Intra-/Intermolecular Bifurcated Chalcogen Bonding in Crystal Structure of Thiazole/Thiadiazole Derived Binuclear (Diaminocarbene)PdII Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Instrumentation
2.2. Synthesis and Characterization
2.3. X-ray Structure Determination
2.4. Details of Hirshfeld Surface Analysis
2.5. Computational Details.
3. Results and Discussion
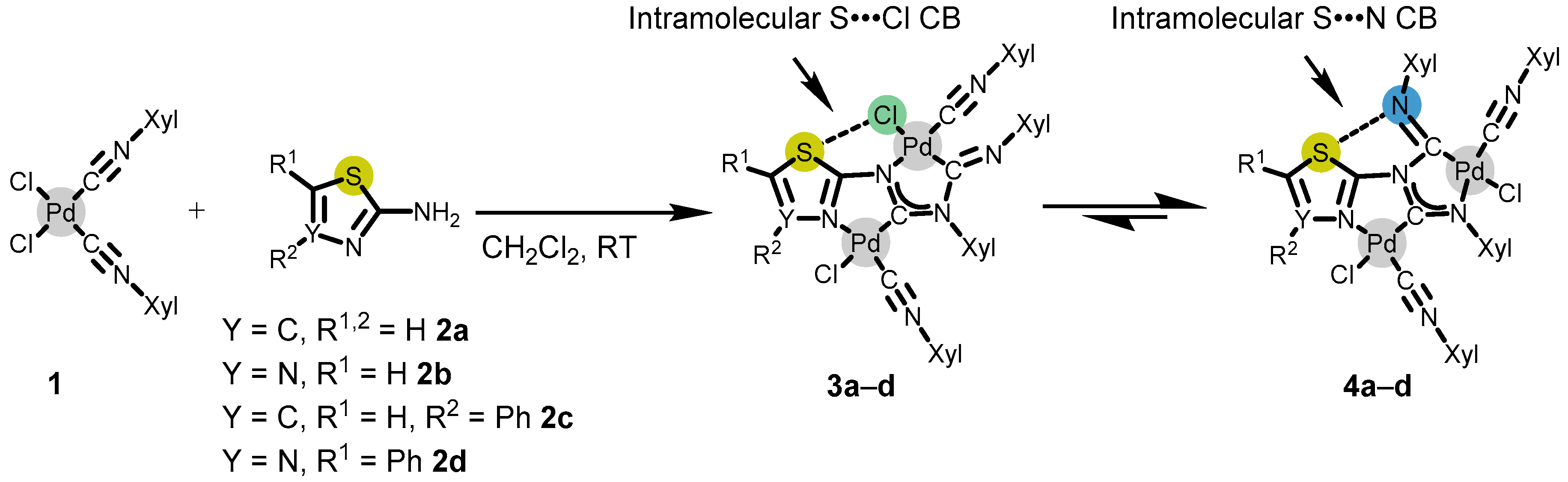
3.1. Synthesis and Crystallization
3.2. Non-Covalent Interactions in the Crystal Structures of 3a–d
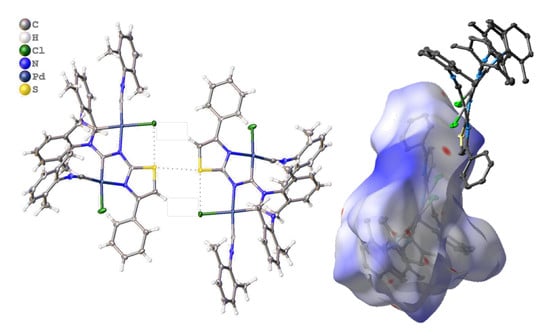
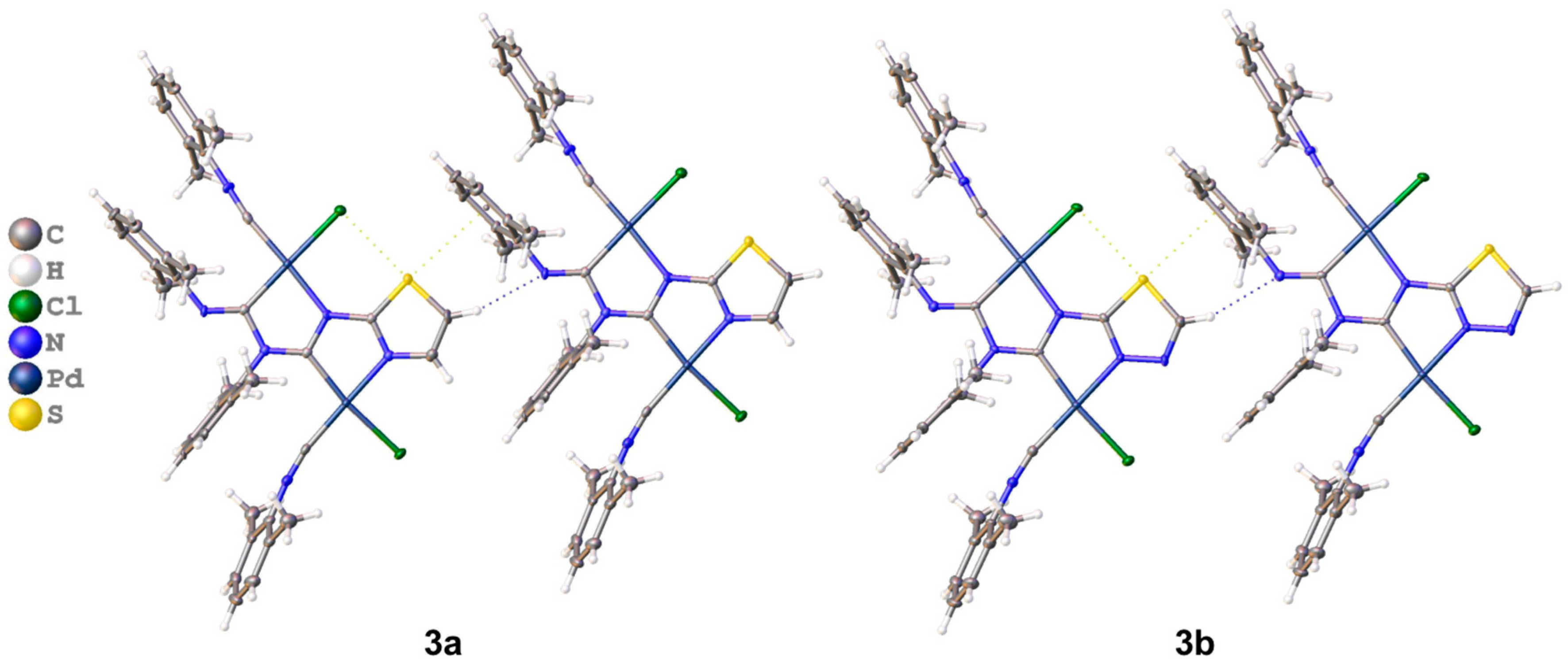
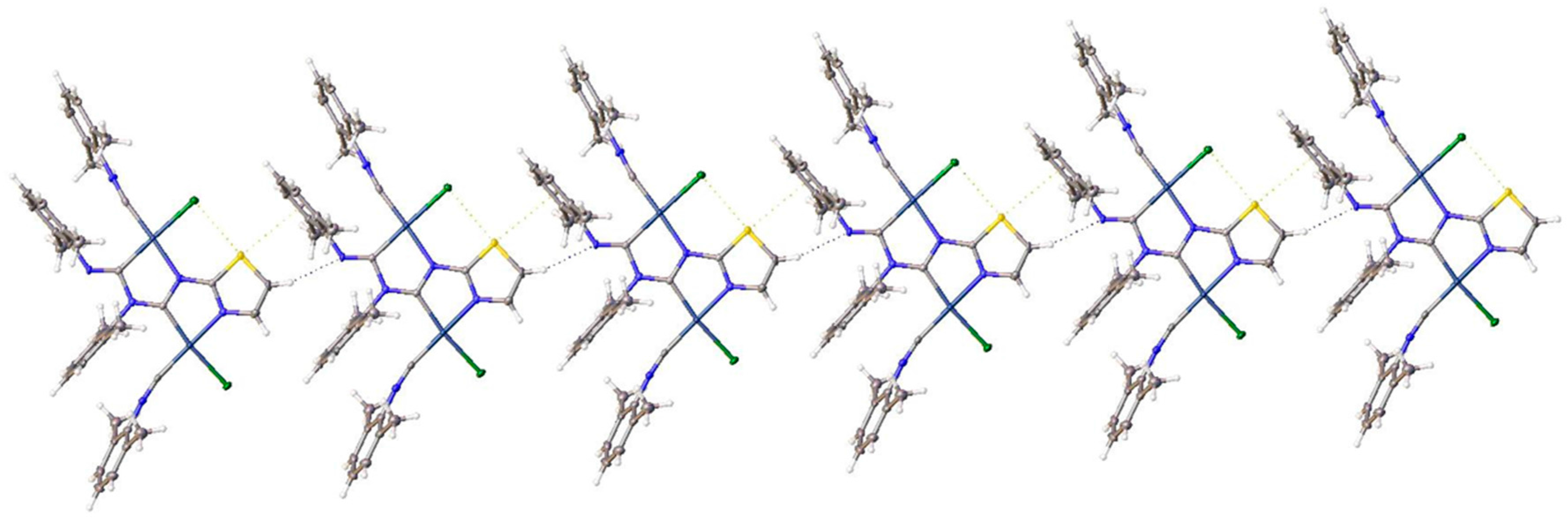
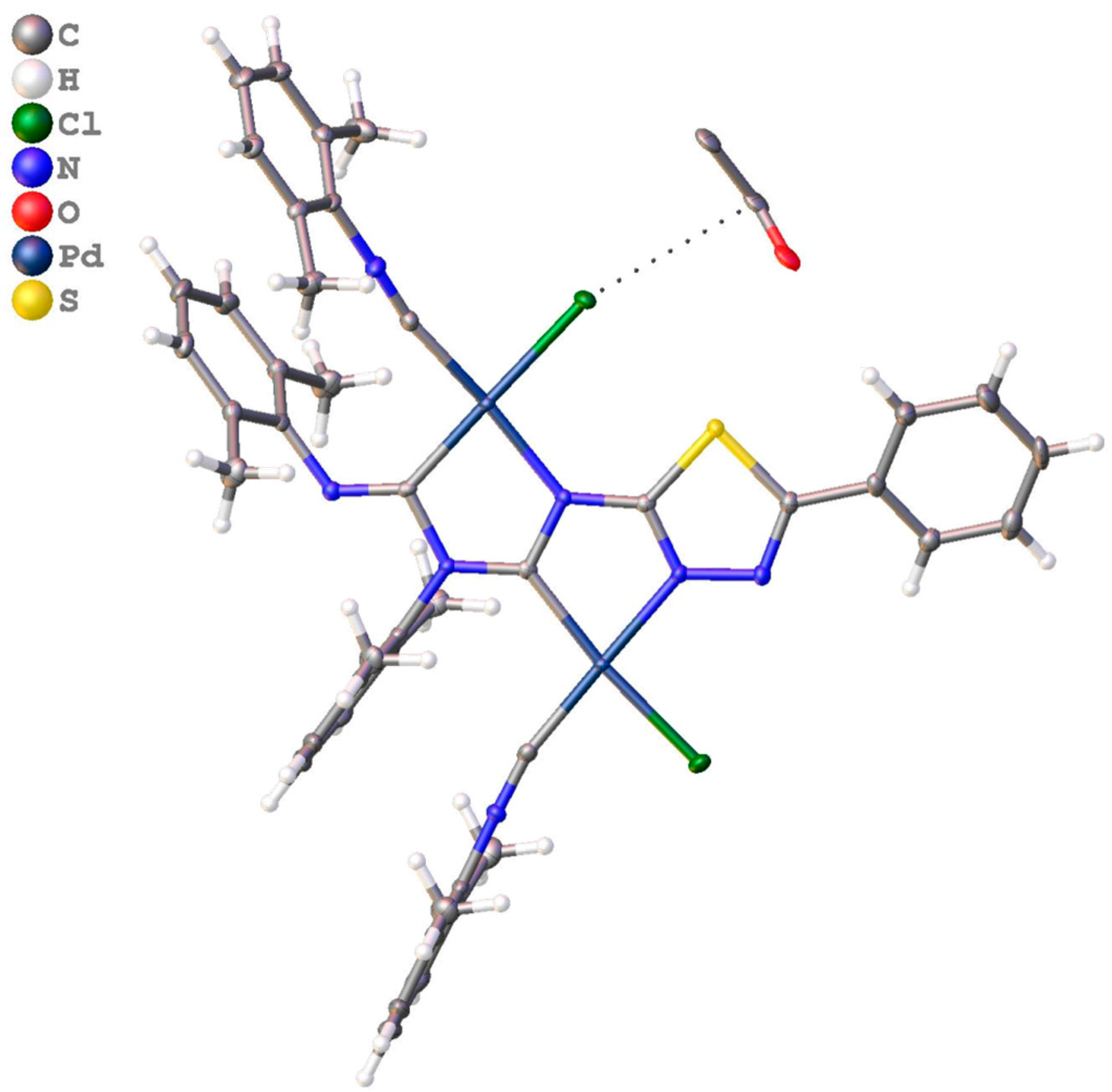
3.2.1. Recognition of S•••Cl/S•••π BCBs in 3a,b
3.2.2. Recognition of S•••Cl/S•••S BCB in 3c
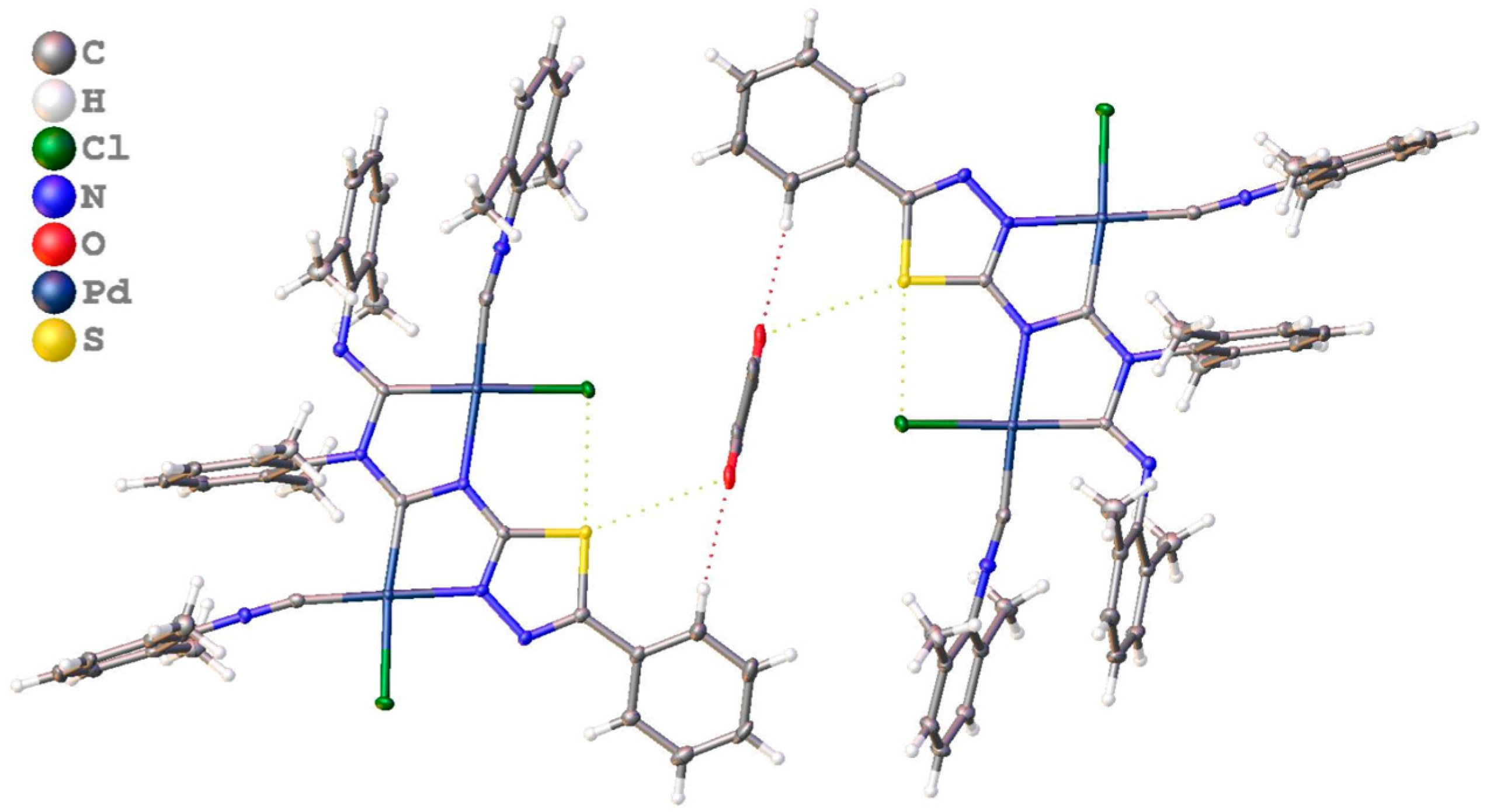
3.2.3. Recognition of S•••Cl/S•••O BCB in 3d
3.3. Theoretical Consideration of Intra-/Intermolecular Bifurcated Chalcogen Bonding in Thiazole/Thiadiazole Derived Binuclear (Diaminocarbene)Pdii Complexes
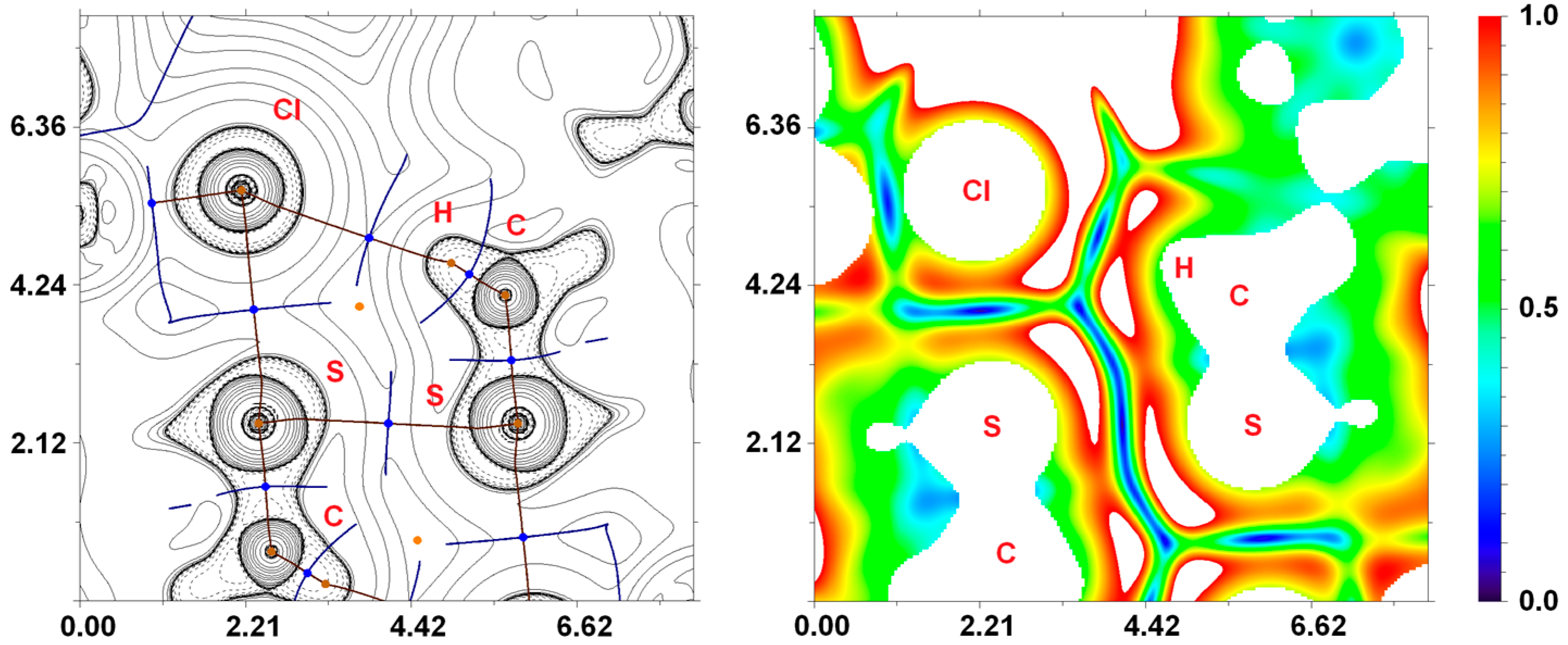
3.3.1. Hirshfeld Surface Analysis for the X-ray Structures of 3a–d
3.3.2. The QTAIM and NBO Analyses of Intra-/Intermolecular Bifurcated Chalcogen Bonding S•••Cl/S•••S in Model Dimeric Associate 3c
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kollman, P.A.; Allen, L.C. Theory of the Hydrogen Bond. Chem. Rev. 1972, 72, 283–303. [Google Scholar] [CrossRef]
- Grabowski, S.J. What Is the Covalency of Hydrogen Bonding? Chem. Rev. 2011, 111, 2597–2625. [Google Scholar] [CrossRef] [PubMed]
- Gilday, L.C.; Robinson, S.W.; Barendt, T.A.; Langton, M.J.; Mullaney, B.R.; Beer, P.D. Halogen Bonding in Supramolecular Chemistry. Chem. Rev. 2015, 115, 7118–7195. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Politzer, P.; Lane, P.; Concha, M.C.; Ma, Y.G.; Murray, J.S. An overview of halogen bonding. J. Mol. Model. 2007, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. Linear Free Energy Relationships in Halogen Bonds. CrystEngComm 2013, 15, 3178–3186. [Google Scholar] [CrossRef]
- Politzer, P.; Riley, K.E.; Bulat, F.A.; Murray, J.S. Perspectives on Halogen Bonding and Other Sigma-Hole Interactions: Lex Parsimoniae (Occam's Razor). Comput. Theor. Chem. 2012, 998, 2–8. [Google Scholar] [CrossRef]
- Politzer, P.; Murray, J.S. Halogen Bonding: An Interim Discussion. ChemPhysChem 2013, 14, 278–294. [Google Scholar] [CrossRef] [PubMed]
- Kolar, M.H.; Hobza, P. Computer Modeling of Halogen Bonds and Other Sigma-Hole Interactions. Chem. Rev. 2016, 116, 5155–5187. [Google Scholar] [CrossRef] [PubMed]
- Fanfrlik, J.; Holub, J.; Ruzickova, Z.; Rezac, J.; Lane, P.D.; Wann, D.A.; Hnyk, D.; Ruzicka, A.; Hobza, P. Competition between Halogen, Hydrogen and Dihydrogen Bonding in Brominated Carboranes. Chemphyschem 2016, 17, 3373–3376. [Google Scholar] [CrossRef] [PubMed]
- Pascoe, D.J.; Ling, K.B.; Cockroft, S.L. The Origin of Chalcogen-Bonding Interactions. J. Am. Chem. Soc. 2017, 139, 15160–15167. [Google Scholar] [CrossRef] [PubMed]
- Mahmudov, K.T.; Kopylovich, M.N.; Guedes da Silva, M.F.C.; Pombeiro, A.J.L. Chalcogen Bonding in Synthesis, Catalysis and Design of Materials. Dalton Trans. 2017, 46, 10121–10138. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Gabbaï, F.P. A Bidentate Lewis Acid with a Telluronium Ion as an Anion-Binding Site. Nat. Chem. 2010, 2, 984–990. [Google Scholar] [CrossRef] [PubMed]
- Iwaoka, M.; Isozumi, N. Hypervalent Nonbonded Interactions of a Divalent Sulfur Atom. Implications in Protein Architecture and the Functions. Molecules 2012, 17, 7266–7283. [Google Scholar] [CrossRef] [PubMed]
- Mikherdov, A.S.; Novikov, A.S.; Kinzhalov, M.A.; Boyarskiy, V.P.; Starova, G.L.; Ivanov, A.Y.; Kukushkin, V.Y. Halides Held by Bifurcated Chalcogen–Hydrogen Bonds. Effect of µ(S,N–H)Cl Contacts on Dimerization of Cl(carbene)PdII Species. Inorg. Chem. 2018, in press. [Google Scholar] [CrossRef]
- Brezgunova, M.E.; Lieffrig, J.; Aubert, E.; Dahaoui, S.; Fertey, P.; Lebegue, S.; Angyan, J.G.; Fourmigue, M.; Espinosa, E. Chalcogen Bonding: Experimental and Theoretical Determinations from Electron Density Analysis. Geometrical Preferences Driven by Electrophilic-Nucleophilic Interactions. Cryst. Growth Des. 2013, 13, 3283–3289. [Google Scholar] [CrossRef]
- Alikhani, E.; Fuster, F.; Madebene, B.; Grabowski, S.J. Topological Reaction Sites—Very Strong Chalcogen Bonds. Phys. Chem. Chem. Phys. 2014, 16, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P.; Veccham, S.P.K.P.; Farrugia, L.J.; Row, T.N.G. “Conformational Simulation” of Sulfamethizole by Molecular Complexation and Insights from Charge Density Analysis: Role of Intramolecular S•••O Chalcogen Bonding. Cryst. Growth Des. 2015, 15, 2110–2118. [Google Scholar] [CrossRef]
- Sánchez-Sanz, G.; Trujillo, C. Improvement of Anion Transport Systems by Modulation of Chalcogen Interactions: The Influence of Solvent. J. Phys. Chem. A 2018, 122, 1369–1377. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.P.; Jayatilaka, D.; Row, T.N.G. S•••O Chalcogen Bonding in Sulfa Drugs: Insights from Multipole Charge Density and X-Ray Wavefunction of Acetazolamide. Phys. Chem. Chem. Phys. 2015, 17, 25411–25420. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Intermolecular Weak Interactions in HTeXH Dimers (X=O, S, Se, Te): Hydrogen Bonds, Chalcogen-Chalcogen Contacts and Chiral Discrimination. Chem. Phys. Chem. 2012, 13, 496–503. [Google Scholar] [CrossRef] [PubMed]
- Nziko, V.D.N.; Scheiner, S. Intramolecular S•••O Chalcogen Bond as Stabilizing Factor in Geometry of Substituted Phenyl-SF3 Molecules. J. Org. Chem. 2015, 80, 2356–2363. [Google Scholar] [CrossRef] [PubMed]
- Shishkin, O.V.; Omelchenko, I.V.; Kalyuzhny, A.L.; Paponov, B.V. Intramolecular S•••O Chalcogen Bond in Thioindirubin. Struct. Chem. 2010, 21, 1005–1011. [Google Scholar] [CrossRef]
- Sanz, P.; Yanez, M.; Mo, O. Resonance-Assisted Intramolecular Chalcogen-Chalcogen Interactions? Chem.-A. Eur. J. 2003, 9, 4548–4555. [Google Scholar] [CrossRef] [PubMed]
- Zahn, S.; Frank, R.; Hey-Hawkins, E.; Kirchner, B. Pnicogen Bonds: A New Molecular Linker? Chem.-A. Eur. J. 2011, 17, 6034–6038. [Google Scholar] [CrossRef] [PubMed]
- Scheiner, S. The Pnicogen Bond: Its Relation to Hydrogen, Halogen, and Other Noncovalent Bonds. Acc. Chem. Res. 2013, 46, 280–288. [Google Scholar] [CrossRef] [PubMed]
- Del Bene, J.E.; Alkorta, I.; Elguero, J.; Sanchez-Sanz, G. Lone-Pair Hole on P: P•••N Pnicogen Bonds Assisted by Halogen Bonds. J. Phys. Chem. A 2017, 121, 1362–1370. [Google Scholar] [CrossRef] [PubMed]
- Alkorta, I.; Elguero, J.; Del Bene, J.E. Unusual Acid-Base Properties of The P-4 Molecule in Hydrogen-, Halogen-, and Pnicogen-Bonded Complexes. Phys. Chem. Chem. Phys. 2016, 18, 32593–32601. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J. Intramolecular Pnicogen Interactions in Phosphorus and Arsenic Analogues of Proton Sponges. Phys. Chem. Chem. Phys. 2014, 16, 15900–15909. [Google Scholar] [CrossRef] [PubMed]
- Sanz, D.; Claramunt, R.M.; Mathey, F.; Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. Intermolecular Spin-Spin Coupling Constants Between P-31 Atoms. C. R. Chim. 2013, 16, 937–944. [Google Scholar] [CrossRef]
- Del Bene, J.E.; Alkorta, I.; Sanchez-Sanz, G.; Elguero, J. P-31-P-31 Spin-Spin Coupling Constants for Pnicogen Homodimers. Chem. Phys. Lett. 2011, 512, 184–187. [Google Scholar] [CrossRef]
- Bauza, A.; Mooibroek, T.J.; Frontera, A. The Bright Future of Unconventional Sigma/-Hole Interactions. Chem. Phys. Chem. 2015, 16, 2496–2517. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E. Local Nature of Substituent Effects in Stacking Interactions. J. Am. Chem. Soc. 2011, 133, 10262–10274. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, S.E. Understanding Substituent Effects in Noncovalent Interactions Involving Aromatic Rings. Acc. Chem. Res. 2013, 46, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.R.; Iverson, B.L. Rethinking the term “pi-stacking”. Chem. Sci. 2012, 3, 2191–2201. [Google Scholar] [CrossRef]
- Trujillo, C.; Sanchez-Sanz, G. A Study of pi-pi Stacking Interactions and Aromaticity in Polycyclic Aromatic Hydrocarbon/Nucleobase Complexes. Chem. Phys. Chem. 2016, 17, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.; Kelly, B.; Sanchez-Sanz, G.; Trujillo, C.; Alkorta, I.; Elguero, J.; Rozas, I. Non-Covalent Interactions: Complexes of Guanidinium with DNA and RNA Nucleobases. J. Phys. Chem. B 2013, 117, 11608–11616. [Google Scholar] [CrossRef] [PubMed]
- Dougherty, D.A.Y. The Cation-π Interaction. Acc. Chem. Res. 2013, 46, 885–893. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.C.; Dougherty, D.A. The Cation-π Interaction. Chem. Rev. 1997, 97, 1303–1324. [Google Scholar] [CrossRef] [PubMed]
- Mahadevi, A.S.; Sastry, G.N. Cation-π Interaction: Its Role and Relevance in Chemistry, Biology, and Material Science. Chem. Rev. 2013, 113, 2100–2138. [Google Scholar] [CrossRef] [PubMed]
- Schottel, B.L.; Chifotides, H.T.; Dunbar, K.R. Anion-π Interactions. Chem. Soc. Rev. 2008, 37, 68–83. [Google Scholar] [CrossRef] [PubMed]
- López-Andarias, J.; Frontera, A.; Matile, S. Anion-π Catalysis on Fullerenes. J. Am. Chem. Soc. 2017, 139, 13296–13299. [Google Scholar] [CrossRef] [PubMed]
- Yam, V.W.-W.; Au, V.K.-M.; Leung, S.Y.-L. Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes. Chem. Rev. 2015, 115, 7589–7728. [Google Scholar] [CrossRef] [PubMed]
- Sculfort, S.; Braunstein, P. Intramolecular d10-d10 Interactions in Heterometallic Clusters of The Transition Metals. Chem. Soc. Rev. 2011, 40, 2741–2760. [Google Scholar] [CrossRef] [PubMed]
- Eryazici, I.; Moorefield, C.N.; Newkome, G.R. Square-Planar Pd(II), Pt(II), and Au(III) Terpyridine Complexes: Their Syntheses, Physical Properties, Supramolecular Constructs, and Biomedical Activities. Chem. Rev. 2008, 108, 1834–1895. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Alkorta, I.; Frontera, A.; Elguero, J. On the Reliability of Pure and Hybrid DFT Methods for the Evaluation of Halogen, Chalcogen, and Pnicogen Bonds Involving Anionic and Neutral Electron Donors. J. Chem. Theory Comput. 2013, 9, 5201–5210. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J. Phys. Chem. A 2009, 113, 8132–8135. [Google Scholar] [CrossRef] [PubMed]
- Bleiholder, C.; Werz, D.B.; Koppel, H.; Gleiter, R. Theoretical Investigations on Chalcogen-Chalcogen Interactions: What Makes These Nonbonded Interactions Bonding? J. Am. Chem. Soc. 2006, 128, 2666–2674. [Google Scholar] [CrossRef] [PubMed]
- Bauza, A.; Quinonero, D.; Deya, P.M.; Frontera, A. Halogen Bonding Versus Chalcogen and Pnicogen Bonding: A Combined Cambridge Structural Database and Theoretical Study. CrystEngComm 2013, 15, 3137–3144. [Google Scholar] [CrossRef]
- Cavallo, G.; Metrangolo, P.; Pilati, T.; Resnati, G.; Terraneo, G. Naming Interactions from the Electrophilic Site. Cryst. Growth Des. 2014, 14, 2697–2702. [Google Scholar] [CrossRef]
- Bora, P.L.; Novak, M.; Novotny, J.; Foroutan-Nejad, C.; Marek, R. Supramolecular Covalence in Bifurcated Chalcogen Bonding. Chem.-A Eur. J. 2017, 23, 7315–7323. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.K.; Kumar, V.; Murray, J.S.; Politzer, P.; Terraneo, G.; Pilati, T.; Metrangolo, P.; Resnati, G. Fluorination Promotes Chalcogen Bonding in Crystalline Solids. CrystEngComm 2017, 19, 4955–4959. [Google Scholar] [CrossRef]
- Lee, L.M.; Corless, V.B.; Tran, M.; Jenkins, H.; Britten, J.F.; Vargas-Baca, I. Synthetic, Structural, and Computational Investigations of N-alkyl benzo-2,1,3-Selenadiazolium Iodides and Their Supramolecular Aggregates. Dalton Trans. 2016, 45, 3285–3293. [Google Scholar] [CrossRef] [PubMed]
- Jackson, N.E.; Savoie, B.M.; Kohlstedt, K.L.; de la Cruz, M.O.; Schatz, G.C.; Chen, L.X.; Ratner, M.A. Controlling Conformations of Conjugated Polymers and Small Molecules: The Role of Nonbonding Interactions. J. Am. Chem. Soc. 2013, 135, 10475–10483. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.M.; Elder, P.J.W.; Cozzolino, A.F.; Yang, Q.; Vargas-Baca, I. An Experimental And Computational Investigation of The Formation and Structures of N-hydro and N,N′-Dihydro-Benzo-2,1,3-Chalcogenadiazolium Chlorides. Main Group Chem. 2010, 9, 117–133. [Google Scholar]
- Schottel, B.L.; Chifotides, H.T.; Shatruk, M.; Chouai, A.; Perez, L.M.; Bacsa, J.; Dunbar, K.R. Anion-pi Interactions as Controlling Elements in Self-Assembly Reactions of Ag(I) Complexes with Pi-Acidic Aromatic Rings. J. Am. Chem. Soc. 2006, 128, 5895–5912. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Fujii, H.; Yamashita, Y.; Kabuto, C.; Tanaka, S.; Harasawa, M.; Mukai, T.; Miyashi, T. Clathrate Formation and Molecular Recognition by Novel Chalcogen Cyano Interactions in Tetracyanoquinodimethanes Fused with Thiadiazole and Selenadiazole Rings. J. Am. Chem. Soc. 1992, 114, 3034–3043. [Google Scholar] [CrossRef]
- Mikherdov, A.S.; Kinzhalov, M.A.; Novikov, A.S.; Boyarskiy, V.P.; Boyarskaya, I.A.; Dar’in, D.V.; Starova, G.L.; Kukushkin, V.Y. Difference in Energy between Two Distinct Types of Chalcogen Bonds Drives Regioisomerization of Binuclear (Diaminocarbene)PdII Complexes. J. Am. Chem. Soc. 2016, 138, 14129–14137. [Google Scholar] [CrossRef] [PubMed]
- Katkova, S.A.; Kinzhalov, M.A.; Tolstoy, P.M.; Novikov, A.S.; Boyarskiy, V.P.; Ananyan, A.Y.; Gushchin, P.V.; Haukka, M.; Zolotarev, A.A.; Ivanov, A.Y.; et al. Diversity of Isomerization Patterns and Protolytic Forms in Aminocarbene PdII and PtII Complexes Formed upon Addition of N,N′-Diphenylguanidine to Metal-Activated Isocyanides. Organometallics 2017, 36, 4145–4159. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Legkodukh, A.S.; Anisimova, T.B.; Novikov, A.S.; Suslonov, V.V.; Luzyanin, K.V.; Kukushkin, V.Y. Tetrazol-5-ylidene Gold(III) Complexes from Sequential [2 + 3] Cycloaddition of Azide to Metal-Bound Isocyanides and N4 Alkylation. Organometallics 2017, 36, 3974–3980. [Google Scholar] [CrossRef]
- Anisimova, T.B.; Kinzhalov, M.A.; Silva, M.F.C.G.d.; Novikov, A.S.; Kukushkin, V.Y.; Pombeiro, A.J.L.; Luzyanin, K.V. Addition of N-Nucleophiles to Gold(III)-Bound Isocyanides Leading to Short-Lived Gold(III) Acyclic Diaminocarbene Complexes. New J. Chem. 2017, 41, 3246–3250. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Starova, G.L.; Boyarskiy, V.P. Interaction of Benzene-1,2-Diamines with Isocyanide Complexes of Palladium(II): Insight into the Mechanism. Inorg. Chim. Acta 2017, 455, 607–612. [Google Scholar] [CrossRef]
- Kinzhalov, M.A.; Timofeeva, S.A.; Luzyanin, K.V.; Boyarskiy, V.P.; Yakimanskiy, A.A.; Haukka, M.; Kukushkin, V.Y. Palladium(II)-Mediated Addition of Benzenediamines to Isocyanides: Generation of Three Types of Diaminocarbene Ligands Depending on the Isomeric Structure of the Nucleophile. Organometallics 2016, 35, 218–228. [Google Scholar] [CrossRef]
- Boyarskiy, V.P.; Bokach, N.A.; Luzyanin, K.V.; Kukushkin, V.Y. Metal-Mediated and Metal-Catalyzed Reactions of Isocyanides. Chem. Rev. 2015, 115, 2698–2779. [Google Scholar] [CrossRef] [PubMed]
- Timofeeva, S.; Kinzhalov, M.; Valishina, E.; Luzyanin, K.; Boyarskiy, V.; Buslaeva, T.; Haukka, M.; Kukushkin, V. Application of Palladium Complexes Bearing Acyclic Amino(Hydrazido)Carbene Ligands as Catalysts for Copper-Free Sonogashira Cross-Coupling. J. Catal. 2015, 329, 449–456. [Google Scholar] [CrossRef]
- Kinzhalov, M.; Boyarskiy, V.; Luzyanin, K.; Dolgushin, F.; Kukushkin, V. Metal-Mediated Coupling of a Coordinated Isocyanide and Indazoles. Dalton Trans. 2013, 42, 10394–10397. [Google Scholar] [CrossRef] [PubMed]
- Valishina, E.A.; Guedes da Silva, M.F.C.; Kinzhalov, M.A.; Timofeeva, S.A.; Buslaeva, T.M.; Haukka, M.; Pombeiro, A.J.L.; Boyarskiy, V.P.; Kukushkin, V.Y.; Luzyanin, K.V. Palladium-ADC Complexes as Efficient Catalysts in Copper-Free and Room Temperature Sonogashira Coupling. J. Mol. Catal. A: Chem. 2014, 395, 162–171. [Google Scholar] [CrossRef]
- Kinzhalov, M.; Luzyanin, K.; Boyarskiy, V.; Haukka, M.; Kukushkin, V. ADC-Based Palladium Catalysts for Aqueous Suzuki Miyaura Cross-Coupling Exhibit Greater Activity than the Most Advantageous Catalytic Systems. Organometallics 2013, 32, 5212–5223. [Google Scholar] [CrossRef]
- Bikbaeva, Z.M.; Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Bokach, N.A.; Kukushkin, V.Y. Electrophilic–Nucleophilic Dualism of Nickel(II) toward Ni···I Noncovalent Interactions: Semicoordination of Iodine Centers via Electron Belt and Halogen Bonding via σ-Hole. Inorg. Chem. 2017, 56, 13562–13578. [Google Scholar] [CrossRef] [PubMed]
- Adonin, S.A.; Gorokh, I.D.; Novikov, A.S.; Abramov, P.A.; Sokolov, M.N.; Fedin, V.P. Halogen Contacts-Induced Unusual Coloring in Bi-III Bromide Complex: Anion-to-Cation Charge Transfer via Br⋅⋅⋅Br Interactions. Chem. Eur. J. 2017, 23, 15612–15616. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, D.M.; Kinzhalov, M.A.; Novikov, A.S.; Ananyev, I.V.; Romanova, A.A.; Boyarskiy, V.P.; Haukka, M.; Kukushkin, V.Y. H2C(X)–X···X– (X = Cl, Br) Halogen Bonding of Dihalomethanes. Cryst. Growth Des. 2017, 17, 1353–1362. [Google Scholar] [CrossRef]
- Novikov, A.S.; Ivanov, D.M.; Avdontceva, M.S.; Kukushkin, V.Y. Diiodomethane as a Halogen Bond Donor Toward Metal-Bound Halides. CrystEngComm 2017, 19, 2517–2525. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Novikov, A.S.; Ananyev, I.V.; Kirina, Y.V.; Kukushkin, V.Y. Halogen Bonding between Metal Centers and Halocarbons. Chem. Commun. 2016, 52, 5565–5568. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Tuikka, M.J.; Hirva, P.; Kukushkin, V.Y.; Novikov, A.S.; Haukka, M. Fine-Tuning Halogen Bonding Properties Of Diiodine Through Halogen-Halogen Charge Transfer—Extended [Ru(2,2'-Bipyridine)(CO)2X2]¬¨‚àëI2 Systems (X = Cl, Br, I). CrystEngComm 2016, 18, 1987–1995. [Google Scholar] [CrossRef]
- Ivanov, D.M.; Novikov, A.S.; Starova, G.L.; Haukka, M.; Kukushkin, V.Y. A Family of Heterotetrameric Clusters of Chloride Species and Halomethanes Held by Two Halogen and Two Hydrogen Bonds. CrystEngComm 2016, 18, 5278–5286. [Google Scholar] [CrossRef]
- Mikhaylov, V.N.; Sorokoumov, V.N.; Korvinson, K.A.; Novikov, A.S.; Balova, I.A. Synthesis and Simple Immobilization of Palladium(II) Acyclic Diaminocarbene Complexes on Polystyrene Support as Efficient Catalysts for Sonogashira and Suzuki–Miyaura Cross-Coupling. Organometallics 2016, 35, 1684–1697. [Google Scholar] [CrossRef]
- Serebryanskaya, T.V.; Novikov, A.S.; Gushchin, P.V.; Haukka, M.; Asfin, R.E.; Tolstoy, P.M.; Kukushkin, V.Y. Identification and H(D)-Bond Energies of C-H(D)Center Dot Center Dot Center Dot CL Interactions in Chloride-Haloalkane Clusters: A Combined X-ray Crystallographic, Spectroscopic, and Theoretical Study. Phys. Chem. Chem. Phys. 2016, 18, 14104–14112. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A: Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef] [PubMed]
- Dolomanov, O.; Bourhis, L.; Gildea, R.; Howard, J.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. J. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- CrysAlisPro; 1.171.36.20; Agilent Technologies: Yarnton, Oxfordshire, UK, 2012.
- Spek, A.L. Platon Squeeze: A Tool for the Calculation of the Disordered Solvent Contribution to the Calculated Structure Factors. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Wolff, S.K.; Grimwood, D.J.; McKinnon, J.J.; Turner, M.J.; Jayatilaka, D.; Spackman, M.A. CrystalExplorer, Version 3.1; University of Western Australia: Perth, Australia, 2012. [Google Scholar]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld Surface Analysis. CrystEngComm 2009, 11, 19–32. [Google Scholar] [CrossRef]
- McKinnon, J.J.; Jayatilaka, D.; Spackman, M.A. Towards Quantitative Analysis of Intermolecular Interactions with Hirshfeld Surfaces. Chem. Commun. 2007, 37, 3814–3816. [Google Scholar] [CrossRef]
- Bondi, A. Van Der Waals Volumes + RadiI. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Nakajima, T.; Hirao, K. The Douglas-Kroll-Hess Approach. Chem. Rev. 2012, 112, 385–402. [Google Scholar] [CrossRef] [PubMed]
- Reiher, M. Relativistic Douglas-Kroll-Hess Theory. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 139–149. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2010. [Google Scholar]
- Bader, R.F.W. A Quantum-Theory of Molecular-Structure and Its Applications. Chem. Rev. 1991, 91, 893–928. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F.W. Multiwfn: A Multifunctional Wavefunction Analyzer. J. Comput. Chem. 2012, 33, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Glendening, E.D.; Landis, C.R.; Weinhold, F. Natural Bond Orbital Methods. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2012, 2, 1–42. [Google Scholar] [CrossRef]
- Rowland, R.S.; Taylor, R. Intermolecular Nonbonded Contact Distances in Organic Crystal Structures: Comparison with Distances Expected from Van Der Waals Radii. J. Phys. Chem. 1996, 100, 7384–7391. [Google Scholar] [CrossRef]
- Burling, F.T.; Goldstein, B.M. Computational Studies of Nonbonded Sulfur Oxygen and Selenium Oxygen Interactions in the Thiazole and Selenazole Nucleosides. J. Am. Chem. Soc. 1992, 114, 2313–2320. [Google Scholar] [CrossRef]
- Rosenfield, R.E.; Parthasarathy, R.; Dunitz, J.D. Directional Preferences of Nonbonded Atomic Contacts with Divalent Sulfur. 1. Electrophiles and Nucleophiles. J. Am. Chem. Soc. 1977, 99, 4860–4862. [Google Scholar] [CrossRef]
- Arunan, E.; Desiraju, G.R.; Klein, R.A.; Sadlej, J.; Scheiner, S.; Alkorta, I.; Clary, D.C.; Crabtree, R.H.; Dannenberg, J.J.; Hobza, P.; et al. Definition of the Hydrogen Bond (IUPAC Recommendations 2011). Pure Appl. Chem. 2011, 83, 1637–1641. [Google Scholar] [CrossRef]
- Shukla, R.; Chopra, D. Crystallographic and Theoretical Investigation on the Nature and Characteristics of Type I C=S•••S=C Interactions. Cryst. Growth Des. 2016, 16, 6734–6742. [Google Scholar] [CrossRef]
- Allinger, N.L.; Zhou, X.F.; Bergsma, J. Molecular Mechanics Parameters. J. Mol. Struct. Theochem 1994, 118, 69–83. [Google Scholar] [CrossRef]
- Newberry, R.W.; Raines, R.T. The n→π* Interaction. Acc. Chem. Res. 2017, 50, 1838–1846. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, W.; Jin, W.J. σ-Hole Bond vs π-Hole Bond: A Comparison Based on Halogen Bond. Chem. Rev. 2016, 116, 5072–5104. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Das, A. The n -> pi* interaction: A Rapidly Emerging Non-Covalent Interaction. Phys. Chem. Chem. Phys. 2015, 17, 9596–9612. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.R.; Keinan, S.; Mori-Sánchez, P.; Contreras-García, J.; Cohen, A.J.; Yang, W. Revealing Noncovalent Interactions. J. Am. Chem. Soc. 2010, 132, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Molins, E.; Lecomte, C. Hydrogen Bond Strengths Revealed by Topological Analyses of Experimentally Observed Electron Densities. Chem. Phys. Lett. 1998, 285, 170–173. [Google Scholar] [CrossRef]
- Vener, M.V.; Egorova, A.N.; Churakov, A.V.; Tsirelson, V.G. Intermolecular Hydrogen Bond Energies in Crystals Evaluated Using Electron Density Properties: DFT Computations with Periodic Boundary Conditions. J. Comput. Chem. 2012, 33, 2303–2309. [Google Scholar] [CrossRef] [PubMed]
- Espinosa, E.; Alkorta, I.; Elguero, J.; Molins, E. From Weak to Strong Interactions: A Comprehensive Analysis of the Topological and Energetic Properties of the Electron Density Distribution Involving X-H•••F-Y Systems. J. Chem. Phys. 2002, 117, 5529–5542. [Google Scholar] [CrossRef]



| Empirical Formula | C45H42N6SCl2Pd2 |
|---|---|
| Formula weight | 982.61 |
| Temperature/K | 100(2) |
| Crystal system | triclinic |
| Space group | P-1 |
| a/Å | 8.2666(4) |
| b/Å | 14.3733(8) |
| c/Å | 19.2538(9) |
| α/° | 99.238(4) |
| β/° | 97.705(4) |
| γ/° | 97.182(4) |
| Volume/Å3 | 2212.40(19) |
| Z | 2 |
| ρcalcg/cm3 | 1.473 |
| μ/mm−1 | 8.390 |
| F(000) | 992.0 |
| Crystal size/mm3 | 0.22 × 0.16 × 0.09 |
| Radiation | CuKα (λ = 1.54184) |
| 2Θ range for data collection/° | 6.3–139.9 |
| Index ranges | −10 ≤ h ≤ 10, −17 ≤ k ≤ 17, −19 ≤ l ≤ 23 |
| Reflections collected | 24325 |
| Independent reflections | 8247 [Rint = 0.0764, Rsigma = 0.0679] |
| Data/restraints/parameters | 8247/0/495 |
| Goodness-of-fit on F2 | 1.019 |
| Final R indexes [I >= 2σ (I)] | R1 = 0.0523, wR2 = 0.1422 |
| Final R indexes [all data] | R1 = 0.0623, wR2 = 0.1592 |
| Largest diff. peak/hole/e Å−3 | 2.25/−1.64 |
| Contact | Parameter | 3a | 3b | Rowland wdV [92] | Bondi wdV [85] |
|---|---|---|---|---|---|
| Intramolecular CB | d(S•••Cl) | 3.097(2) | 3.0927(9) | 3.57 | 3.55 |
| ∠(Y–S•••Cl) | 172.0(3) | 172.79(12) | |||
| Intermolecular CB | d(S•••C) | 3.445(7) | 3.425(3) | 3.58 | 3.50 |
| d(S•••Xylcentr) | 3.351(3) | 3.2906(13) | |||
| ∠(Y–S•••Xyl) | 172.0(3) | 172.79(12) | |||
| ∠(S•••Xylplane) | 88.6(3) | 91.45(11) | |||
| Intermolecular HB | d(H•••N) | 2.51(5) | 2.50(5) | 2.74 | 2.75 |
| d(C•••N) | 3.361(9) | 3.349(4) | 3.41 | 3.25 | |
| ∠(C–H•••N) | 151(1) | 151(1) |
| X-ray structure | Contributions of Different Intermolecular Contacts to the Molecular Hirshfeld Surface * |
|---|---|
| 3a | H–H 52.8%, Cl–H 15.3%, C–H 13.8%, N–H 5.7%, Pd–H 3.6%, S–H 3.4%, C–C 2.5%, S–C 2.4% |
| 3b | H–H 49.3%, Cl–H 16.0%, C–H 11.9%, N–H 9.2%, Pd–H 3.7%, S–H 3.3%, C–C 2.6%, S–C 2.5%, N–C 1.1% |
| 3c | H–H 53.1%, C–H 20.4%, Cl–H 11.3%, N–H 2.9%, S–H 2.2%, Pd–H 2.1%, C–C 1.2% |
| 3d | H–H 45.9%, C–H 23.9%, Cl–H 11.9%, N–H 6.2%, Pd–H 3.2%, S–H 2.9%, O–H 2.6%, C–C 1.9%, Cl–C 1.0%, |
| * The contributions of all other intermolecular contacts do not exceed 1%. | |
| Contact | ρ(r) | ∇2ρ(r) | Hb | V(r) | G(r) | Eint a | Eint b | WI |
|---|---|---|---|---|---|---|---|---|
| S•••Cl | 0.015 | 0.049 | 0.002 | −0.009 | 0.010 | 2.8 | 2.7 | 0.02 |
| S•••S | 0.007 | 0.024 | 0.001 | −0.003 | 0.005 | 0.9 | 1.3 | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mikherdov, A.S.; Novikov, A.S.; Kinzhalov, M.A.; Zolotarev, A.A.; Boyarskiy, V.P. Intra-/Intermolecular Bifurcated Chalcogen Bonding in Crystal Structure of Thiazole/Thiadiazole Derived Binuclear (Diaminocarbene)PdII Complexes. Crystals 2018, 8, 112. https://doi.org/10.3390/cryst8030112
Mikherdov AS, Novikov AS, Kinzhalov MA, Zolotarev AA, Boyarskiy VP. Intra-/Intermolecular Bifurcated Chalcogen Bonding in Crystal Structure of Thiazole/Thiadiazole Derived Binuclear (Diaminocarbene)PdII Complexes. Crystals. 2018; 8(3):112. https://doi.org/10.3390/cryst8030112
Chicago/Turabian StyleMikherdov, Alexander S., Alexander S. Novikov, Mikhail A. Kinzhalov, Andrey A. Zolotarev, and Vadim P. Boyarskiy. 2018. "Intra-/Intermolecular Bifurcated Chalcogen Bonding in Crystal Structure of Thiazole/Thiadiazole Derived Binuclear (Diaminocarbene)PdII Complexes" Crystals 8, no. 3: 112. https://doi.org/10.3390/cryst8030112