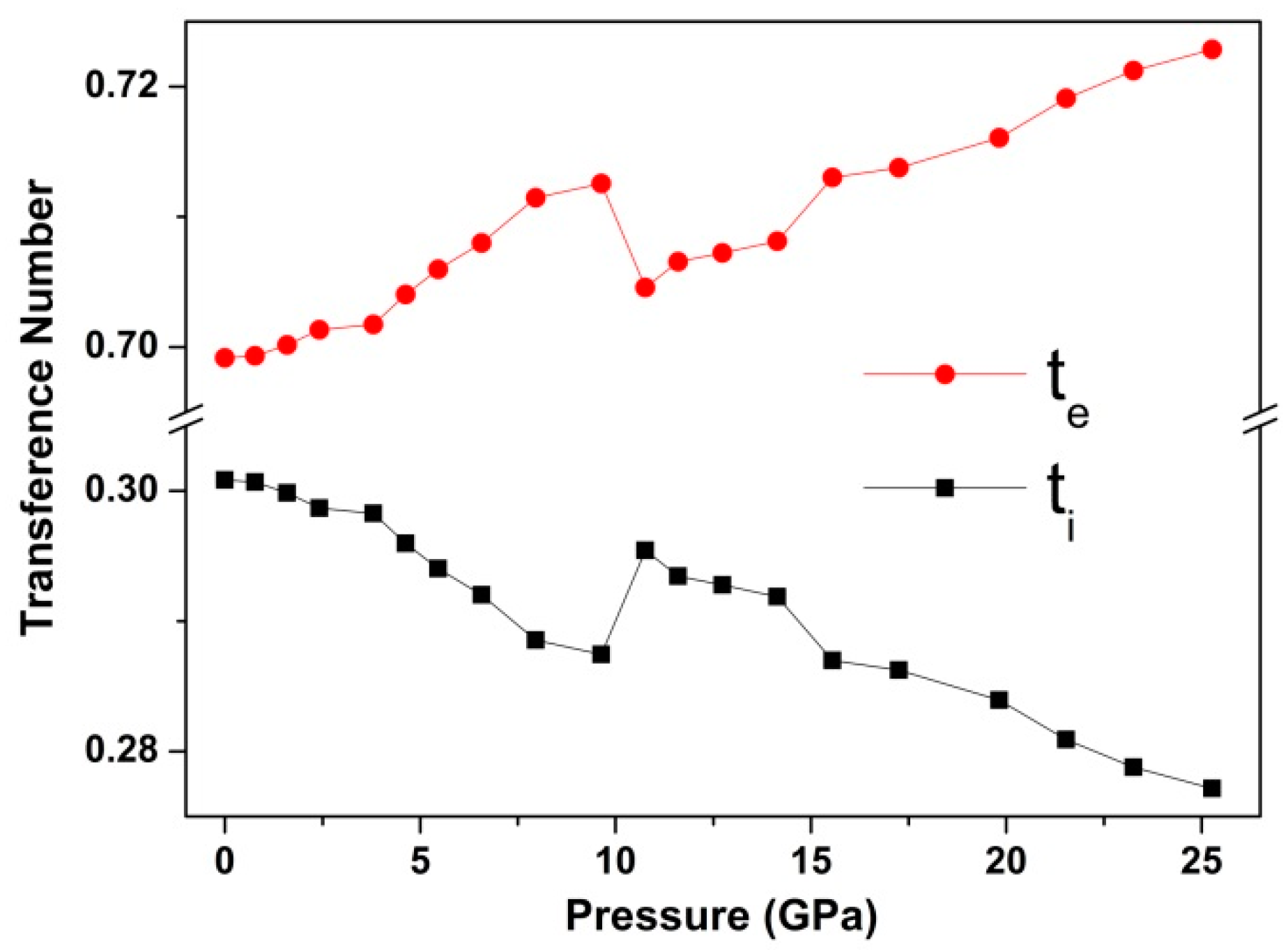
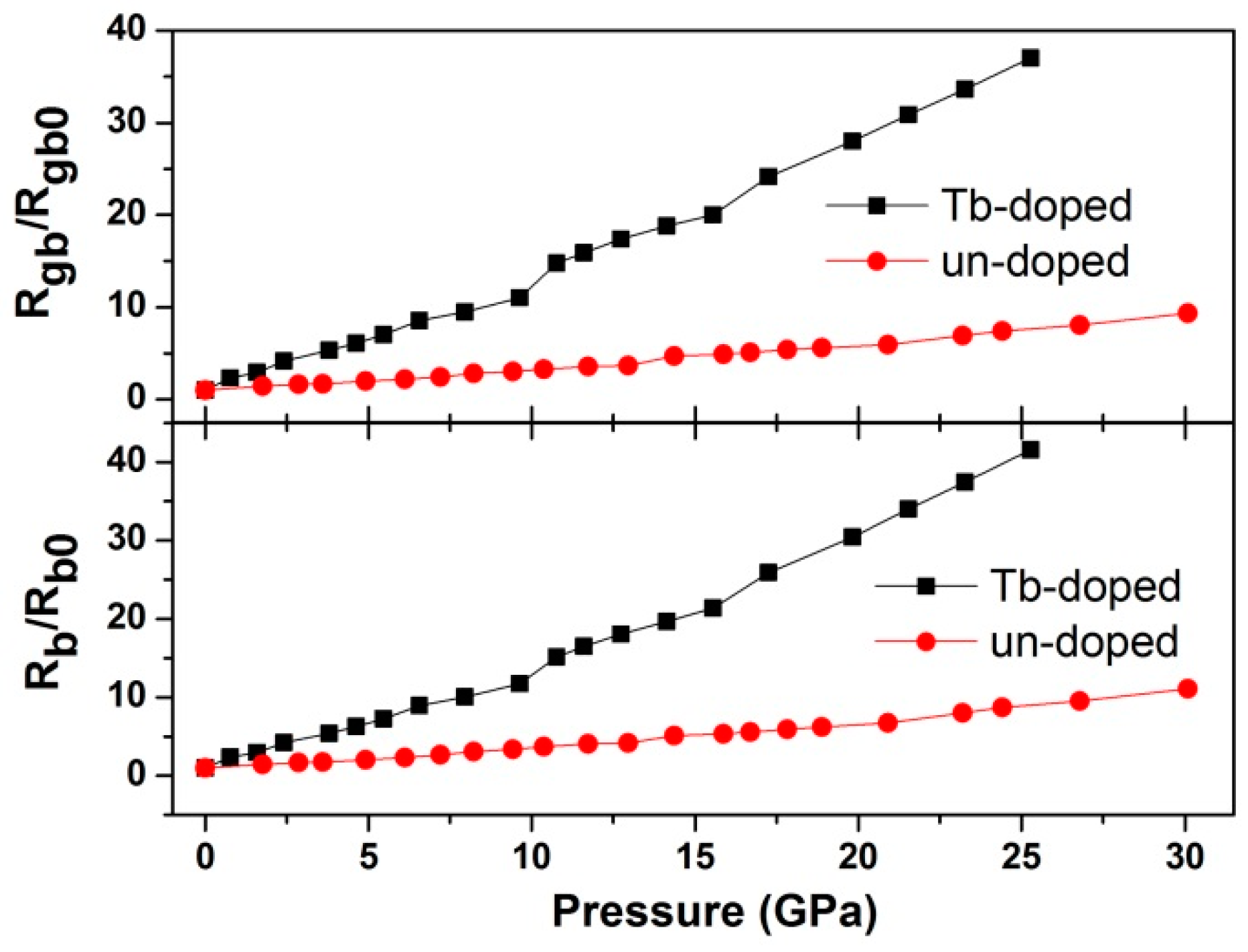
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Bazzi, R.; Flores, M.A.; Louis, C.; Lebbou, K.; Zhang, W.; Dujardin, C.; Roux, S.; Mercier, B.; Ledoux, G.; Bernstein, E.; et al. Synthesis and properties of europium-based phosphors on the nanometer scale: Eu2O3, Gd2O3: Eu, and Y2O3: Eu. J. Colloid Interface Sci. 2004, 273, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Nishi, M.; Tanabe, S.; Inoue, M.; Takahashi, M.; Fujita, K.; Hirao, K. Optical-telecommunication-band fluorescence properties of Er3+-doped YAG nanocrystals synthesized by glycothermal method. Opt. Mater. 2005, 27, 655–662. [Google Scholar] [CrossRef]
- Rulison, A.J.; Flagan, R.C. Synthesis of yttria powders by electrospray pyrolysis. J. Am. Ceram. Soc. 1994, 77, 3244–3250. [Google Scholar] [CrossRef]
- Patra, A.; Friend, C.S.; Kapoor, R.; Prasad, P.N. Fluorescence upconversion properties of Er3+-doped TiO2 and BaTiO3 nanocrystallites. Chem. Mater. 2003, 15, 3650–3655. [Google Scholar] [CrossRef]
- Masenelli, B.; Melinon, P.; Nicolas, D.; Bernstein, E.; Prevel, B.; Kapsa, J.; Boisron, O.; Perezl, A.; Ledoux, G.; Mercier, B.; et al. Rare earth based clusters for nanoscale light source. Eur. Phys. J. D 2005, 34, 139–143. [Google Scholar] [CrossRef]
- Wei, Z.G.; Sun, L.D.; Liao, C.S.; Yan, C.H. Fluorescence intensity and color purity improvement in nanosized YBO3: Eu. Appl. Phys. Lett. 2002, 80, 1447–1449. [Google Scholar] [CrossRef]
- Matsuura, D. Red, green, and blue upconversion luminescence of trivalent-rare-earth ion-doped Y2O3 nanocrystals. Appl. Phys. Lett. 2002, 81, 4526–4528. [Google Scholar] [CrossRef]
- Barber, D.B.; Pollock, C.R.; Beecroft, L.L.; Ober, C.K. Amplification by optical composites. Opt. Lett. 1997, 22, 1247–1249. [Google Scholar] [CrossRef] [PubMed]
- Yi, G.; Lu, H.; Zhao, S.; Ge, Y.; Yang, W.; Chen, D.; Guo, L. Synthesis, characterization, and biological application of size-controlled nanocrystalline NaYF4:Yb, Er infrared-to-visible up-conversion phosphors. Nano Lett. 2004, 4, 2191–2196. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, H.; Hu, H.; Yu, M.; Li, F.; Zhang, Q.; Zhou, Z.; Yi, T.; Huang, C. Versatile synthesis strategy for carboxylic acid−functionalized upconverting nanophosphors as biological labels. J. Am. Chem. Soc. 2008, 130, 3023–3029. [Google Scholar] [CrossRef] [PubMed]
- Fujihara, S.; Kadota, Y.; Kimura, T. Role of organic additives in the sol-gel synthesis of porous CaF2 anti-reflective coatings. J. Sol-Gel Sci. Technol. 2002, 24, 147–154. [Google Scholar] [CrossRef]
- McKeever, S.W.S.; Brown, M.D.; Abbundi, R.J.; Chan, H.; Mathur, V.K. Characterization of optically active sites in CaF2: Ce, Mn from optical spectra. J. Appl. Phys. 1986, 60, 2505–2510. [Google Scholar] [CrossRef]
- Fukuda, Y. Thermoluminescence in sintered CaF2:Tb. J. Radiat. Res. 2002, 43, S67–S69. [Google Scholar] [CrossRef] [PubMed]
- Pote, S.S.; Joshi, C.P.; Moharil, S.V.; Muthal, P.L.; Dhopte, S.M. Luminescence of Ce3+ in Ca0.65 La0.35F2.35 host. J. Lumin. 2010, 130, 666–668. [Google Scholar] [CrossRef]
- Cazorla1, C.; Errandonea, D. Superionicity and polymorphism in calcium fluoride at high pressure. Phys. Rev. Lett. 2014, 113, 235902. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, D.C.; West, A.R. Impedance and modulus spectroscopy of semiconducting BaTiO3 showing positive temperature coefficient of resistance. J. Appl. Phys. 1989, 66, 3850–3856. [Google Scholar] [CrossRef]
- Sinclair, D.C.; Adams, T.B.; Morrison, F.D.; West, A.R. CaCu3Ti4O12: One-step internal barrier layer capacitor. Appl. Phys. Lett. 2002, 80, 2153–2155. [Google Scholar] [CrossRef]
- Dutta, S.; Choudhary, R.N.P.; Sinha, P.K.; Thakur, A.K. Microstructural studies of (PbLa)(ZrTi)O3 ceramics using complex impedance spectroscopy. J. Appl. Phys. 2004, 96, 1607–1613. [Google Scholar] [CrossRef]
- Hsu, H.S.; Huang, J.C.A.; Chen, S.F.; Liu, C.P. Role of grain boundary and grain defects on ferromagnetism in Co : ZnO films. Appl. Phys. Lett. 2007, 90, 102506. [Google Scholar] [CrossRef]
- Dualeh, A.; Moehl, T.; Tetreault, N.; Teuscher, J.; Gao, P.; Nazeeruddin, M.K.; Gratzel, M. Impedance spectroscopic analysis of lead iodide perovskite-sensitized solid-state solar cells. ACS Nano 2013, 8, 362–373. [Google Scholar] [CrossRef] [PubMed]
- Huggins, R.A. Simple method to determine electronic and ionic components of the conductivity in mixed conductors: A review. Ionics 2002, 8, 300–313. [Google Scholar] [CrossRef]
- Teraoka, Y.; Zhang, H.; Okamoto, K.; Yamazoe, N. Mixed ionic-electronic conductivity of La1−xSrxCo1−yFeyO3−δ perovskite-type oxides. Mater. Res. Bull. 1988, 23, 51–58. [Google Scholar] [CrossRef]
- Riess, I. Measurements of electronic and ionic partial conductivities in mixed conductors, without the use of blocking electrodes. Solid State Ionics 1991, 44, 207–214. [Google Scholar] [CrossRef]
- Riess, I. Review of the limitation of the Hebb-Wagner polarization method for measuring partial conductivities in mixed ionic electronic conductors. Solid State Ion. 1996, 91, 221–232. [Google Scholar] [CrossRef]
- Adler, S.B.; Lane, J.; Steele, B. Electrode kinetics of porous mixed-conducting oxygen electrodes. J. Electrochem. Soc. 1996, 143, 3554–3564. [Google Scholar] [CrossRef]
- Riess, I. Mixed ionic–electronic conductors—Material properties and applications. Solid State Ion. 2003, 157, 1–17. [Google Scholar] [CrossRef]
- Cui, X.Y.; Hu, T.J.; Wang, J.S.; Zhang, J.K.; Zhao, R.; Li, X.F.; Yang, J.H.; Gao, C.X. Mixed conduction in BaF2 nanocrystals under high pressure. RSC Adv. 2017, 7, 12098–12102. [Google Scholar] [CrossRef]
- Cui, X.Y.; Hu, T.J.; Wang, J.S.; Zhang, J.K.; Li, X.F.; Yang, J.H.; Gao, C.X. High pressure impedance spectroscopy of SrF2 nanocrystals. High Press. Res. 2017, 37, 312–318. [Google Scholar] [CrossRef]
- Hu, T.J.; Cui, X.Y.; Wang, J.S.; Zhang, J.K.; Li, X.F.; Yang, J.H.; Gao, C.X. Transport properties of mixing conduction in CaF2 nanocrystals under high pressure. Chin. Phys. B 2018, 27, 016401. [Google Scholar] [CrossRef]
- Mao, H.K.; Bell, P.M. High-pressure physics: The 1-megabar mark on the ruby R1 static pressure scale. Science 1976, 191, 851–852. [Google Scholar] [CrossRef] [PubMed]
- Errandonea, D.; Muñoz, A.; Gonzalez-Platas, J. Comment on “High-pressure X-ray diffraction study of YBO3/Eu3+, GdBO3, and EuBO3: Pressure-induced amorphization in GdBO3” [J. Appl. Phys. 115, 043507 (2014)]. J. Appl. Phys. 2014, 115, 216101. [Google Scholar] [CrossRef]
- Errandonea, D.; Segura, A.; Martínez-García, D.; Muñoz-San Jose, V. Hall-effect and resistivity measurements in CdTe and ZnTe at high pressure: Electronic structure of impurities in the zinc-blende phase and the semimetallic or metallic character of the high-pressure phases. Phys. Rev. B 2009, 79, 125203. [Google Scholar] [CrossRef]
- Wang, J.S.; Hao, J.; Wang, Q.S.; Jin, Y.X.; Li, F.F.; Liu, B.; Li, Q.J.; Liu, B.B.; Cui, Q.L. Pressure-induced structural transition in CaF2 nanocrystals. Phys. Status Solidi B 2011, 248, 1115–1118. [Google Scholar] [CrossRef]
- Wang, Q.L.; Liu, C.L.; Gao, Y.; Ma, Y.Z.; Han, Y.H.; Gao, C.X. Mixed conduction and grain boundary effect in lithium niobate under high pressure. Appl. Phys. Lett. 2015, 106, 132902. [Google Scholar] [CrossRef]
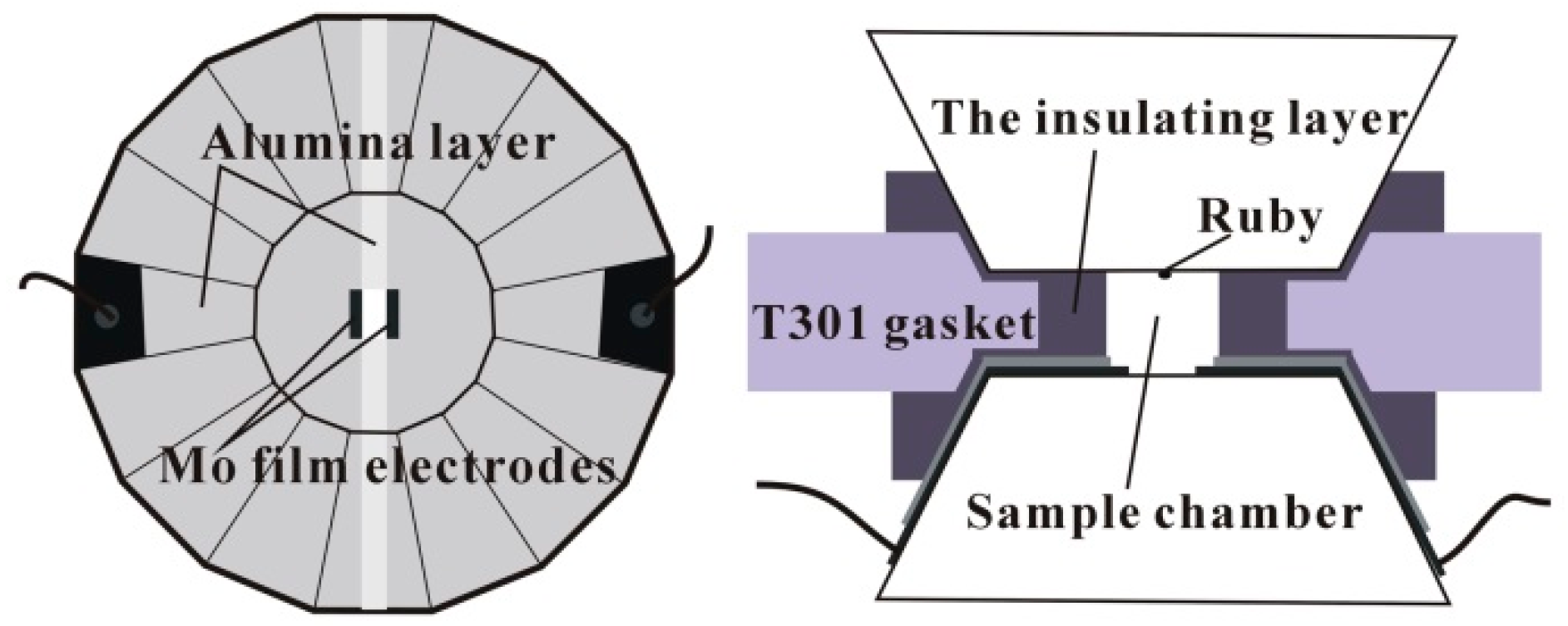
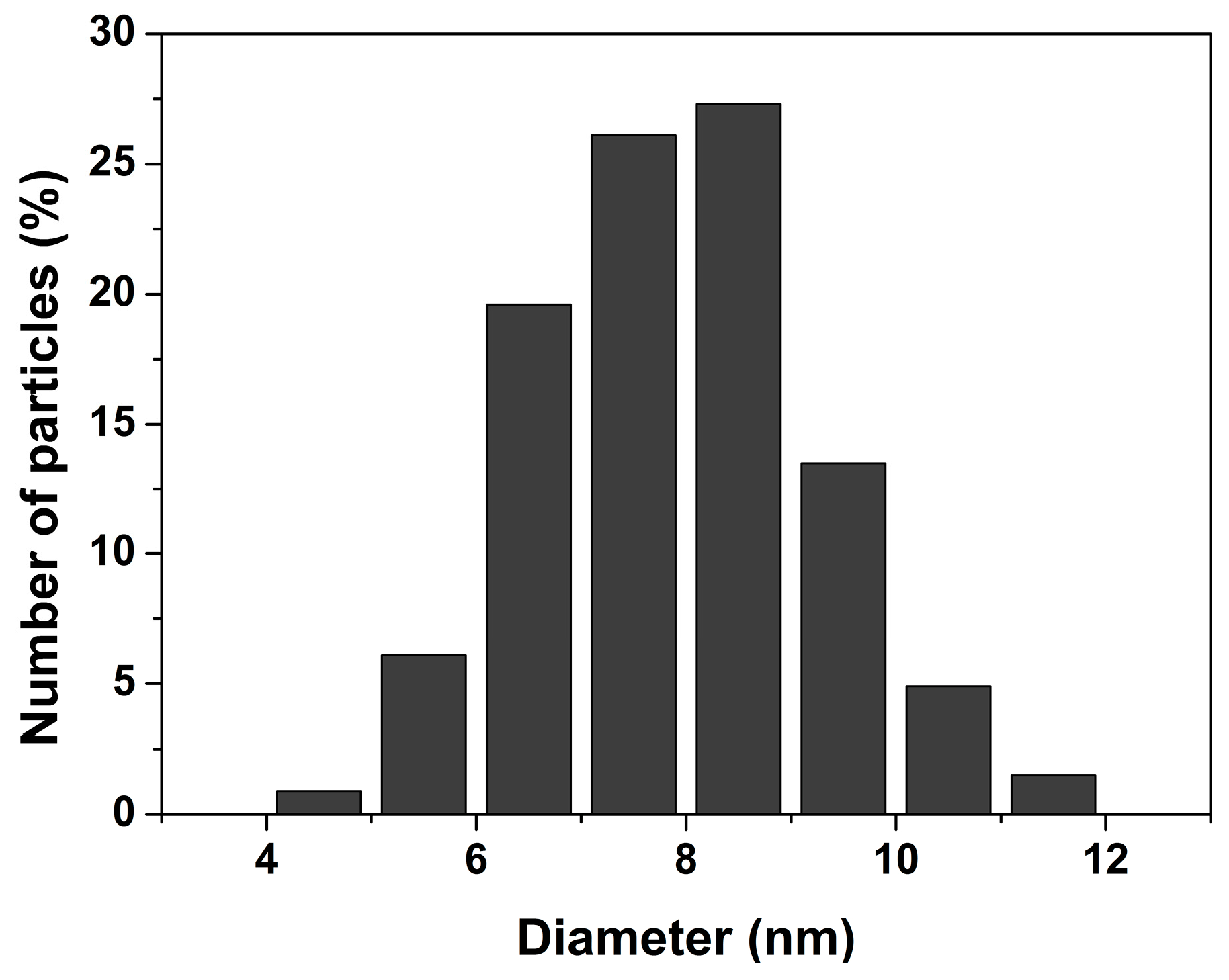
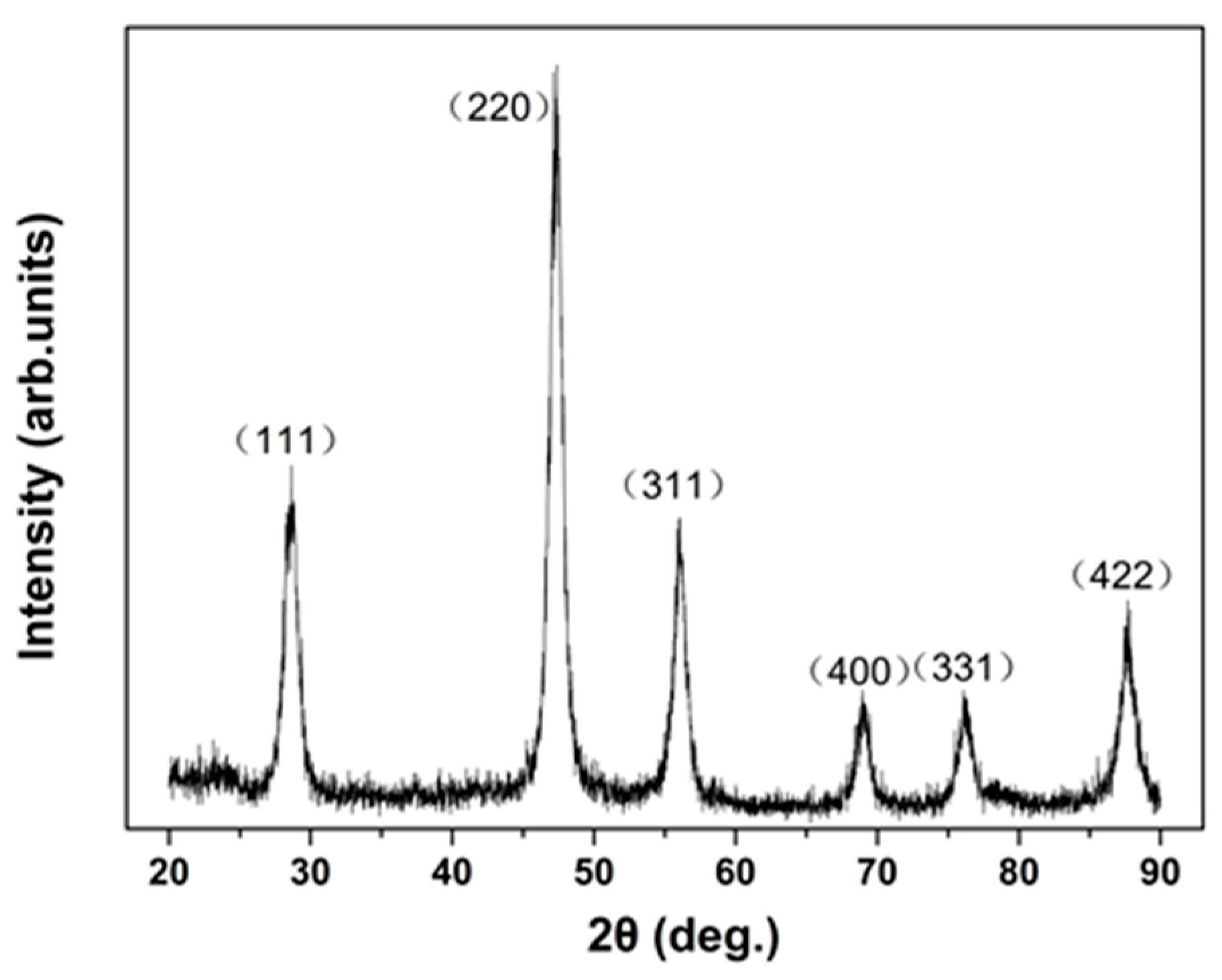
| Phase | dH/dp (meV/GPa) (Tb-dDoped) | dH/dp (meV/GPa) (Un-Doped) |
|---|---|---|
| Fm3m | 4.70 | 3.12 |
| Pnma | 2.97 | 1.44 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, T.; Cui, X.; Wang, J.; Zhong, X.; Chen, Y.; Zhang, J.; Li, X.; Yang, J.; Gao, C. The Electrical Properties of Tb-Doped CaF2 Nanoparticles under High Pressure. Crystals 2018, 8, 98. https://doi.org/10.3390/cryst8020098
Hu T, Cui X, Wang J, Zhong X, Chen Y, Zhang J, Li X, Yang J, Gao C. The Electrical Properties of Tb-Doped CaF2 Nanoparticles under High Pressure. Crystals. 2018; 8(2):98. https://doi.org/10.3390/cryst8020098
Chicago/Turabian StyleHu, Tingjing, Xiaoyan Cui, Jingshu Wang, Xin Zhong, Yinzhu Chen, Junkai Zhang, Xuefei Li, Jinghai Yang, and Chunxiao Gao. 2018. "The Electrical Properties of Tb-Doped CaF2 Nanoparticles under High Pressure" Crystals 8, no. 2: 98. https://doi.org/10.3390/cryst8020098





