Direct Rehydrogenation of LiBH4 from H-Deficient Li2B12H12−x
Abstract
:1. Introduction
2. Experimental
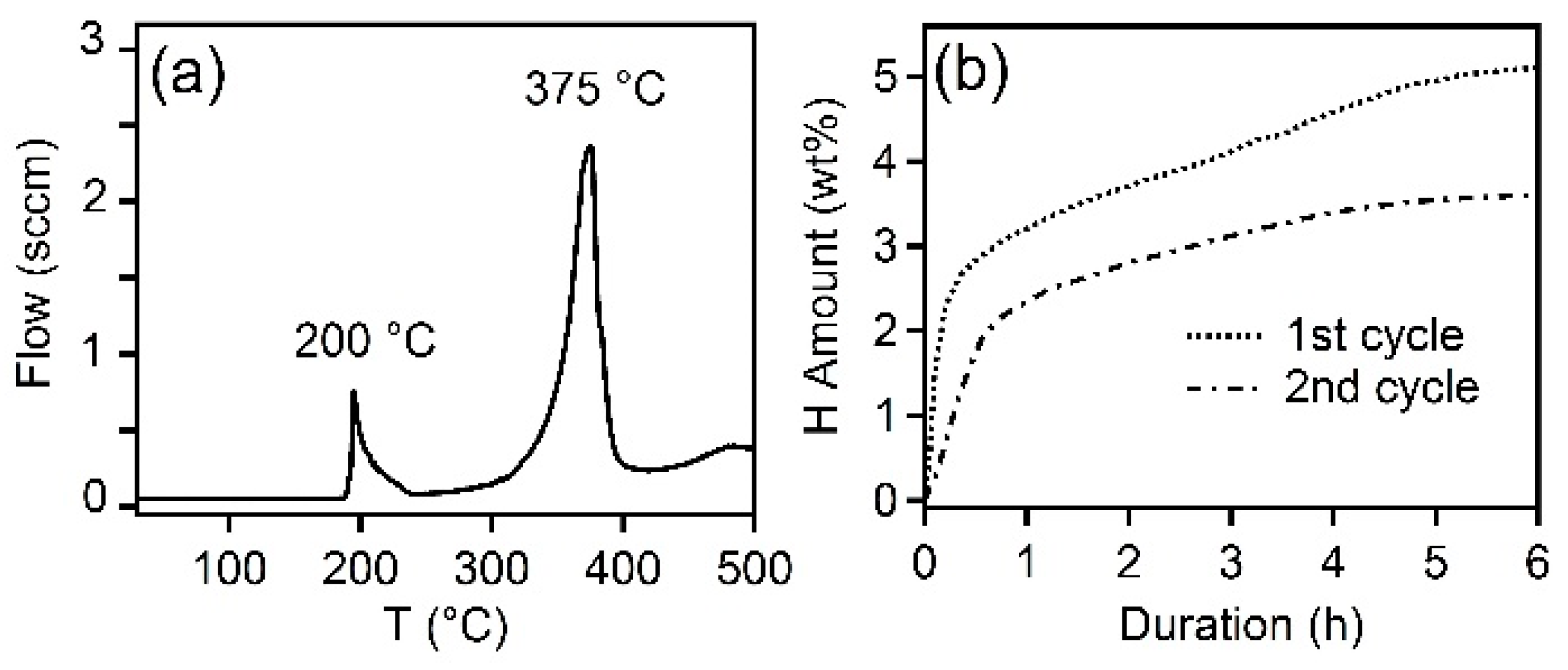
3. Results
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yu, X.; Tang, Z.; Sun, D.; Ouyang, L.; Zhu, M. Recent advances and remaining challenges of nanostructured materials for hydrogen storage applications. Prog. Mater. Sci. 2017, 88, 1–48. [Google Scholar] [CrossRef]
- Paskevicius, M.; Jepsen, L.H.; Schouwink, P.; Cerny, R.; Ravnsbaek, D.B.; Filinchuk, Y.; Dornheim, M.; Besenbacherf, F.; Jensen, T.R. Metal borohydrides and derivatives - synthesis, structure and properties. Chem. Soc. Rev. 2017, 46, 1565–1634. [Google Scholar] [CrossRef] [PubMed]
- Moller, K.T.; Jensen, T.R.; Akiba, E.; Li, H.W. Hydrogen—A sustainable energy carrier. Prog. Nat. Sci.-Mater. 2017, 27, 34–40. [Google Scholar] [CrossRef]
- He, T.; Pachfule, P.; Wu, H.; Xu, Q.; Chen, P. Hydrogen carriers. Nat. Rev. Mater. 2016, 1, 16059. [Google Scholar] [CrossRef]
- Lai, Q.; Paskevicius, M.; Sheppard, D.A.; Buckley, C.E.; Thornton, A.W.; Hill, M.R.; Gu, Q.; Mao, J.; Huang, Z.; Liu, H.K.; et al. Hydrogen storage materials for mobile and stationary applications: Current state of the art. Chemsuschem 2015, 8, 2789–2825. [Google Scholar] [CrossRef] [PubMed]
- Orimo, S.I.; Nakamori, Y.; Eliseo, J.R.; Zuttel, A.; Jensen, C.M. Complex hydrides for hydrogen storage. Chem. Rev. 2007, 107, 4111–4132. [Google Scholar] [CrossRef] [PubMed]
- Soulie, J.P.; Renaudin, G.; Cerny, R.; Yvon, K. Lithium boro-hydride LiBH4. J. Alloy Compd. 2002, 346, 200–205. [Google Scholar] [CrossRef]
- Zuttel, A.; Rentsch, S.; Fischer, P.; Wenger, P.; Sudan, P.; Mauron, P.; Emmenegger, C. Hydrogen storage properties of LiBH4. J. Alloy Compd. 2003, 356, 515–520. [Google Scholar] [CrossRef]
- Orimo, S.; Nakamori, Y.; Kitahara, G.; Miwa, K.; Ohba, N.; Towata, S.; Züttel, A. Dehydriding and rehydriding reactions of LiBH4. J. Alloy Compd. 2005, 404, 427–430. [Google Scholar] [CrossRef]
- Orimo, S.I.; Nakamori, Y.; Ohba, N.; Miwa, K.; Aoki, M.; Towata, S.; Zuttel, A. Experimental studies on intermediate compound of LiBH4. Appl. Phys. Lett. 2006, 89, 021920. [Google Scholar] [CrossRef]
- Mauron, P.; Buchter, F.; Friedrichs, O.; Remhof, A.; Bielmann, M.; Zwicky, C.N.; Zuttel, A. Stability and reversibility of LiBH4. J. Phys. Chem. B 2008, 112, 906–910. [Google Scholar] [CrossRef] [PubMed]
- Her, J.H.; Yousufuddin, M.; Zhou, W.; Jalisatgi, S.S.; Kulleck, J.G.; Zan, J.A.; Hwang, S.J.; Bowman, R.C.; Udovic, T.J. Crystal structure of Li2B12H12: A possible intermediate species in the decomposition of LiBH4. Inorg. Chem. 2008, 47, 9757–9759. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.G.; Remhof, A.; Hwang, S.J.; Li, H.W.; Mauron, P.; Orimo, S.; Zuttel, A. Pressure and temperature dependence of the decomposition pathway of LiBH4. Phys. Chem. Chem. Phys. 2012, 14, 6514–6519. [Google Scholar] [CrossRef] [PubMed]
- Pitt, M.P.; Paskevicius, M.; Brown, D.H.; Sheppard, D.A.; Buckley, C.E. Thermal stability of Li2B12H12 and its role in the decomposition of LiBH4. J. Am. Chem. Soc. 2013, 135, 6930–6941. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Wang, H.; Liu, J.; Jiao, L.; Wang, Y.; Ouyang, L.; Sun, T.; Sun, D.; Wang, H.; Yao, X.; et al. Towards easy reversible dehydrogenation of LiBH4 by catalyzing hierarchic nanostructured cob. Nano Energy 2014, 10, 235–244. [Google Scholar] [CrossRef]
- Shao, J.; Xiao, X.Z.; Fan, X.L.; Huang, X.; Zhai, B.; Li, S.Q.; Ge, H.W.; Wang, Q.D.; Chen, L.X. Enhanced hydrogen storage capacity and reversibility of LiBH4 nanoconfined in the densified zeolite-templated carbon with high mechanical stability. Nano Energy 2015, 15, 244–255. [Google Scholar] [CrossRef]
- White, J.L.; Newhouse, R.J.; Zhang, J.Z.; Udovic, T.J.; Stavila, V. Understanding and mitigating the effects of stable dodecahydro-closo-dodecaborate intermediates on hydrogen-storage reactions. J. Phys. Chem. C 2016, 120, 25725–25731. [Google Scholar] [CrossRef]
- Ngene, P.; Verkuijlen, M.H.W.; Barre, C.; Kentgens, A.P.M.; de Jongh, P.E. Reversible li-insertion in nanoscaffolds: A promising strategy to alter the hydrogen sorption properties of li-based complex hydrides. Nano Energy 2016, 22, 169–178. [Google Scholar] [CrossRef]
- Vajo, J.J.; Skeith, S.L.; Mertens, F. Reversible storage of hydrogen in destabilized LiBH4. J. Phys. Chem. B 2005, 109, 3719–3722. [Google Scholar] [CrossRef] [PubMed]
- Pinkerton, F.E.; Meyer, M.S.; Meisner, G.P.; Balogh, M.P.; Vajo, J.J. Phase boundaries and reversibility of LiBH4/MgH2 hydrogen storage material. J. Phys. Chem. C 2007, 111, 12881–12885. [Google Scholar] [CrossRef]
- Au, M.; Jurgensen, A.R.; Spencer, W.A.; Anton, D.L.; Pinkerton, F.E.; Hwang, S.J.; Kim, C.; Bowman, R.C. Stability and reversibility of lithium borohydrides doped by metal halides and hydrides. J. Phys. Chem. C 2008, 112, 18661–18671. [Google Scholar] [CrossRef]
- Blanchard, D.; Shi, Q.; Boothroyd, C.B.; Vegge, T. Reversibility of al/ti modified LiBH4. J. Phys. Chem. C 2009, 113, 14059–14066. [Google Scholar] [CrossRef]
- Nielsen, T.K.; Bosenberg, U.; Gosalawit, R.; Dornheim, M.; Cerenius, Y.; Besenbacher, F.; Jensen, T.R. A reversible nanoconfined chemical reaction. ACS Nano 2010, 4, 3903–3908. [Google Scholar] [CrossRef] [PubMed]
- Shim, J.H.; Lim, J.H.; Rather, S.U.; Lee, Y.S.; Reed, D.; Kim, Y.; Book, D.; Cho, Y.W. Effect of hydrogen back pressure on dehydrogenation behavior of LiBH4-based reactive hydride composites. J. Phys. Chem. Lett. 2010, 1, 59–63. [Google Scholar] [CrossRef]
- Choi, Y.J.; Lu, J.; Sohn, H.Y.; Fang, Z.Z.; Kim, C.; Bowman, R.C.; Hwang, S.J. Reaction mechanisms in the Li3AlH6/LiBH4 and Al/LiBH4 systems for reversible hydrogen storage. Part 2: Solid-state NMR studies. J. Phys. Chem. C 2011, 115, 6048–6056. [Google Scholar] [CrossRef]
- Cai, W.T.; Wang, H.; Sun, D.L.; Zhu, M. Nanosize-controlled reversibility for a destabilizing reaction in the LiBH4-NdH2+x system. J. Phys. Chem. C 2013, 117, 9566–9572. [Google Scholar] [CrossRef]
- Javadian, P.; Sheppard, D.A.; Buckley, C.E.; Jensen, T.R. Hydrogen storage properties of nanoconfined LiBH4-Ca(BH4)2. Nano Energy 2015, 11, 96–103. [Google Scholar] [CrossRef]
- Puszkiel, J.A.; Riglos, M.V.C.; Karimi, F.; Santoru, A.; Pistidda, C.; Klassen, T.; von Colbe, J.M.B.; Dornheim, M. Changing the dehydrogenation pathway of LiBH4-MgH2 via nanosized lithiated TiO2. Phys. Chem. Chem. Phys. 2017, 19, 7455–7460. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.-D.; Wang, P.; Ma, L.-P.; Cheng, H.-M. Reversible hydrogen storage in LiBH4 destabilized by milling with al. Appl. Phys. A 2007, 89, 963–966. [Google Scholar] [CrossRef]
- Song, D.; Wang, Y.; Wang, Y.; Jiao, L.; Yuan, H. Preparation and characterization of novel structure Co–B hydrogen storage alloy. Electrochem. Commun. 2008, 10, 1486–1489. [Google Scholar] [CrossRef]
- Wang, Q.; Jiao, L.; Du, H.; Song, D.; Peng, W.; Si, Y.; Wang, Y.; Yuan, H. Facile preparation and good electrochemical hydrogen storage properties of chain-like and rod-like Co-B nanomaterials. Electrochim. Acta 2010, 55, 7199–7203. [Google Scholar] [CrossRef]
- Massiot, D.; Fayon, F.; Capron, M.; King, I.; LeCalve, S.; Alonso, B.; Durand, J.O.; Bujoli, B.; Gan, Z.H.; Hoatson, G. Modelling one- and two-dimensional solid-state nmr spectra. Magn. Reson. Chem. 2002, 40, 70–76. [Google Scholar] [CrossRef]
- Yan, Y.G.; Remhof, A.; Mauron, P.; Rentsch, D.; Lodziana, Z.; Lee, Y.S.; Lee, H.S.; Cho, Y.W.; Zuttel, A. Controlling the dehydrogenation reaction toward reversibility of the LiBH4-Ca(BH4)2 eutectic system. J. Phys. Chem. C 2013, 117, 8878–8886. [Google Scholar] [CrossRef]
- Bykova, E.; Tsirlin, A.A.; Gou, H.; Dubrovinsky, L.; Dubrovinskaia, N. Novel non-magnetic hard boride Co5B16 synthesized under high pressure. J. Alloy Compd. 2014, 608, 69–72. [Google Scholar] [CrossRef]
- Chikazumi, S. Physics of Ferromagnetism, 2nd ed.; Oxford University Press: Oxford, UK, 1997. [Google Scholar]
- Wu, C.; Wu, F.; Bai, Y.; Yi, B.; Zhang, H. Cobalt boride catalysts for hydrogen generation from alkaline nabh4 solution. Mater. Lett. 2005, 59, 1748–1751. [Google Scholar] [CrossRef]
- Tang, W.S.; Wu, G.; Liu, T.; Wee, A.T.S.; Yong, C.K.; Xiong, Z.; Hor, A.T.S.; Chen, P. Cobalt-catalyzed hydrogen desorption from the LiNH2-LiBH4 system. Dalton Trans. 2008, 0, 2395–2399. [Google Scholar] [CrossRef] [PubMed]
- Masa, J.; Weide, P.; Peeters, D.; Sinev, I.; Xia, W.; Sun, Z.; Somsen, C.; Muhler, M.; Schuhmann, W. Amorphous cobalt boride (Co2B) as a highly efficient nonprecious catalyst for electrochemical water splitting: Oxygen and hydrogen evolution. Adv. Energy Mater. 2016, 6, 15023131. [Google Scholar] [CrossRef]
- Fernandes, R.; Patel, N.; Miotello, A.; Filippi, M. Studies on catalytic behavior of Co–Ni–B in hydrogen production by hydrolysis of NaBH4. J. Mol. Catal. A 2009, 298, 1–6. [Google Scholar] [CrossRef]
- Carenco, S.; Portehault, D.; Boissière, C.; Mézailles, N.; Sanchez, C. Nanoscaled metal borides and phosphides: Recent developments and perspectives. Chem. Rev. 2013, 113, 7981–8065. [Google Scholar] [CrossRef] [PubMed]

© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Y.; Wang, H.; Zhu, M.; Cai, W.; Rentsch, D.; Remhof, A. Direct Rehydrogenation of LiBH4 from H-Deficient Li2B12H12−x. Crystals 2018, 8, 131. https://doi.org/10.3390/cryst8030131
Yan Y, Wang H, Zhu M, Cai W, Rentsch D, Remhof A. Direct Rehydrogenation of LiBH4 from H-Deficient Li2B12H12−x. Crystals. 2018; 8(3):131. https://doi.org/10.3390/cryst8030131
Chicago/Turabian StyleYan, Yigang, Hui Wang, Min Zhu, Weitong Cai, Daniel Rentsch, and Arndt Remhof. 2018. "Direct Rehydrogenation of LiBH4 from H-Deficient Li2B12H12−x" Crystals 8, no. 3: 131. https://doi.org/10.3390/cryst8030131





