Transition Metal Selenite Halides: A Fascinating Family of Magnetic Compounds
Abstract
:1. Introduction
2. Crystal Structures and Magnetic Properties Overview
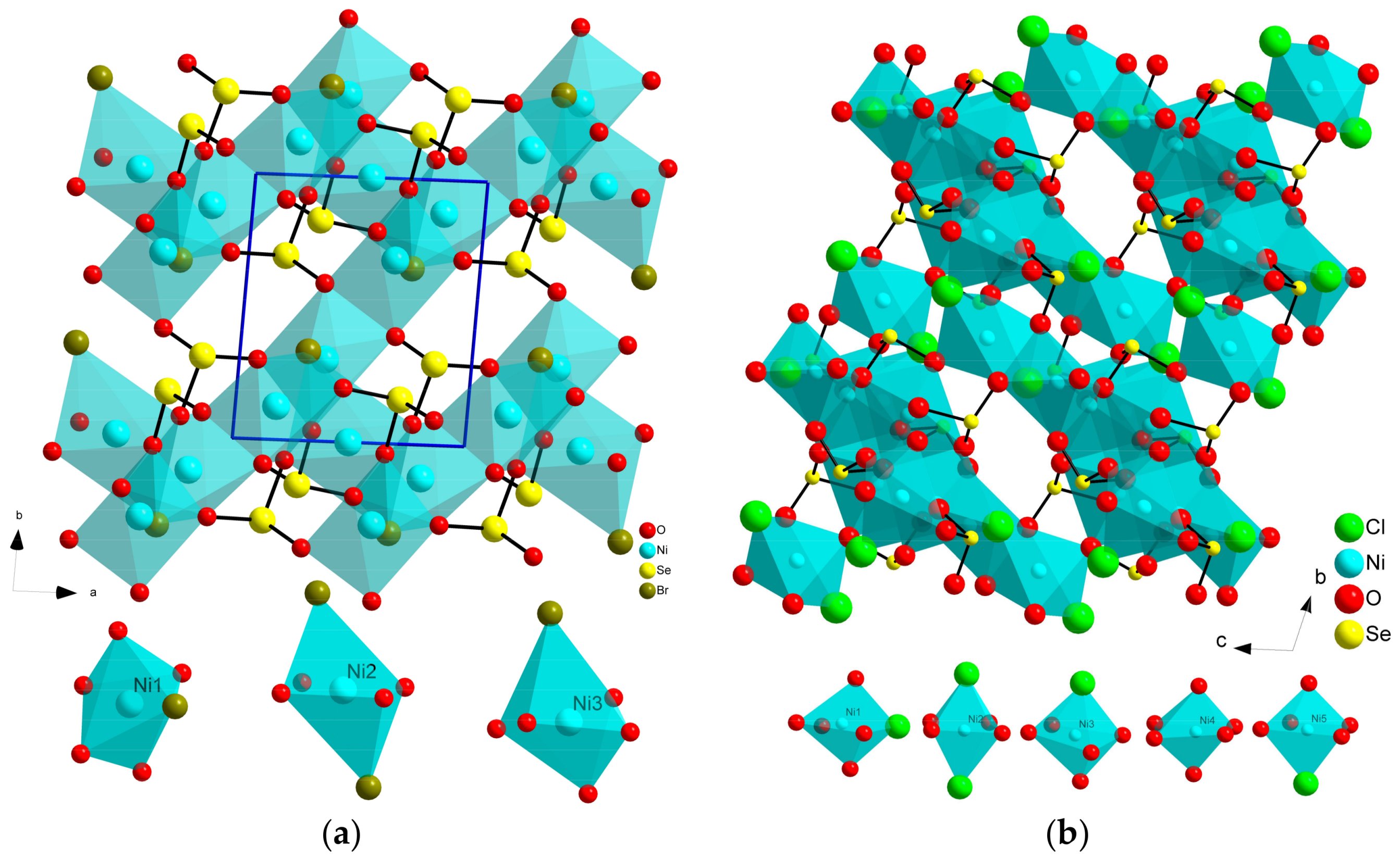
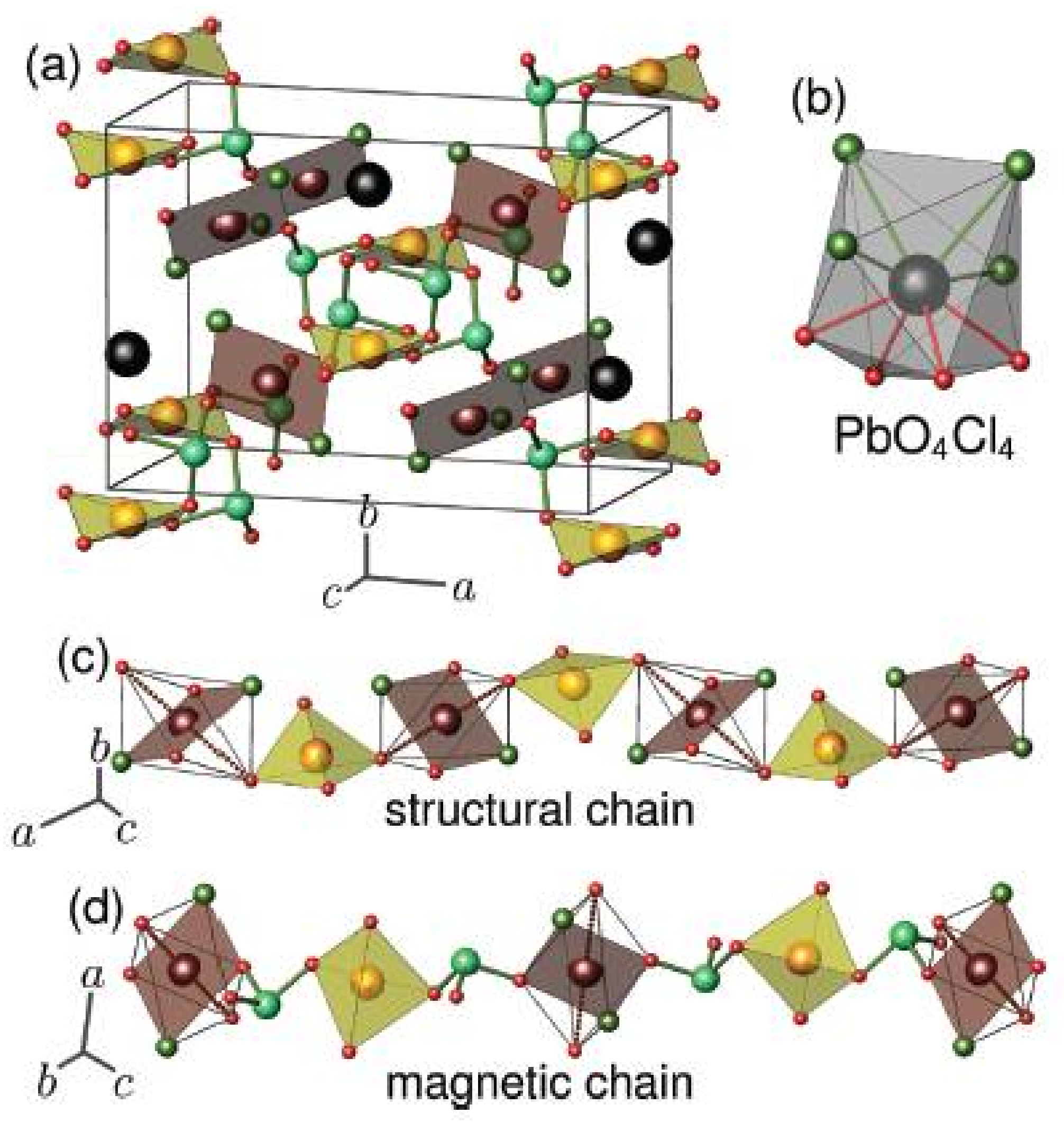
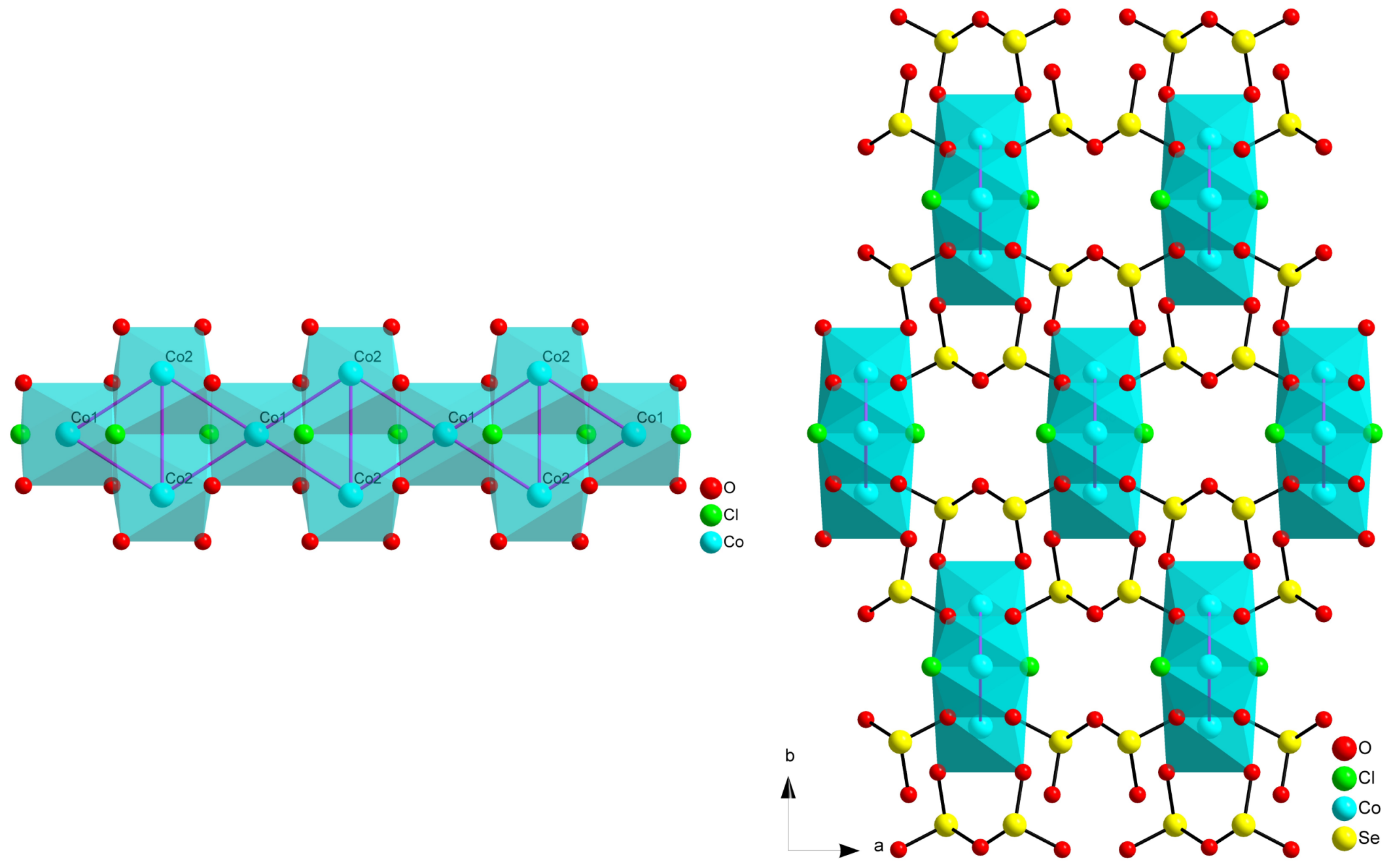
2.1. Composition MII5(SeO3)4X2
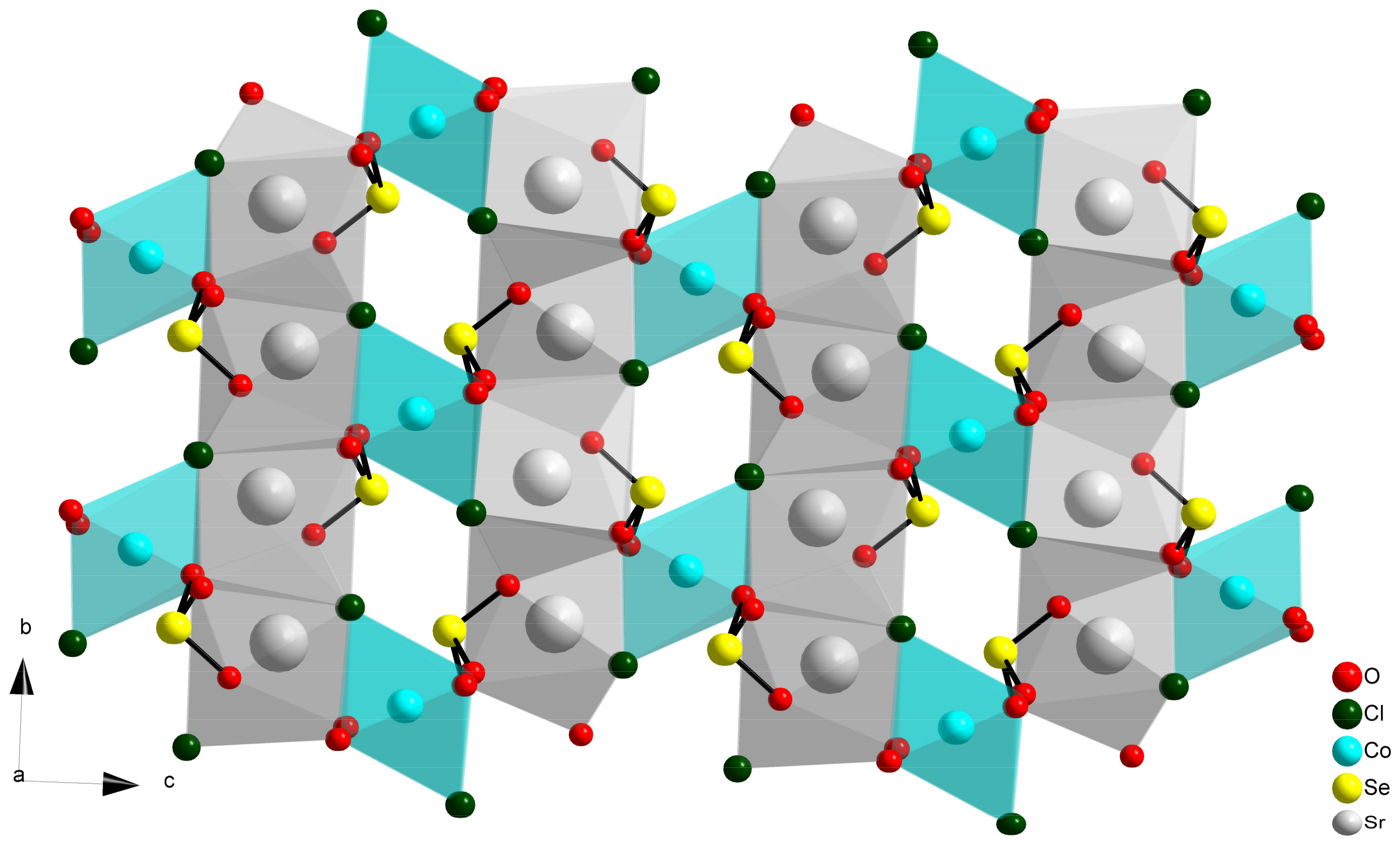
2.2. Compositions Sr2MII(SeO3)2Cl2 and MM”2(SeO3)2Cl2
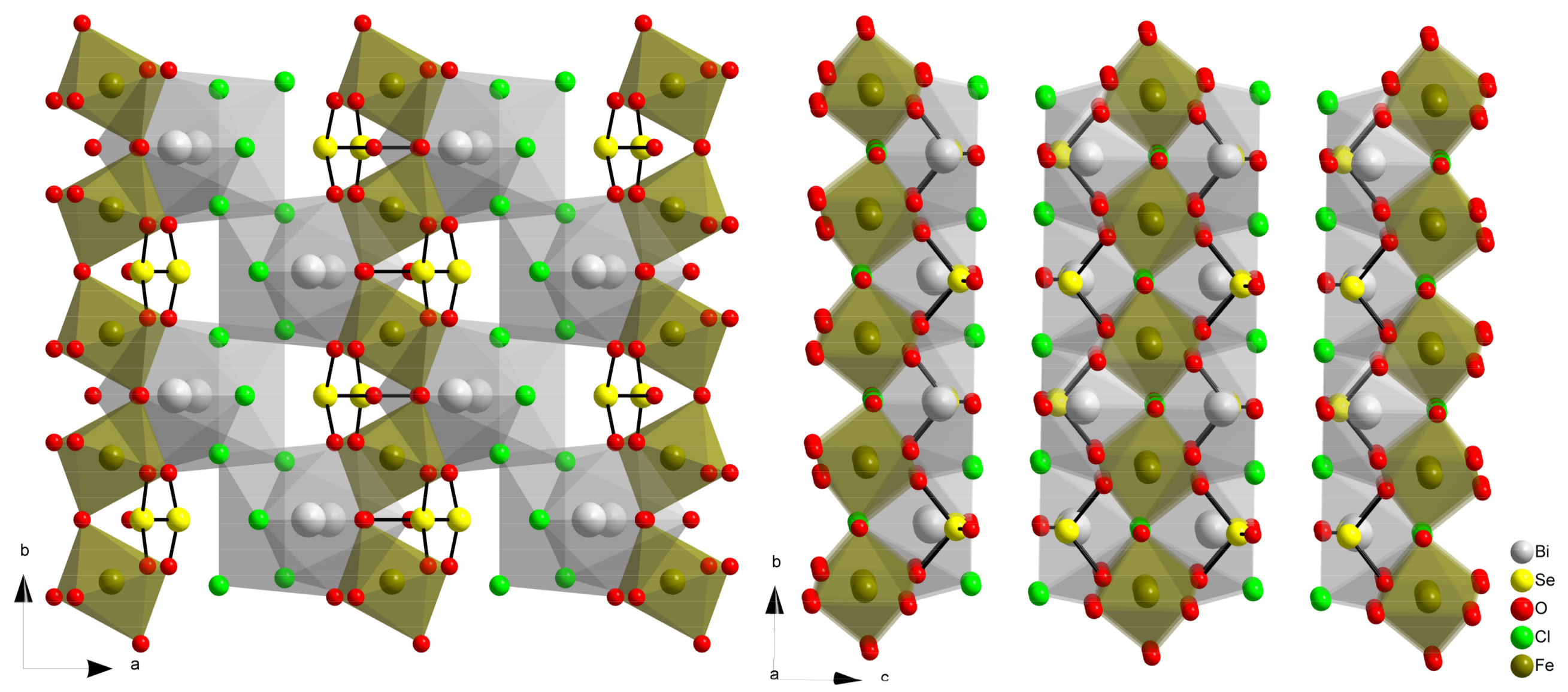
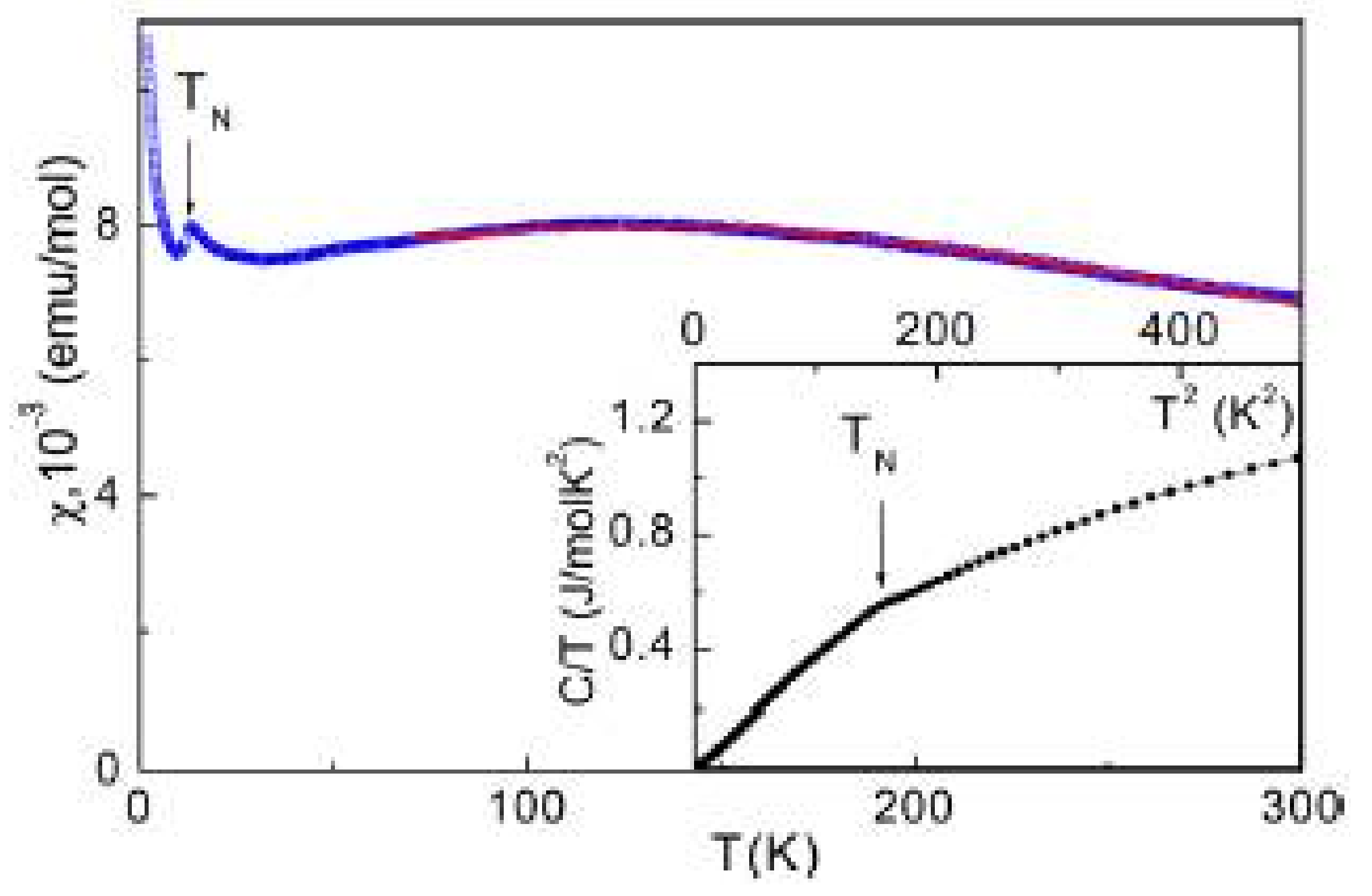
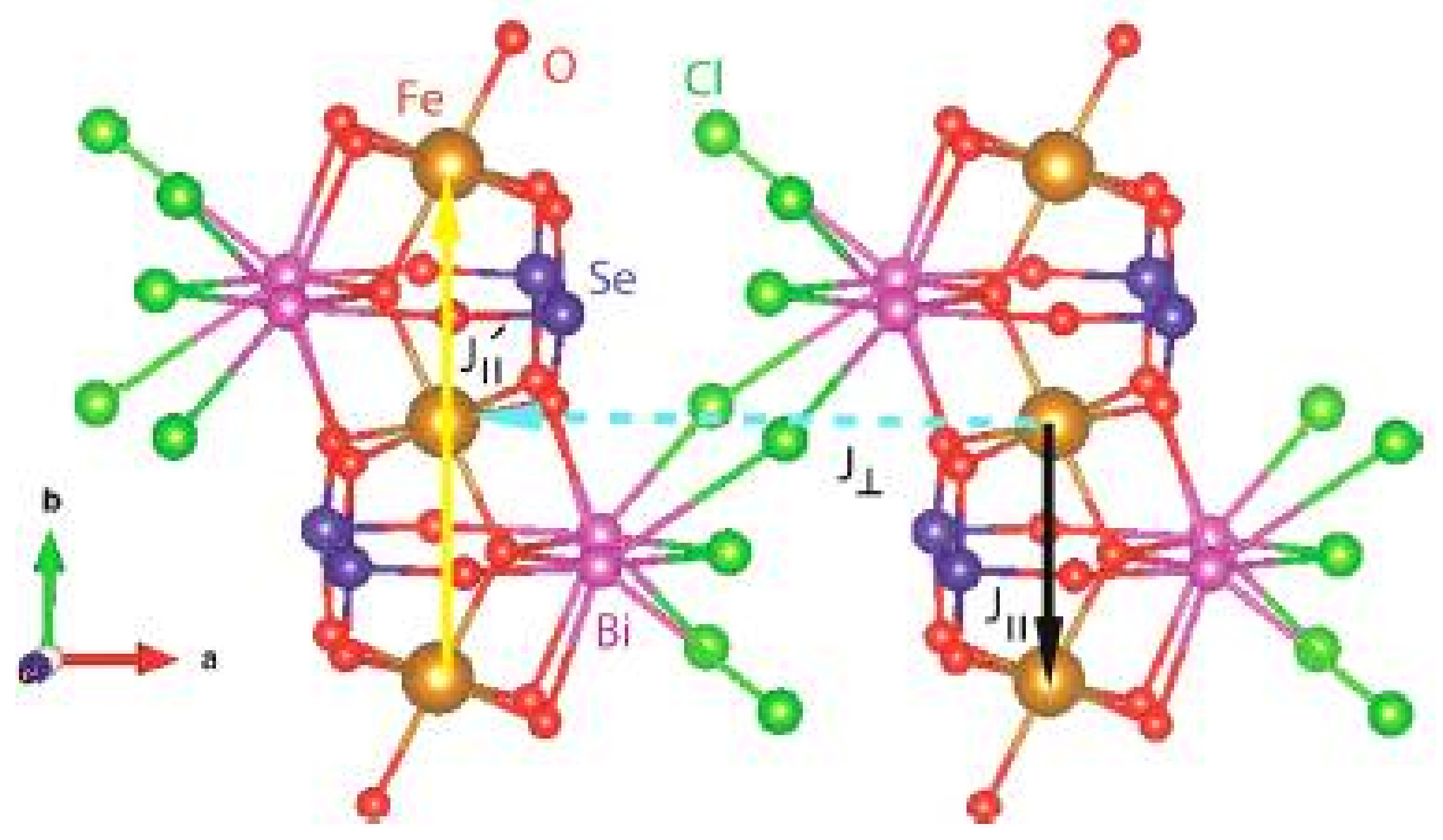
2.3. Compound Bi2Fe(SeO3)2OCl3
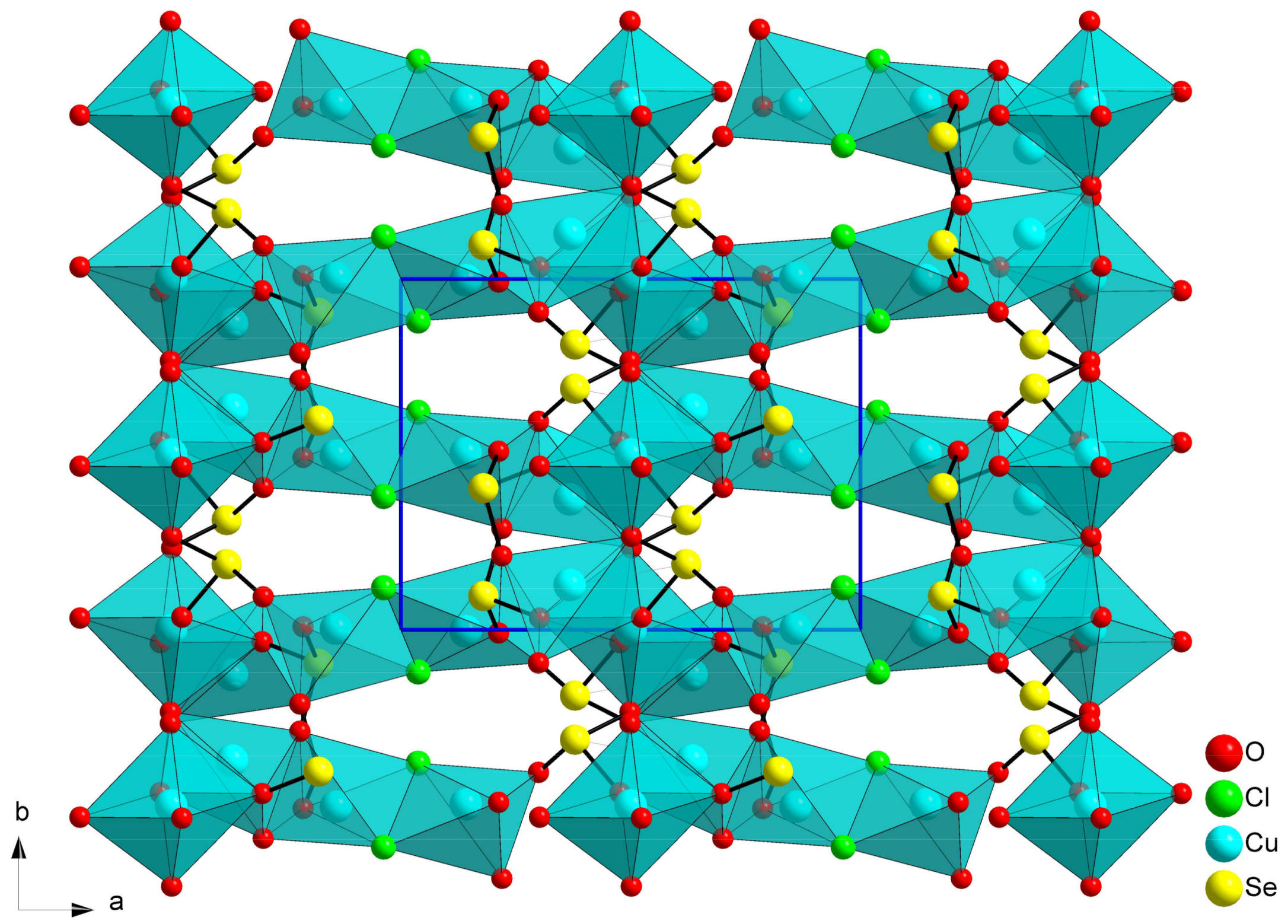
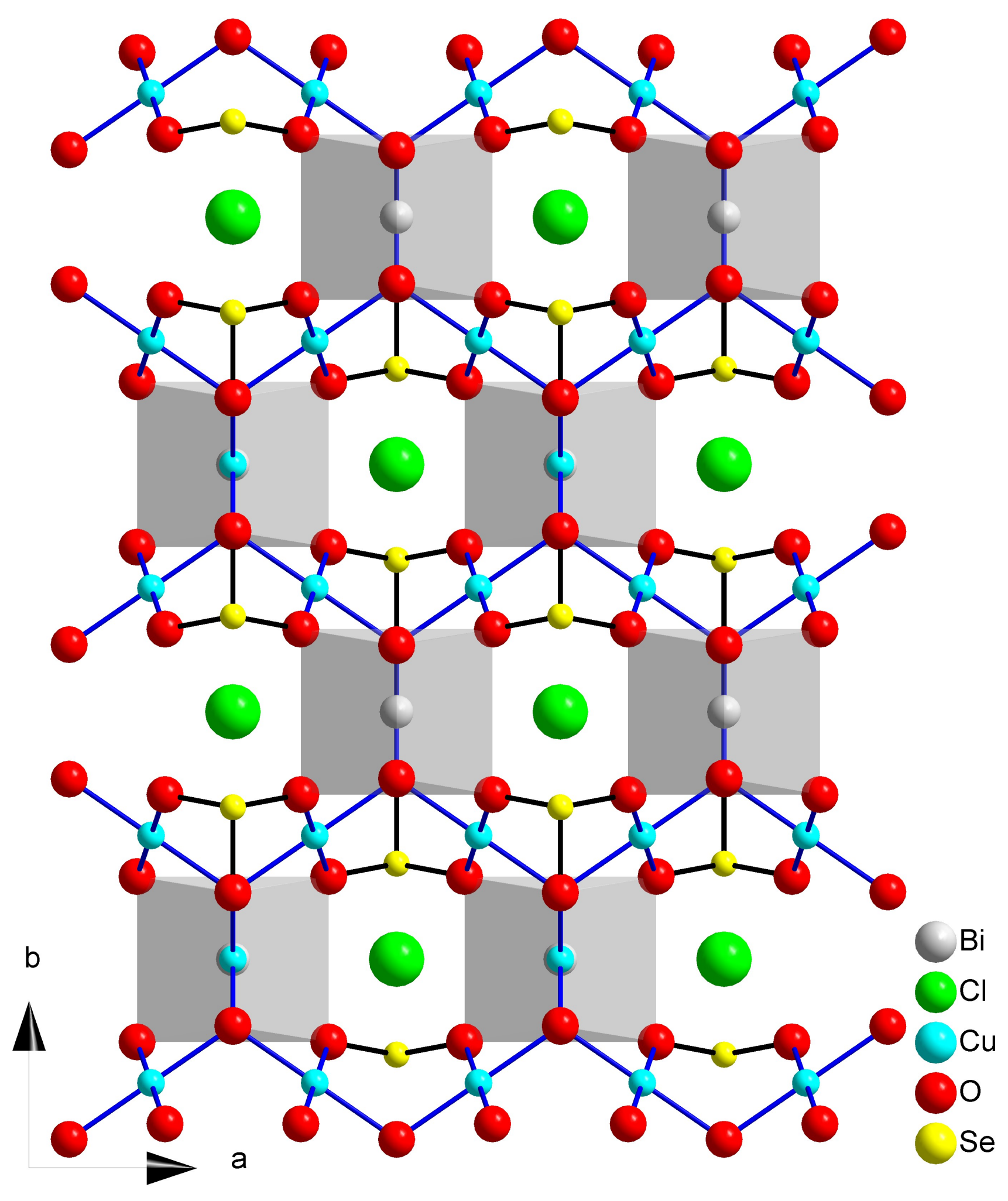
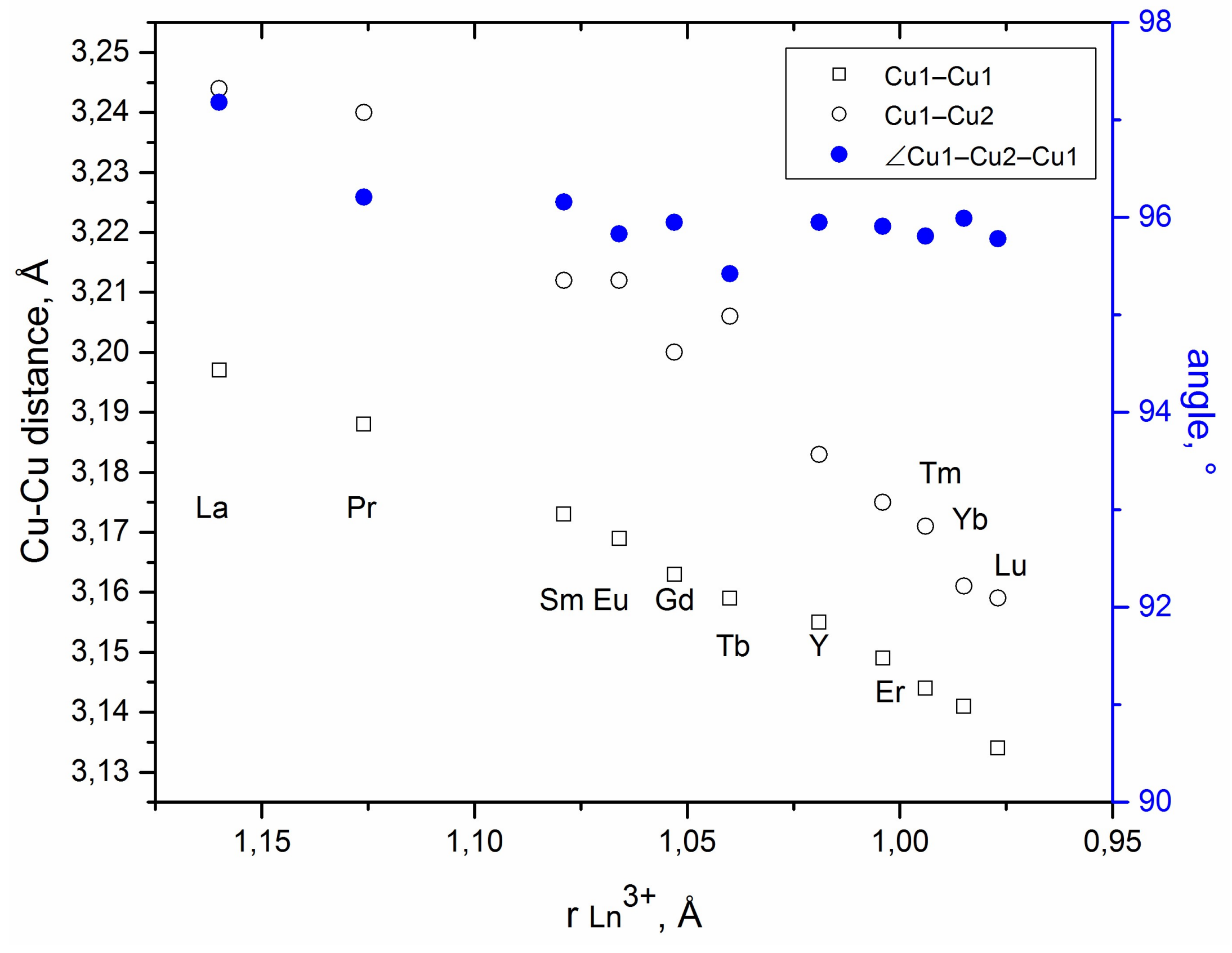
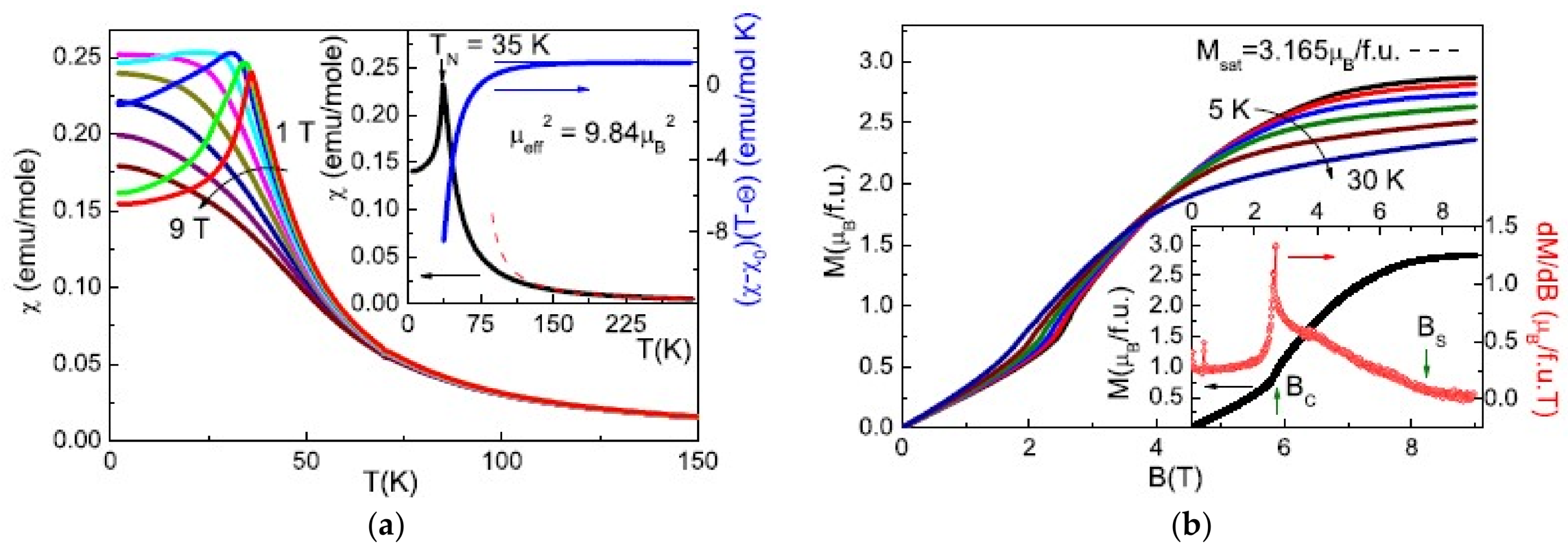
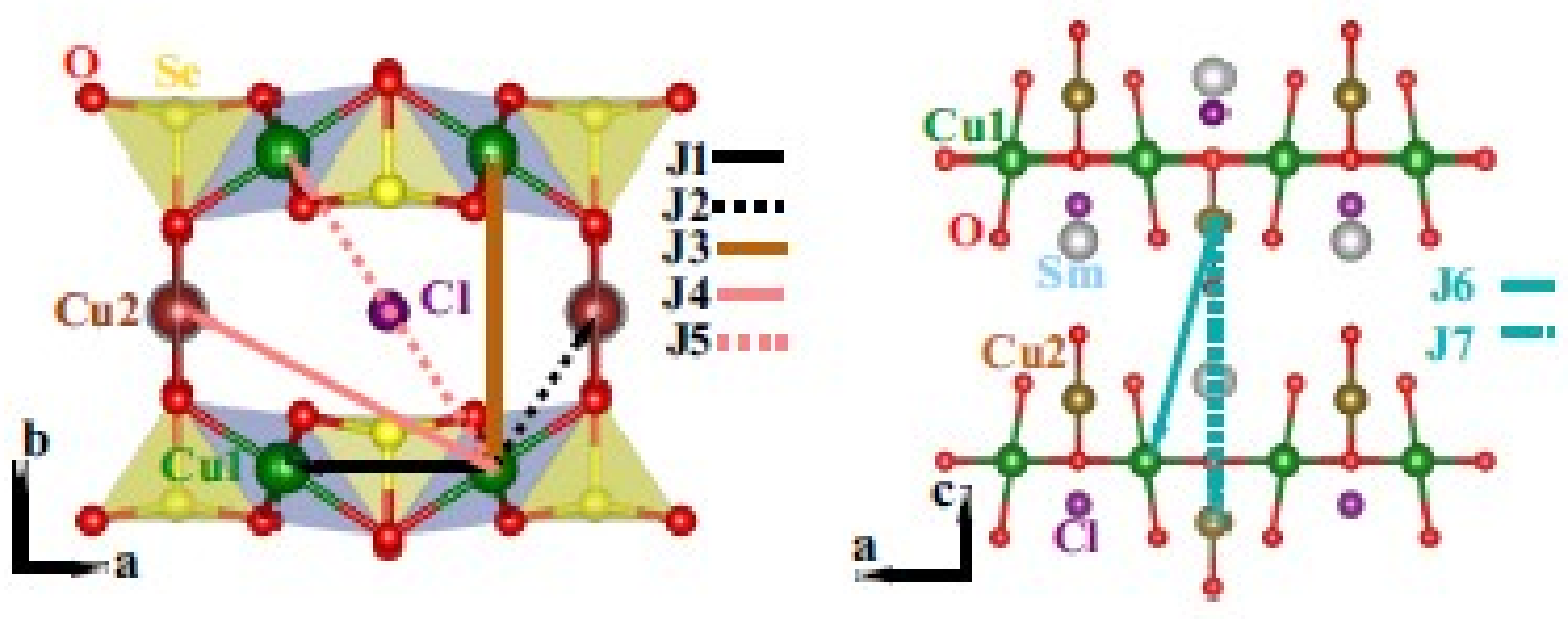
2.4. Francisite Like Compounds Cu3M(SeO3)2O2X
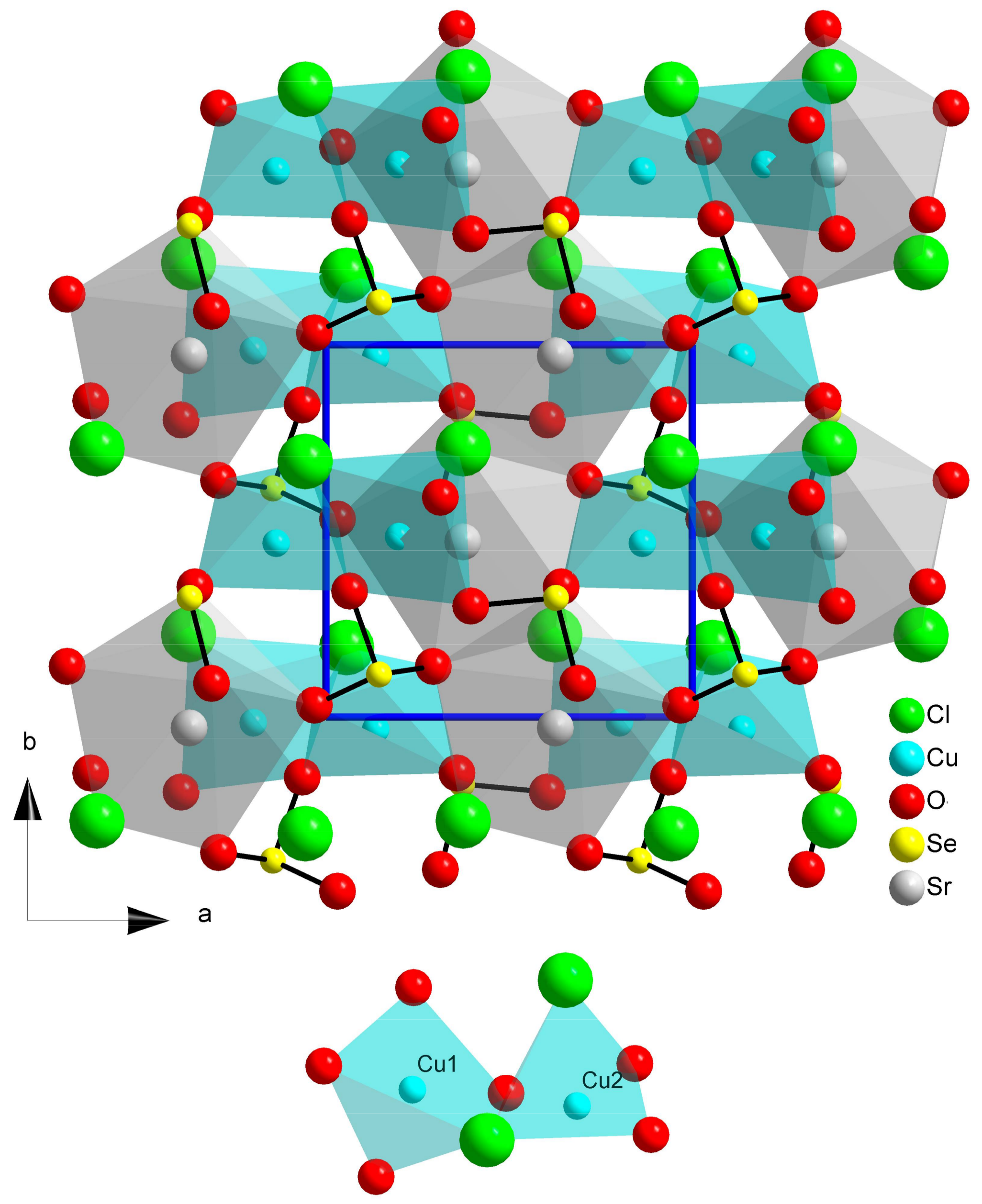
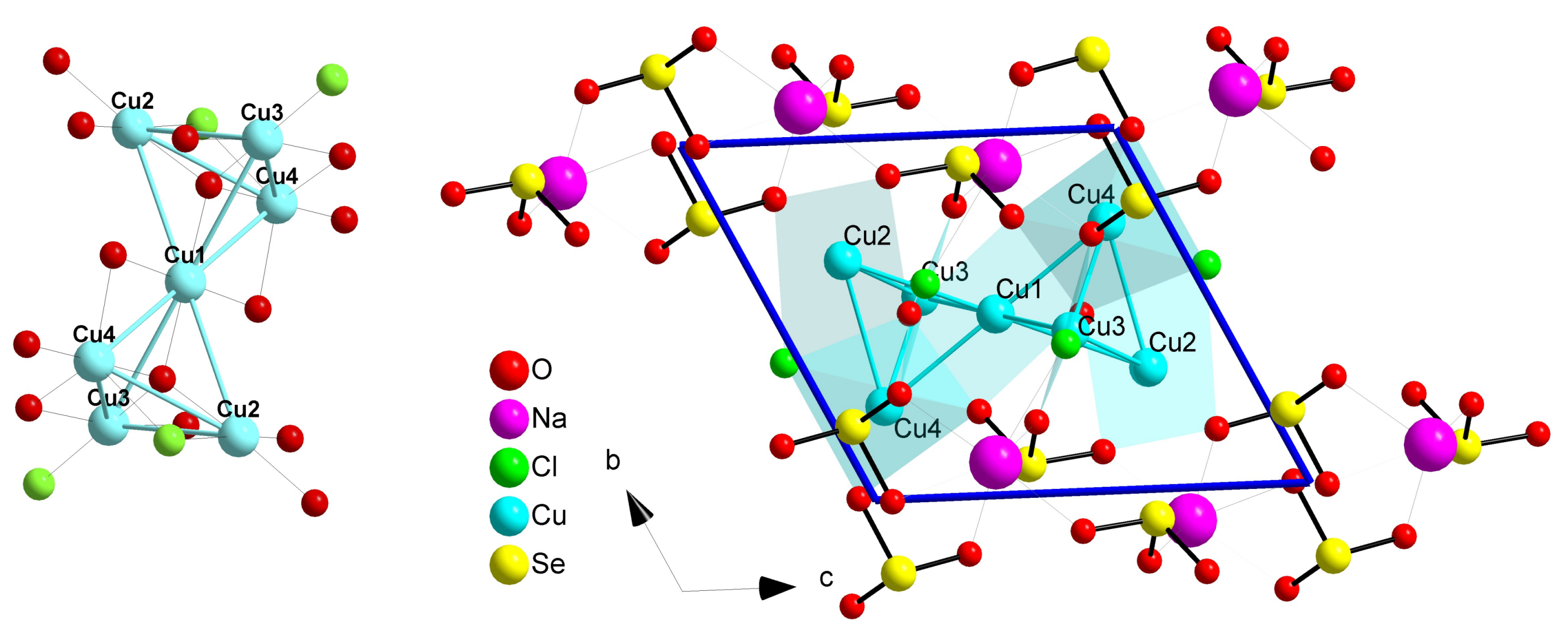
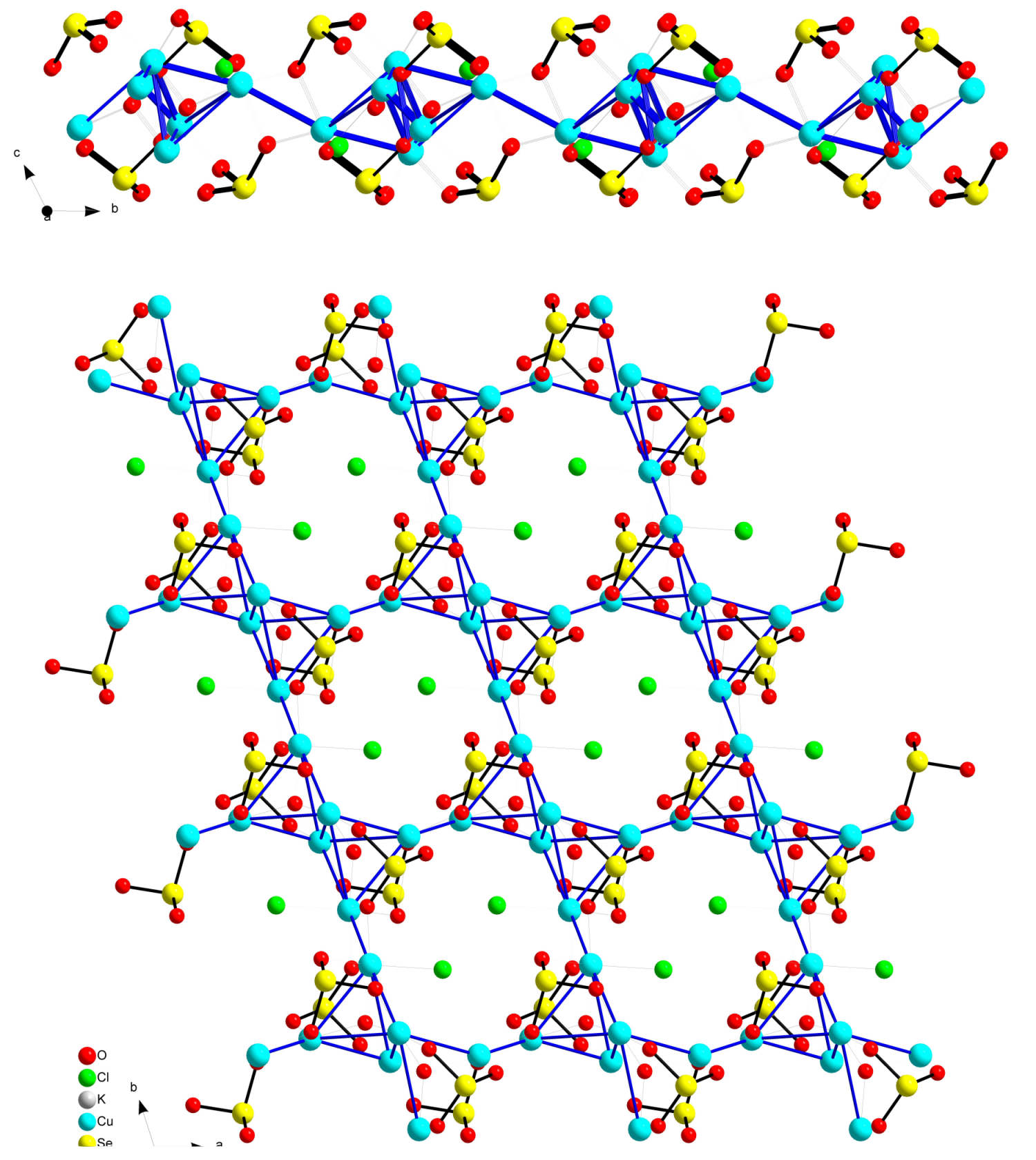
2.5. Ilinskite Like Compounds MCu5(SeO3)2O2Cl3
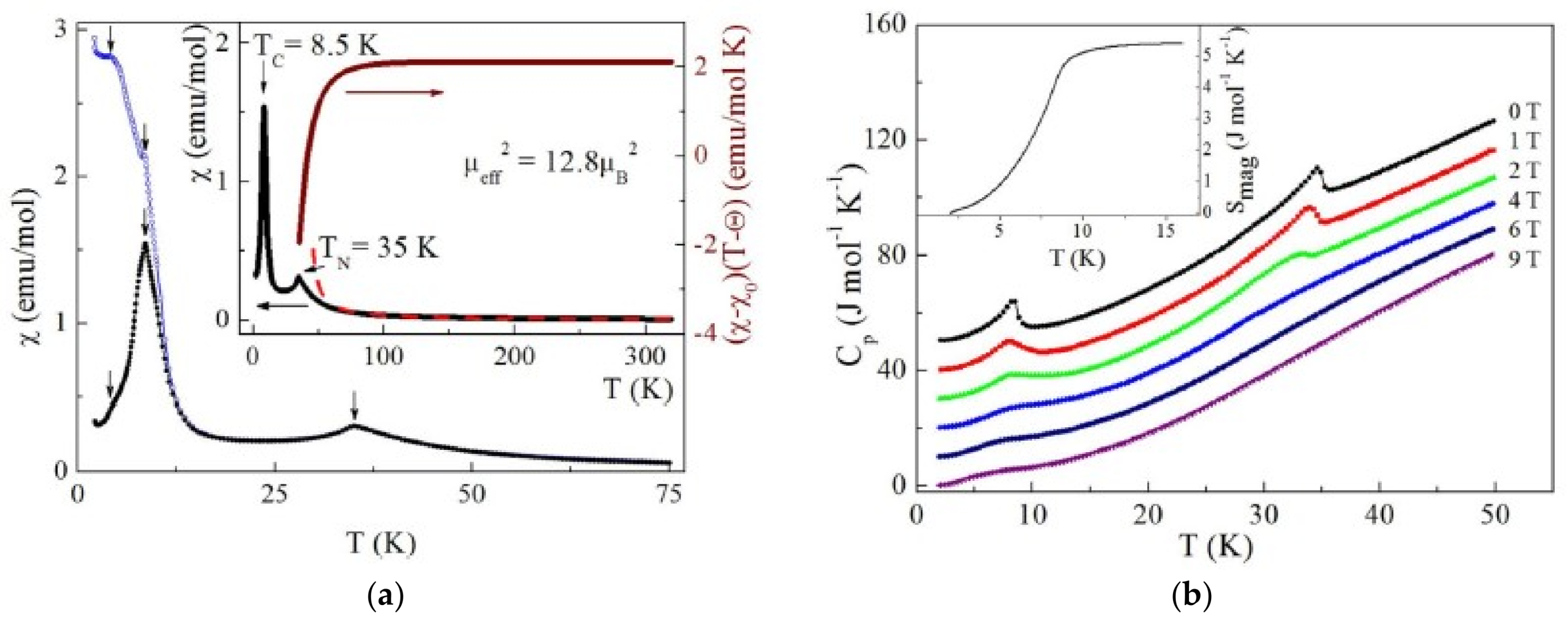
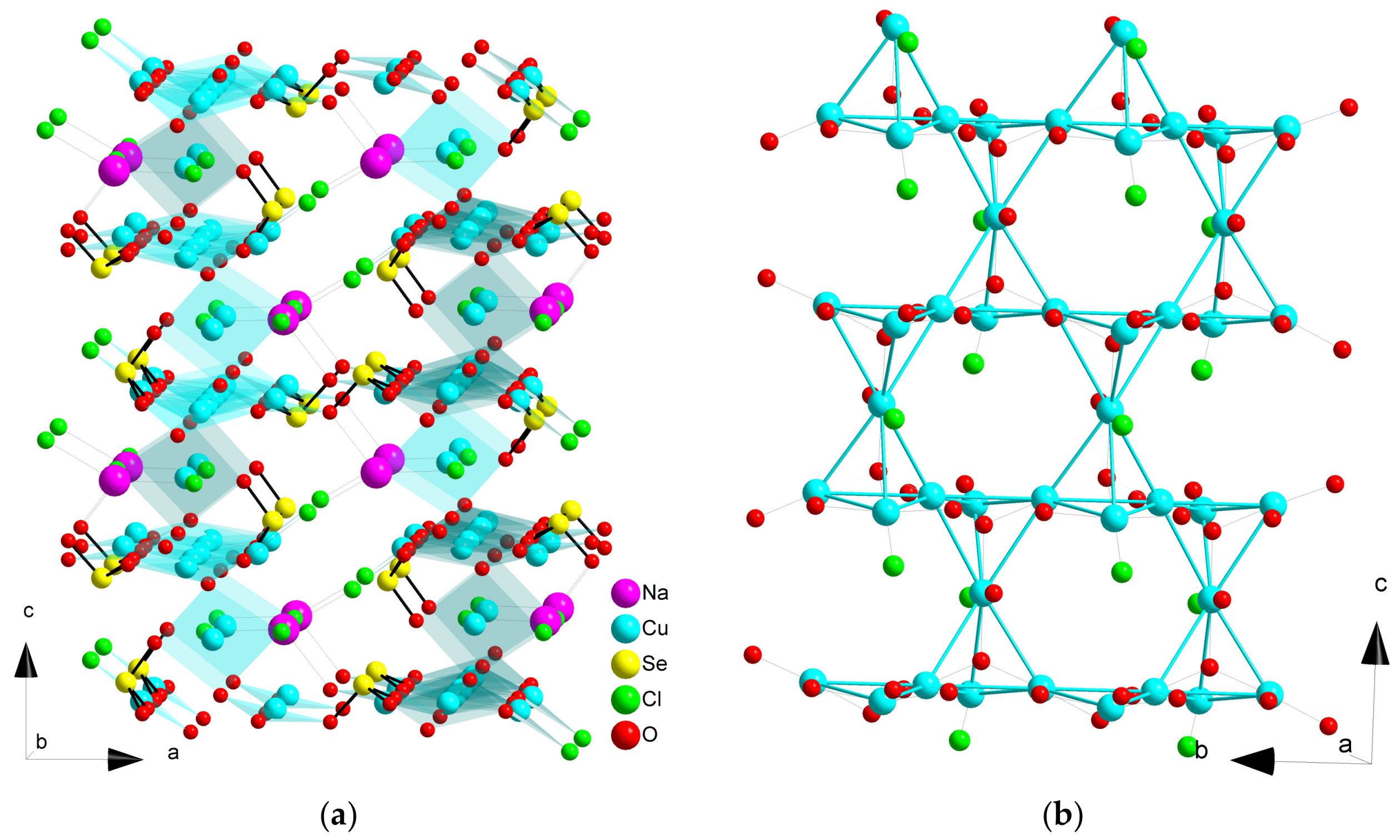
2.6. Compound Na2Cu7(SeO3)4O2Cl4
3. Conclusions and Outlook
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schiffer, P. Condensed-matter physics: Magnetic frustration squeezed out. Nature 2002, 420, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Balents, L. Spin liquids in frustrated magnets. Nature 2010, 464, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Hess, C. Heat conduction in low-dimensional quantum magnets. Eur. Phys. J. Spec. Top. 2007, 151, 73–83. [Google Scholar] [CrossRef]
- Gillespie, R.J.; Hargittai, I. The VSEPR Model of Molecular Geometry; Prentice-Hall: Upper Saddle River, NJ, USA, 1991; 6990p, ISBN 978-0205123698. [Google Scholar]
- Gillespie, R.J. The VSEPR model revisited. Chem. Soc. Rev. 1992, 21, 59–69. [Google Scholar] [CrossRef]
- Galy, J.; Meunier, G.; Andersson, G.; Åstrtöm, A. Stéréochimie des Eléments Comportant des Paires Non Liées: Ge (II), As (III), Se(IV), Br (v), Sn (II), Sb(III), Te (IV), I (V), Xe(VI), Tl (I), Pb (II), et Bi (III) (Oxydes, Fluorures et Oxyfluorures). J. Solid State Chem. 1975, 13, 142–159. [Google Scholar] [CrossRef]
- Mao, J.-G.; Jiang, H.-L.; Kong, F. Structures and Properties of Functional Metal Selenites and Tellurites. Inorg. Chem. 2008, 47, 8498–8510. [Google Scholar] [CrossRef] [PubMed]
- Wickleder, M.S.; Logemann, C. Compounds Containing the Chalcogen Oxygen E–O Bond (E = S, Se, Te). In Handbook of Chalcogen Chemistry: New Perspectives in Sulfur, Selenium and Tellurium, 2nd ed.; Devillanova, F.A., du Mont, W.W., Eds.; RSC Publishing: Cambridge, UK, 2013; Volume 1, pp. 307–345. ISBN 978-1-84973-623-7. [Google Scholar]
- Vanýsek, P. Electrochemical series. In CRC Handbook of Chemistry and Physics, 97th ed.; W.M. Haynes CRC Press: Boca Raton, FL, USA, 2016; pp. 5-78–5-84. ISBN 978-1-4987-5429-3. [Google Scholar]
- Christy, A.G.; Mills, S.J.; Kampf, A.R. A review of the structural architecture of tellurium oxycompounds. Mineral. Mag. 2016, 80, 415–545. [Google Scholar] [CrossRef]
- Johnsson, M.; Törnroos, K.W.; Mila, F.; Millet, P. Tetrahedral Clusters of Copper(II): Crystal Structures and Magnetic Properties of Cu2Te2O5X2 (X = Cl, Br). Chem. Mater. 2000, 12, 2853–2857. [Google Scholar] [CrossRef]
- Johnsson, M.; Törnroos, K.W.; Lemmens, P.; Millet, P. Crystal Structure and Magnetic Properties of a New Two-Dimensional S = 1 Quantum Spin System Ni5(TeO3)4X2 (X = Cl, Br). Chem. Mater. 2003, 15, 68–73. [Google Scholar] [CrossRef]
- Becker, R.; Johnsson, M.; Kremer, R.K.; Klauss, H.-H.; Lemmens, P. Crystal Structure and Magnetic Properties of FeTe2O5X (X = Cl, Br): A Frustrated Spin Cluster Compound with a New Te(IV) Coordination Polyhedron. J. Am. Chem. Soc. 2006, 128, 15469–15475. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Johnsson, M.; Kremer, R.; Lemmens, P. Crystal structure and magnetic properties of Cu3(TeO3)2Br2—A layered compound with a new Cu(II) coordination polyhedron. J. Solid State Chem. 2005, 178, 2024–2029. [Google Scholar] [CrossRef]
- Millet, P.; Bastide, B.; Johnsson, M. Cu3(SeO3)2Cl2: A new oxochloride of copper(II) and selenium(IV). Solid State Commun. 2000, 113, 719–723. [Google Scholar] [CrossRef]
- Lemmens, P.; Millet, P. Spin—Orbit—Topology, a triptych. In Quantum Magnetism; Lecture Notes in Physics; Schollwöck, U., Richter, J., Farnell, D.J.J., Bishop, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 645, pp. 433–477. ISBN 978-3-540-40066-0. [Google Scholar] [CrossRef]
- Cao, X.-L.; Hu, C.-L.; Xu, X.; Kong, F.; Mao, J.-G. Pb2TiOF(SeO3)2Cl and Pb2NbO2(SeO3)2Cl: Small changes in structure induced a very large SHG enhancement. Chem. Commun. 2013, 49, 9965–9967. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Johnsson, M.; Law, J.M.; Bettis, J.L., Jr.; Whangbo, M.-H.; Kremer, R.K. Crystal Structure and Magnetic Properties of the S = 1/2 Quantum Spin System Cu7(TeO3)6F2 with Mixed Dimensionality. Inorg. Chem. 2014, 53, 4250–4256. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Johnsson, M. Synthesis and crystal structure of two synthetic oxofluoride framework compounds—Co2TeO3F2 and Co2SeO3F2. Dalton Trans. 2012, 41, 12786–12789. [Google Scholar] [CrossRef] [PubMed]
- Orive, J.; Balda, R.; Fernández, J.; Lezamad, L.; Arriortua, M.I. Low temperature red luminescence of a fluorinated Mn-doped zinc selenite. Dalton Trans. 2013, 42, 12481–12494. [Google Scholar] [CrossRef] [PubMed]
- Liang, M.-L.; Ma, Y.-X.; Hu, C.-L.; Kong, F.; Mao, J.-G. A(VO2F)(SeO3) (A = Sr, Ba) and Ba(MOF2)(TeO4) (M = Mo, W): First examples of alkali-earth selenites/tellurites with a fluorinated d0-TM octahedron. Dalton Trans. 2018, 47, 1513–1519. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Berger, H.; Kremer, R.K.; Wulferding, D.; Lemmens, P.; Johnsson, M. Synthesis, Crystal Structure, and Magnetic Properties of the Copper Selenite Chloride Cu5(SeO3)4Cl2. Inorg. Chem. 2010, 49, 9683–9688. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.; Prester, M.; Berger, H.; Lin, P.H.; Johnsson, M.; Drobac, D.; Zivkovic, I. Crystal structure and magnetic properties of two new cobalt selenite halides: Co5(SeO3)4X2 (X = Cl, Br). J. Solid State Chem. 2007, 180, 1051–1059. [Google Scholar] [CrossRef]
- Shen, Y.-L.; Mao, J.-G.; Jiang, H.-L. Synthesis, crystal structure and magnetic property of a new nickel selenite chloride: Ni5(SeO3)4Cl2. J. Solid State Chem. 2005, 178, 2942–2946. [Google Scholar] [CrossRef]
- Jiang, H.-L.; Mao, J.-G. New Members in the Nin+1(QO3)nX2 Family: Unusual 3D Network Based on Ni4ClO3 Cubane-like Clusters in Ni7(TeO3)6Cl. Inorg. Chem. 2006, 45, 7593–7599. [Google Scholar] [CrossRef] [PubMed]
- Berdonosov, P.S.; Olenev, A.V.; Kuznetsov, A.N.; Dolgikh, V.A. A group of new selenite-chlorides of strontium and d-metals (Co, Ni): Synthesis, thermal behavior and crystal chemistry. J. Solid State Chem. 2009, 182, 77–82. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Olenev, A.V.; Dolgikh, V.A. Strontium–copper selenite–chlorides: Synthesis and structural investigation. J. Solid State Chem. 2009, 182, 2368–2373. [Google Scholar] [CrossRef]
- Janson, O.; Tsirlin, A.A.; Osipova, E.S.; Berdonosov, P.S.; Olenev, A.V.; Dolgikh, V.A.; Rosner, H. CaCu2(SeO3)2Cl2: Spin-1/2 Heisenberg chain compound with complex frustrated interchain couplings. Phys. Rev. 2011, B83, 144423. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Janson, O.; Olenev, A.V.; Krivovichev, S.V.; Rosner, H.; Dolgikh, V.A.; Tsirlin, A.A. Crystal structures and variable magnetism of PbCu2(XO3)2Cl2 with X = Se, Te. Dalton Trans. 2013, 42, 9547–9554. [Google Scholar] [CrossRef] [PubMed]
- Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A.; Sobolev, A.V.; Presniakov, I.A.; Olenev, A.V.; Rahaman, B.; Saha-Dasgupta, T.; Zakharov, K.V.; Zvereva, E.A.; et al. Crystal Structure, Physical Properties, and Electronic and Magnetic Structure of the Spin S = 5/2 Zigzag Chain Compound Bi2Fe(SeO3)2OCl3. Inorg. Chem. 2014, 53, 5830–5838. [Google Scholar] [CrossRef] [PubMed]
- Millet, P.; Bastide, B.; Pashchenko, V.; Gnatchenko, S.; Gapon, V.; Ksarid, Y.; Stepanov, A. Syntheses, crystal structures and magnetic properties of francisite compounds Cu3Bi(SeO3)2O2X (X = Cl, Br and I). J. Mater. Chem. 2001, 11, 1152–1157. [Google Scholar] [CrossRef]
- Zakharov, K.V.; Zvereva, E.A.; Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A.; Clark, L.; Black, C.; Lightfoot, P.; Kockelmann, W.; Pchelkina, Z.V.; et al. Thermodynamic properties, electron spin resonance, and underlying spin model in Cu3Y(SeO3)2O2Cl. Phys. Rev. 2014, B90, 214417. [Google Scholar] [CrossRef]
- Markina, M.M.; Zakharov, K.V.; Zvereva, E.A.; Denisov, R.S.; Berdonosov, P.S.; Dolgikh, V.A.; Kuznetsova, E.S.; Olenev, A.V.; Vasiliev, A.N. Static and dynamic magnetic properties of two synthetic francisites Cu3La(SeO3)2O2X (X = Br and Cl). Phys. Chem. Miner. 2017, 44, 277–285. [Google Scholar] [CrossRef]
- Zakharov, K.V.; Zvereva, E.A.; Markina, M.M.; Stratan, M.I.; Kuznetsova, E.S.; Dunaev, S.F.; Berdonosov, P.S.; Dolgikh, V.A.; Olenev, A.V.; Klimin, S.A.; et al. Magnetic, resonance, and optical properties of Cu3Sm(SeO3)2O2Cl: A rare-earth francisite compound. Phys. Rev. 2016, B94, 054401. [Google Scholar] [CrossRef]
- Zakharov, K.V.; Zvereva, E.A.; Kuznetsova, E.S.; Berdonosov, P.S.; Dolgikh, V.A.; Markina, M.M.; Olenev, A.V.; Shakin, A.A.; Volkova, O.S.; Vasiliev, A.N. Two new lanthanide members of francisite family Cu3Ln(SeO3)2O2Cl (Ln = Eu, Lu). J. Alloys Comp. 2016, 685, 442–447. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Dolgikh, V.A. Copper Lanthanide Selenite Oxohalides with Francisite Structure: Synthesis and Structural Characteristics. Russ. J. Inorg. Chem. 2008, 53, 1353–1358. [Google Scholar] [CrossRef]
- Markina, M.M.; Zakharov, K.V.; Ovchenko, E.A.; Berdonosov, P.S.; Dolgikh, V.A.; Kuznetsova, E.S.; Olenev, A.V.; Klimin, S.A.; Kashchenko, M.A.; Budkin, I.V.; et al. Interplay of rare-earth and transition-metal subsystems in Cu3Yb(SeO3)2O2Cl. Phys. Rev. 2017, B94, 134422. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Vergasova, L.P. The crystal structure of ilinskite, NaCu5O2(SeO3)2Cl3, and review of mixed-ligand CuOmCln coordination geometries in minerals and inorganic compounds. Miner. Petrol. 2013, 107, 235–242. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Siidra, O.I.; Colmont, M.; Mentré, O.; Krivovichev, S.V. Emulating exhalative chemistry: Synthesis and structural characterization of ilinskite, Na[Cu5O2](SeO3)2Cl3, and its K-analogue. Miner. Petrol. 2015, 109, 421–430. [Google Scholar] [CrossRef]
- Tang, Y.; Guo, W.; Zhang, S.; Xiang, H.; Cui, M.; He, Z. Na2Cu7(SeO3)4O2Cl4: A selenite chloride compound with Cu7 units showing spin-frustration and a magnetization plateau. Dalton Trans. 2016, 45, 8324–8326. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.; Wan, W.; Rabbani, F.; Su, J.; Xu, H.; Hovmöler, S.; Johnsson, M.; Zou, X. Phase identification and structure determination from multiphase crystalline powder samples by rotation electron diffraction. J. Appl. Cryst. 2014, 47, 2048–2054. [Google Scholar] [CrossRef]
- Becker, R.M.; Prester, H.; Berger, M.; Johnsson, D.; Drobac, I. Zivkovic Crystal structure and magnetic properties of the new cobalt tellurite halide Co5(TeO3)4X2 (X = Cl, Br). Solid State Sci. 2007, 9, 223–230. [Google Scholar] [CrossRef]
- Takagi, R.; Johnsson, M.; Kremer, R.K.; Lemmens, P. Crystal structure and magnetic properties of the coupled spin dimer compound SrCu2(TeO3)2Cl2. J. Solid State Chem. 2006, 179, 3763–3767. [Google Scholar] [CrossRef]
- Feger, C.R.; Kolis, J.W. Synthesis and Characterization of Two New Copper Tellurites, Ba2Cu4Te4O11Cl4 and BaCu2Te2O6Cl2, in Supercritical H2O. Inorg. Chem. 1998, 37, 4046–4051. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Cryst. 1976, A32, 751–767. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Dolgikh, V.A.; Tsirlin, A.A.; Janson, O. Copper(II) selenate(IV) chlorides as low-dimensional magnets. In Proceedings of the 18th International Conference on Solid Compounds of Transition Elements, Lisbon, Portugal, 31 March–5 April 2012; p. 215. [Google Scholar]
- .Pring, A.; Gatehouse, B.M.; Birch, W.D. Francisite, Cu3Bi(SeO3)2O2Cl, a new mineral from Iron Monarch, South Australia: Description and crystal structure. Am. Mineral. 1990, 75, 1421–1425. [Google Scholar]
- Becker, R.; Johnsson, M. Crystal structure of Cu3Bi(TeO3)2O2Cl: A Kagomé lattice type compound. Solid State Sci. 2006, 7, 375–380. [Google Scholar] [CrossRef]
- Pregelj, M.; Zaharko, O.; Günther, A.; Loidl, A.; Tsurkan, V.; Guerrero, S. Magnetic ground state and two-dimensional behavior in pseudo-kagome layered system Cu3Bi(SeO3)2O2Br. Phys. Rev. B 2012, 86, 144409. [Google Scholar] [CrossRef]
- Miller, K.H.; Stephens, P.W.; Martin, C.; Constable, E.; Lewis, R.A.; Berger, H.; Carr, G.L.; Tanner, D.B. Infrared phonon anomaly and magnetic excitations in single-crystal Cu3Bi(SeO3)2O2Cl. Phys. Rev. 2012, B86, 174104. [Google Scholar] [CrossRef]
- Nikolaev, S.A.; Mazurenko, V.V.; Tsirlin, A.A.; Mazurenko, V.G. First-principles study of the magnetic ground state and magnetization process of the kagome francisites Cu3Bi(SeO3)2O2X (X = Cl, Br). Phys. Rev. 2016, B94, 144412. [Google Scholar] [CrossRef]
- Miller, K.H.; Constable, E.; Berger, H.; Tanner, D.B.; Horvat, J. Complementary techniques for probing terahertz magnetic excitations in Cu3Bi(SeO3)2O2Cl. In Proceedings of the International Conference on Infrared, Millimeter, and Terahertz Waves, IRMMW-THz, Wollongong, NSW, Australia, 23–28 September 2012; pp. 1–2. [Google Scholar]
- Wang, Z.; Schmidt, M.; Goncharov, Y.; Tsurkan, V.; Krug von Nidda, H.-A.; Loidl, A.; Deisenhofer, J. Terahertz spectroscopy in the pseudo-Kagome system Cu3Bi(SeO3)2O2Br. Phys. Rev. 2012, B86, 174411. [Google Scholar] [CrossRef]
- Pregelj, M.; Zaharko, O.; Zorko, A.; Gomilšek, M.; Sendetskyi, O.; Günther, A.; Ozerov, M.; Zvyagin, S.A.; Luetkens, H.; Baines, C.; et al. Controllable Broadband Absorption in the Mixed Phase of Metamagnets. Adv. Funct. Mater. 2015, 25, 3634–3640. [Google Scholar] [CrossRef]
- Rousochatzakis, I.; Richter, J.; Zinke, R.; Tsirlin, A.A. Frustration and Dzyaloshinsky-Moriya anisotropy in the kagome francisites Cu3Bi(SeO3)2O2X (X = Br, Cl). Phys. Rev. 2015, B91, 024416. [Google Scholar] [CrossRef]
- Zorko, A.; Gomilšek, M.; Pregelj, M.; Ozerov, M.; Zvyagin, S.A.; Ozarowski, A.; Tsurkan, V.; Loidl, A.; Zaharko, O. Electron spin resonance insight into broadband absorption of the Cu3Bi(SeO3)2O2Br metamagnet. AIP Adv. 2016, 6, 056210. [Google Scholar] [CrossRef]
- Wu, H.C.; Tseng, W.J.; Yang, P.Y.; Chandrasekhar, K.D.; Berger, H.; Yang, H.D. Anisotropic pressure effects on the Kagome Cu3Bi(SeO3)2O2Cl metamagnet. J. Phys. D Appl. Phys. 2017, 50, 265002. [Google Scholar] [CrossRef]
- Prishchenko, D.A.; Tsirlin, A.A.; Tsurkan, V.; Loidl, A.; Jesche, A.; Mazurenko, V.G. Antiferroelectric instability in the kagome francisites Cu3Bi(SeO3)2O2X (X = Cl, Br). Phys. Rev. 2017, B95, 0264102. [Google Scholar] [CrossRef]
- Wu, H.C.; Chandrasekhar, K.D.; Yuan, J.K.; Huang, J.R.; Lin, J.-Y.; Berger, H.; Yang, H.D. Anisotropic spin-flip-induced multiferroic behavior in kagome Cu3Bi(SeO3)2O2Cl. Phys. Rev. 2017, B95, 125121. [Google Scholar] [CrossRef]
- Gnezdilov, V.; Pashkevich, Y.; Lemmens, P.; Kurnosov, V.; Berdonosov, P.; Dolgikh, V.; Kuznetsova, E.; Pryadun, V.; Zakharov, K.; Vasiliev, A. Lattice and magnetic instabilities in Cu3Bi(SeO3)2O2X (X = Br, Cl). Phys. Rev. 2017, B96, 115144. [Google Scholar] [CrossRef]
- Constable, E.; Raymond, S.; Petit, S.; Ressouche, E.; Bourdarot, F.; Debray, J.; Josse, M.; Fabelo, O.; Berger, H.; de Brion, S.; et al. Magnetic and dielectric order in the kagome-like francisite Cu3Bi(SeO3)2O2Cl. Phys. Rev. 2017, B96, 014413. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Semenova, T.F.; Shuvalov, R.R.; Filatov, S.K.; Anan’lyev, V.V. Ilinskite NaCu5O2(SeO3)2Cl3—A new mineral of volcanic exhalations. Trans. Russ. Acad. Sci.-Earth Sci. Sect. 1997, 353, 641–644. (In Russian) [Google Scholar]
- Badrtdinov, D.I.; Kuznetsova, E.S.; Verchenko, V.Y.; Berdonosov, P.S.; Dolgikh, V.A.; Mazurenko, V.V.; Tsirlin, A.A. Magnetism of coupled spin tetrahedra in ilinskite-type KCu5O2(SeO3)2Cl3. Sci. Rep. 2018, 8, 2379. [Google Scholar] [CrossRef] [PubMed]
- Charkin, D.O.; Kayukov, R.A.; Zagidullin, K.A.; Siidra, O.I. Chemical vapor transport and solid-state exchange synthesis of new copper selenite bromides. Solid State Sci. 2017, 64, 109–113. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Colmont, M.; Mentré, O.; Siidra, O.I. Dimers of oxocentred [OCu4]6+ tetrahedra in two novel copper selenite chlorides, K[Cu3O](SeO3)2Cl and Na2[Cu7O2](SeO3)4Cl4, and related minerals and inorganic compounds. Miner. Mag. 2016, 80, 227–238. [Google Scholar] [CrossRef]
- Becker, R.; Berger, H.; Johnsson, M. Monoclinic Cu3(SeO3)2Cl2: An oxohalide with an unusual CuO4Cl trigonal–bipyramidal coordination. Acta Cryst. 2007, C63, i4–i6. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Shuvalov, R.R.; Semenova, T.F.; Filatov, S.K. Crystal chemistry of inorganic compounds based on chains of oxocentered tetrahedral III. Crystal structure of georgbokiite, Cu5O2(SeO3)2Cl2. Z. Kristallogr. 1999, 214, 135–138. [Google Scholar] [CrossRef]
- Galy, J.; Bonnet, J.J.; Andersson, S. The Crystal Structure of a New Oxide Chloride of Copper (II) and Selenium (IV): Cu5Se2O8Cl2. Acta Chem. Scand. 1979, A33, 383–389. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Burns, P.C.; Vergasova, L.P. The crystal structure of parageorgbokiite, β-Cu5O2(SeO3)2Cl2. Can. Miner. 2007, 45, 929–934. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Semenova, T.F.; Krivovichev, S.V.; Filatov, S.K.; Zolotarev, A.A., Jr.; Ananiev, V.V. Nicksobolevite, Cu7(SeO3)2O2Cl6, a new complex copper oxoselenite chloride from Tolbachik fumaroles, Kamchatka peninsula, Russia. Eur. J. Mineral. 2014, 26, 439–449. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Semenova, T.F.; Rozhdestvenskaya, L.V. Crystal chemistry of inorganic compounds based on chains of oxocentered tetrahedral, I. Crystal structure of chloromenite, Cu9O2(SeO3)4Cl6. Z. Kristallogr. 1998, 213, 645–649. [Google Scholar] [CrossRef]
- Vergasova, L.; Krivovichev, S.; Semenova, T.; Filatov, S.; Ananiev, V. Chloromenite, Cu9O2(SeO3)4Cl6, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Eur. J. Mineral. 1999, 11, 119–123. [Google Scholar] [CrossRef]
- Bastide, B.; Millet, P.; Johnsson, M.; Galy, J. Synthesis of copper(II) and selenium(IV) oxochlorides by chemical transport reaction: Crystal structure of Cu9O2(SeO3)4Cl6. Mater. Res. Bull. 2000, 35, 847–855. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Armbruster, T.; Pankratova, O.Y. Crystal Structure of Cu(I)Cu(II)4O(SeO3)Cl5, a New Heterovalent Copper Compound. Dokl. Chem. 2004, 399, 226–228. [Google Scholar] [CrossRef]
- Rabbani, F.; Svengren, H.; Zimmermann, I.; Hu, S.; Laine, T.; Hao, W.; Åkermark, B.; Åkermark, T.; Johnsson, M. Cobalt selenium oxohalides: Catalysts for water oxidation. Dalton Trans. 2014, 43, 3984–3989. [Google Scholar] [CrossRef] [PubMed]
- Johnston, M.G.; Harrison, W.T.A. Barium cobalt chloride selenite, Ba2CoCl2(SeO3)2. Acta Cryst. 2002, E58, i49–i51. [Google Scholar] [CrossRef]
- Hu, S.; Johnsson, M. Synthesis and crystal structure of Fe6Ca2(SeO3)9Cl4—A porous oxohalide. Dalton Trans. 2013, 42, 7859–7862. [Google Scholar] [CrossRef] [PubMed]
- Hamida, M.B.; Wickleder, M.S. {[CoCl2/2O4/1]}-Dimere in der Kristallstruktur von CoNd10(SeO3)12Cl8. Z. Kristallogr. 2005, S22, 141b. [Google Scholar]
- Wickleder, M.S.; Hamida, M.B. CoSm(SeO3)2Cl, CuGd(SeO3)2Cl, MnSm(SeO3)2Cl, CuGd2(SeO3)4 und CuSm2(SeO3)4: Übergangsmetallhaltige Selenite von Samarium und Gadolinum. Z. Anorg. Allg. Chem. 2003, 629, 556–562. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Burns, P.C.; Vergasova, L.P. The Crystal Structure of Allochalcoselite, Cu+Cu2+5PbO2(SeO3)2Cl5, A Mineral With Well-Defined Cu+ And Cu2+ Positions. Can. Miner. 2006, 44, 507–514. [Google Scholar] [CrossRef]
- Burns, P.C.; Krivovichev, S.V.; Filatov, S.K. New Cu2+ coordination polyhedra in the crystal structure of burnsite, KCdCu7O2(SeO3)2Cl9. Can. Miner. 2002, 40, 1587–1595. [Google Scholar] [CrossRef]
- Shuvalov, R.R.; Vegasova, L.P.; Semenova, T.F.; Filatov, S.K.; Krivovichev, S.V.; Siidra, O.I.; Rudashevsky, N.S. Prewittite, KPb1.5Cu6Zn(SeO3)2O2Cl10, a new mineral from Tolbachik fumaroles, Kamchatka peninsula, Russia: Description and crystal structure. Am. Miner. 2013, 98, 463–469. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Siidra, O.I.; Mentré, O.; Krivovichev, S.V. Structural variety of novel Pb and Bi selenites. Acta Cryst. 2013, A69, s134. [Google Scholar] [CrossRef]
- Kovrugin, V.M. Crystal Chemistry of Novel Oxide Compounds of Se4+ and Se6+ PhD Thesis Saint Petersburg 2015. Available online: https://disser.spbu.ru/files/phdspsu2015/Kovrugin.pdf (accessed on 3 April 2018).
- Kovrugin, V.M.; Colmont, M.; Siidra, O.I.; Mentré, O.; Al-Shuray, A.; Gurzhiy, V.V.; Krivovichev, S.V. Oxocentered Cu (ii) lead selenite honeycomb lattices hosting Cu (i) Cl 2 groups obtained by chemical vapor transport reactions. Chem. Commun. 2015, 51, 9563–9566. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.-L.; Kong, F.; Hu, C.-L.; Xu, X.; Mao, J.-G. Pb4V6O16(SeO3)3(H2O), Pb2VO2(SeO3)2Cl, and PbVO2(SeO3)F: New Lead(II)−Vanadium(V) Mixed-Metal Selenites Featuring Novel Anionic Skeletons. Inorg. Chem. 2014, 53, 8816–8824. [Google Scholar] [CrossRef] [PubMed]
- Gemmi, M.; Campostrini, I.; Demartin, F.; Gorelik, T.E.; Gramaccioli, C.M. Structure of the new mineral sarrabusite, Pb5CuCl4(SeO3)4, solved by manual electrondiffraction tomography. Acta Cryst. 2012, B68, 15–23. [Google Scholar] [CrossRef] [PubMed]
- Vergasova, L.P.; Semenova, T.F.; Filatov, S.K.; Krivovichev, S.V.; Shuvalov, R.R.; Ananiev, V.V. Georgbokiite Cu5O2(SeO3)2Cl2—A new mineral from volcanic sublimates. In Doklady Earth Sciences; Springer: Berlin/Heidelberg, Germany, 1999; Volume 364, pp. 527–531. (In Russian) [Google Scholar]
- Semenova, T.F.; Rozhdestvenskaya, I.V.; Filatov, S.K.; Vergasova, L.P. Crystal Structure and Physical Properties of Sophiite, Zn2(SeO3)Cl2, a New Mineral. Miner. Mag. 1992, 56, 241–245. [Google Scholar] [CrossRef]
- Johnsson, M.; Törnroos, K.W. Zinc selenium oxochloride, b-Zn2(SeO3)Cl2, a synthetic polymorph of the mineral sophiite. Acta Cryst. 2007, C63, i34–i36. [Google Scholar] [CrossRef]
- Akhrorov, A.Y.; Kuznetsova, E.S.; Aksenov, S.M.; Berdonosov, P.S.; Kuznetsov, A.N.; Dolgikh, V.A. Synthesis and crystal structure of Fe[(Te1.5Se0.5)O5]Cl, the first iron compound with selenate(IV) and tellurate(IV) groups. Solid State Sci. 2017, 74, 37–43. [Google Scholar] [CrossRef]
- Pregelj, M.; Zaharko, O.; Zorko, A.; Kutnjak, Z.; Jeglič, P.; Brown, P.J.; Jagodič, M.; Jagličić, Z.; Berger, H.; Arčon, D. Spin Amplitude Modulation Driven Magnetoelectric Coupling in the New Multiferroic FeTe2O5Br. Phys. Rev. Lett. 2009, 103, 147202. [Google Scholar] [CrossRef] [PubMed]
- Pregelj, M.; Zorko, A.; Zaharko, O.; Arčon, D.; Komelj, M.; Hillier, A.D.; Berger, H. Persistent Spin Dynamics Intrinsic to Amplitude-Modulated Long-Range Magnetic Order. Phys. Rev. Lett. 2012, 109, 227202. [Google Scholar] [CrossRef] [PubMed]
- Zaharko, O.; Pregelj, M.; Arčon, D.; Brown, P.J.; Chernyshov, D.; Stuhr, U.; Berger, H. FeTe2O5Br system: New ferroelectric with an incommensurate spin modulation. J. Phys. Conf. Ser. 2010, 211, 012002. [Google Scholar] [CrossRef]
- Choi, K.-Y.; Choi, I.H.; Lemmens, P.; van Tol, J.; Berger, H. Magnetic, structural, and electronic properties of the multiferroic compound FeTe2O5Br with geometrical frustration. J. Phys. Condens. Matter 2014, 26, 086001. [Google Scholar] [CrossRef] [PubMed]
- Lafront, A.-M.; Bonvoisin, J.; Trombe, J.-C. Synthesis, Crystal Structure, and Magnetic Measurement of Two New Diselenites:M2(Se2O5)3 with M = Fe(III), Cr(III). J. Solid State Chem. 1996, 122, 130–138. [Google Scholar] [CrossRef]
- Wildner, M. Crystal Structure of Mn(II)Mn(III)2O(SeO3)3. J. Solid State Chem. 1994, 113, 252–256. [Google Scholar] [CrossRef]
- Berdonosov, P.S.; Olenev, A.V.; Dolgikh, V.A. Lead (II) selenite halides Pb3(SeO3)2X2 (X = Br, I): Synthesis and crystal structure. Crystallogr. Rep. 2012, 57, 200–204. [Google Scholar] [CrossRef]
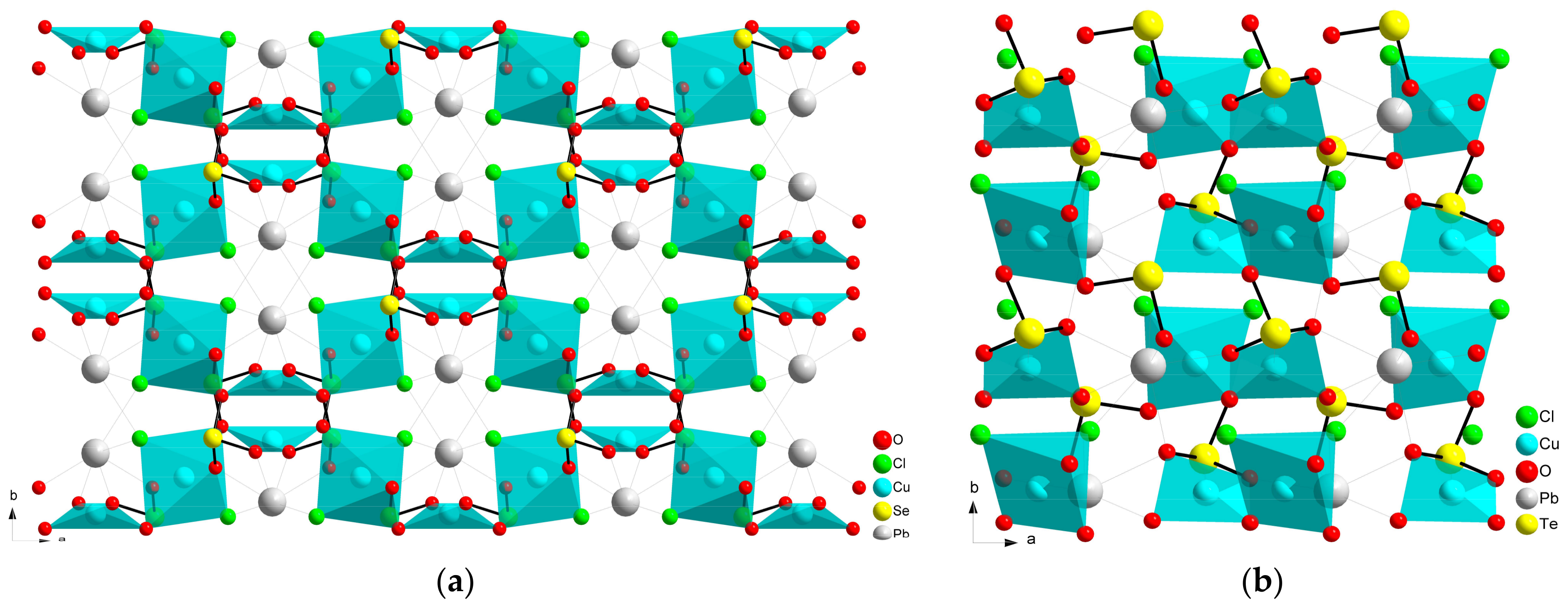
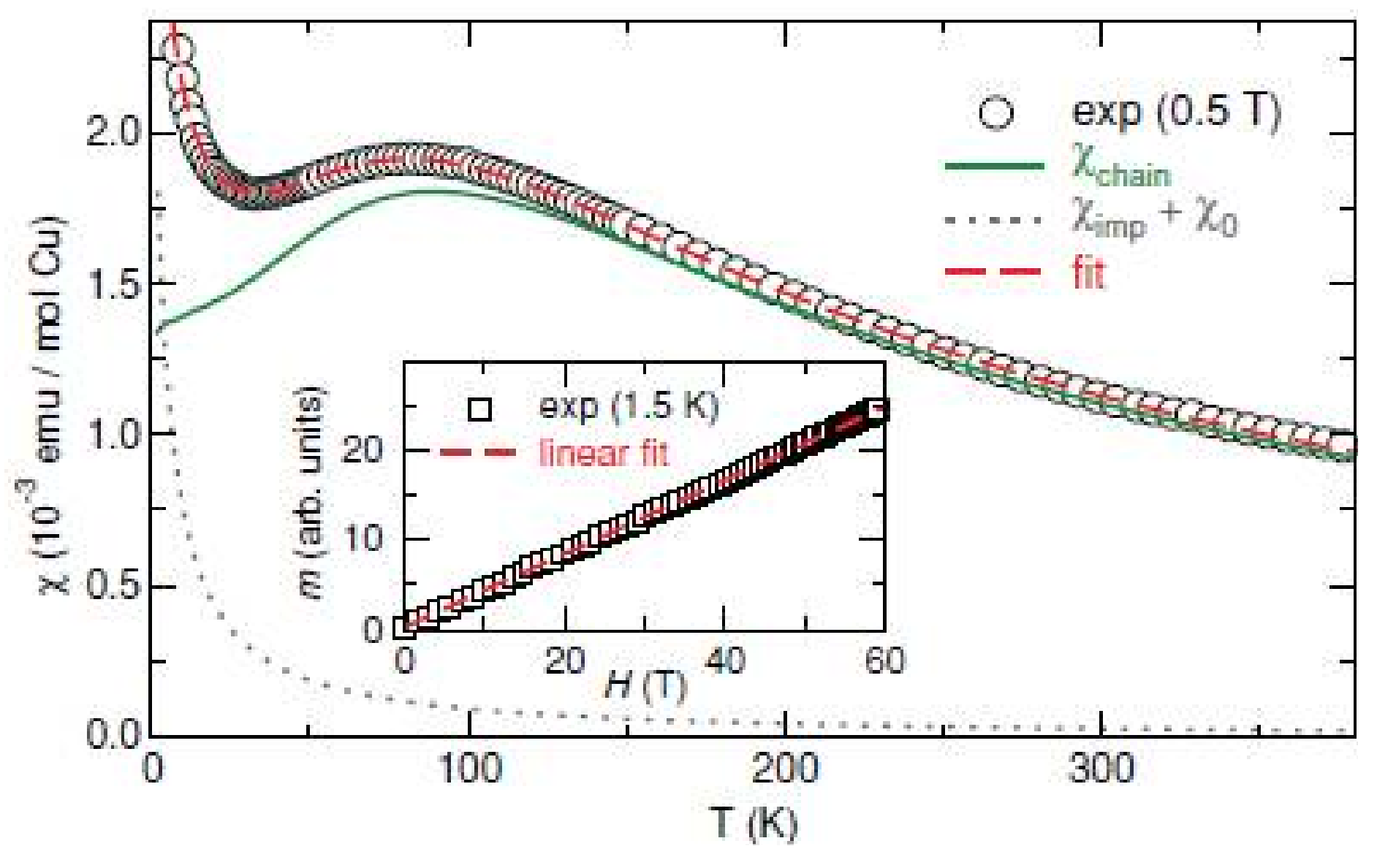

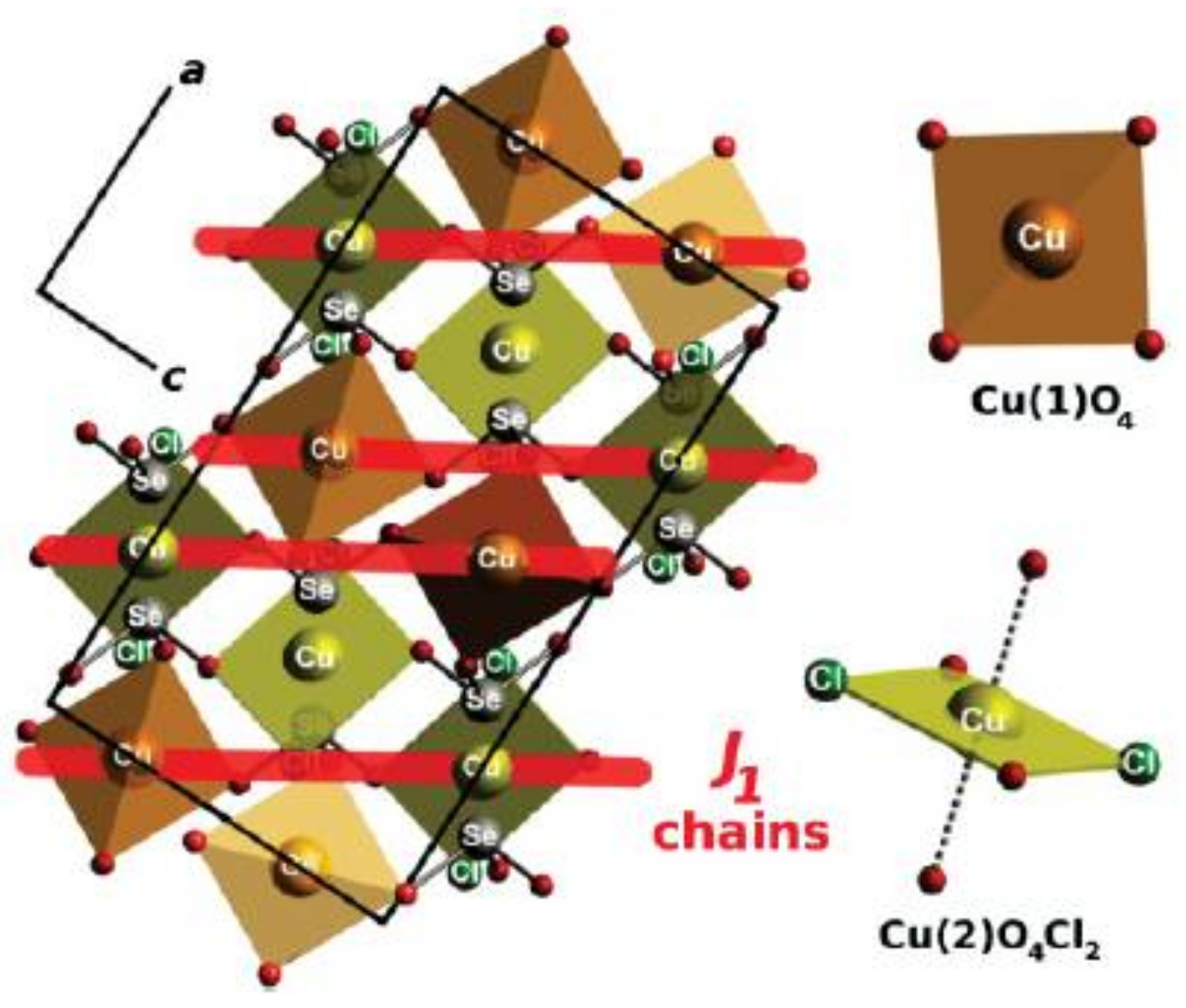
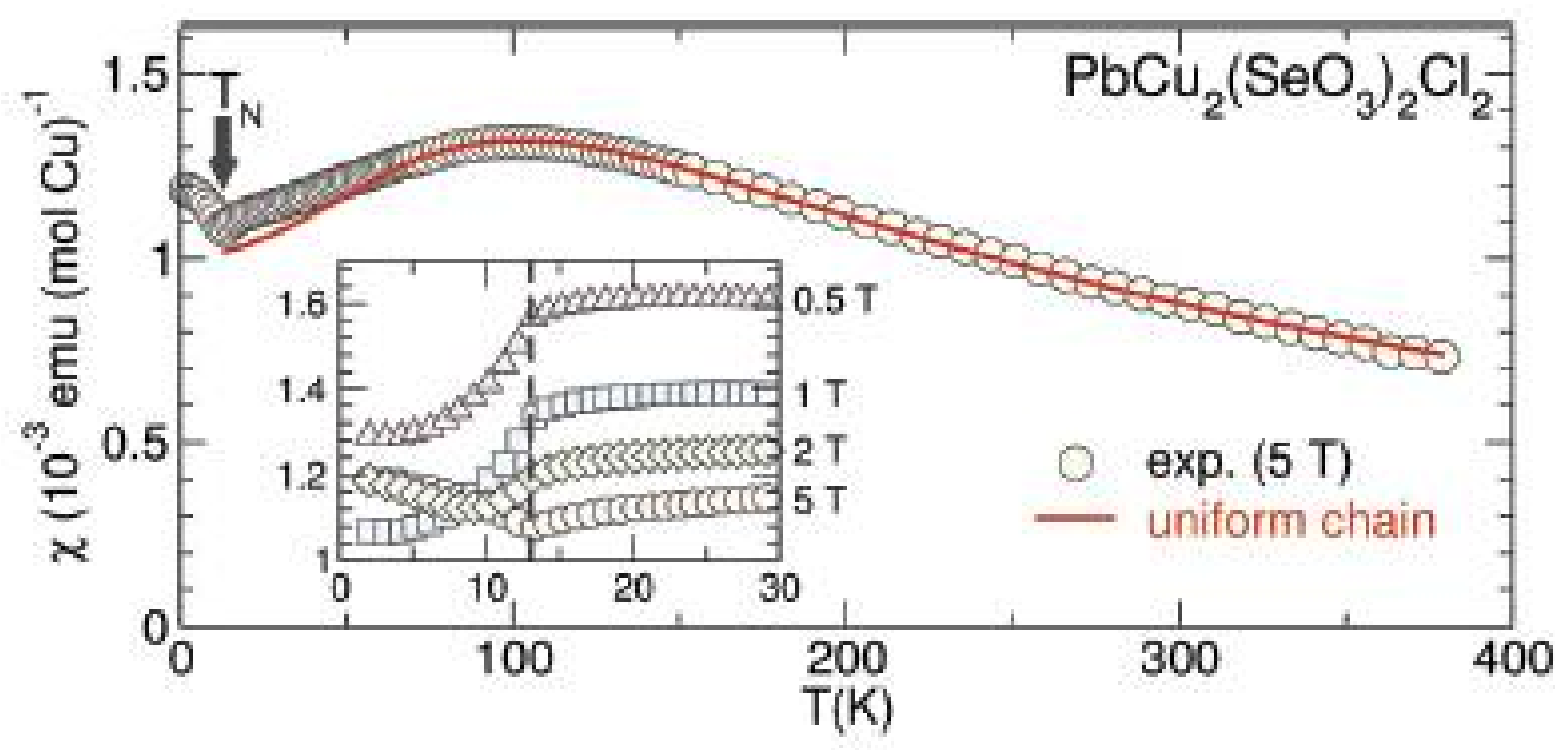



| # | Composition | Space Group | Z | Cell Constants | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| a, Å | b, Å | c, Å | Angles, ° | |||||
| 1 | Cu5(SeO3)4Cl2 | P21/c | 2 | 10.9104(8) | 8.3134(6) | 7.5490(6) | β = 90.715(6) | [22] |
| 2 | Co5(SeO3)4Cl2 | P-1 | 1 | 6.4935(8) | 7.7288(8) | 7.7443(10) | α = 66.051(11) | [23] |
| β = 73.610(11) | ||||||||
| γ = 81.268(9) | ||||||||
| 3 | Co5(SeO3)4Br2 | P-1 | 1 | 6.4897(9) | 7.7574(10) | 7.7552(10) | α = 66.850(13) | [23] |
| β = 73.960(12) | ||||||||
| γ = 81.350(11) | ||||||||
| 4 | Ni5(SeO3)4Cl2 | P-1 | 2 | 8.076(2) | 9.288(2) | 9.376(2) | α = 101.97(3) | [24] |
| β = 105.60(3) | ||||||||
| γ = 91.83(3) | ||||||||
| 5 | Ni5(SeO3)4Br2 | P-1 | 1 | 6.430(3) | 7.632(3) | 7.658(3) | α = 68.017(16) | [25] |
| β = 74.181(16) | ||||||||
| γ = 81.465(19) | ||||||||
| 6 | Sr2Co(SeO3)2Cl2 | P21/n | 2 | 5.3400(10) | 6.4279(6) | 12.3220(10) | β = 92.440(10) | [26] |
| 7 | Sr2Ni(SeO3)2Cl2 | P21/n | 2 | 5.3254(11) | 6.4363(13) | 12.197(2) | β = 92.53(3) | [26] |
| 8 | Sr2Cu(SeO3)2Cl2 | P21/n | 2 | 5.22996(3) | 6.50528(4) | 12.34518(7) | β = 91.3643(2) | [27] |
| 9 | CaCu2(SeO3)2Cl2 | C2/c | 4 | 12.759(3) | 9.0450(18) | 6.9770(14) | β = 91.03(3) | [28] |
| 10 | SrCu2(SeO3)2Cl2 | P21 | 2 | 7.1630(14) | 7.2070(14) | 8.0430(16) | β = 95.92(3) | [27] |
| 11 | PbCu2(SeO3)2Cl2 | C2/c | 4 | 13.056(1) | 9.5567(9) | 6.9006(6) | β = 90.529(7) | [29] |
| 12 | Bi2Fe(SeO3)2OCl3 | P21/m | 2 | 8.570(2) | 7.137(2) | 8.604(2) | β = 107.090(3) | [30] |
| 13 | Cu3Bi(SeO3)2O2Cl | Pmmn | 2 | 6.3540(4) | 9.6350(5) | 7.2330(4) | [31] | |
| 14 | Cu3Bi(SeO3)2O2Br | Pmmn | 2 | 6.3900(3) | 9.6940(4) | 7.2870(3) | [31] | |
| 15 | Cu3Bi(SeO3)2O2I | Pmmn | 2 | 6.4360(2) | 9.7510(4) | 7.3770(3) | [31] | |
| 16 | Cu3Y(SeO3)2O2Cl | Pmmn | 2 | 6.2991(1) | 9.4411(1) | 6.9724(1) | [32] | |
| 17 | Cu3La(SeO3)2O2Cl | Pmmn | 2 | 6.39407(18) | 9.7310(3) | 7.1547(2) | [33] | |
| 18 | Cu3Nd(SeO3)2O2Cl | Pmmn | 2 | 6.37775(10) | 9.62685(16) | 7.09341(11) | [31] | |
| 19 | Cu3Sm(SeO3)2O2Cl | Pmmn | 2 | 6.34616(4) | 9.56090(7) | 7.04377(5) | [34] | |
| 20 | Cu3Eu(SeO3)2O2Cl | Pmmn | 2 | 6.3384 (1) | 9.5341(2) | 7.0273(1) | [35] | |
| 21 | Cu3Gd(SeO3)2O2Cl | Pmmn | 2 | 6.3220(6) | 9.501(1) | 7.0202(8 | [36] | |
| 22 | Cu3Dy(SeO3)2O2Cl | Pmmn | 2 | 6.313(1) | 9.465(2) | 6.987(2) | [36] | |
| 23 | Cu3Ho(SeO3)2O2Cl | Pmmn | 2 | 6.2999(6) | 9.440(1) | 6.9723(8) | [36] | |
| 24 | Cu3Er(SeO3)2O2Cl | Pmmn | 2 | 6.299(1) | 9.432(3) | 6.967(2) | [36] | |
| 25 | Cu3Yb(SeO3)2O2Cl | Pmmn | 2 | 6.28278(3) | 9.39486(5) | 6.93291(3) | [37] | |
| 26 | Cu3Lu(SeO3)2O2Cl | Pmmn | 2 | 6.2681(1) | 9.3756(2) | 6.9326(1) | [35] | |
| 27 | Cu3La(SeO3)2O2Br | Pmmn | 2 | 6.40071(5) | 9.75675(7) | 7.17800(5) | [33] | |
| 28 | Cu3Nd(SeO3)2O2Br | Pmmn | 2 | 6.382(2) | 9.698(3) | 7.091(2) | [36] | |
| 29 | Cu3Sm(SeO3)2O2Br | Pmmn | 2 | 6.348(1) | 9.581(2) | 7.079(2) | [36] | |
| 30 | Cu3Gd(SeO3)2O2Br | Pmmn | 2 | 6.337(1) | 9.5515(8) | 7.0540(9) | [36] | |
| 31 | NaCu5(SeO3)2O2Cl3 | Pnma | 4 | 17.769(7) | 6.448(3) | 10.522(4) | [38] | |
| 32 | КCu5(SeO3)2O2Cl3 | Pnma | 4 | 18.1691(6) | 6.4483(2) | 10.5684(4) | [39] | |
| 33 | Na2Cu7(SeO3)4O2Cl4 | P-1 | 1 | 7.446(2) | 8.349(3) | 9.137(3) | α = 110.335(7) | [40] |
| β = 106.166(3) | ||||||||
| γ = 105.161(7) | ||||||||
| TN, K | BC, T | Ref. | |
|---|---|---|---|
| Cu3Bi(SeO3)2O2Br | 27.4 | 0.8 | [49] |
| Cu3La(SeO3)2O2Cl | 31.2 | 2.4 | [33] |
| Cu3La(SeO3)2O2Br | 34.3 | 2.45 | [33] |
| Cu3Eu(SeO3)2O2Cl | 36 | 2.6 | [35] |
| Cu3Y(SeO3)2O2Cl | 36.3 | 2.6 | [32] |
| Cu3Lu(SeO3)2O2Cl | 38 | 3.0 | [35] |
| # | Composition | Space Group | Z | Cell Constants | Ref. | |||
|---|---|---|---|---|---|---|---|---|
| a, Å | b, Å | c, Å | Angles, ° | |||||
| 1 | α-Cu5(SeO3)4Br2 | P21/c | 2 | 11.1089(18) | 8.3233(13) | 7.5668(12) | β = 90.893(3) | [64] |
| 2 | β-Cu5(SeO3)4Br2 | P-1 | 1 | 6.2096(13) | 7.8553(16) | 7.9006(17) | α = 65.538(6) | [64] |
| β = 83.111(7) | ||||||||
| γ = 75.291(7) | ||||||||
| 3 | Na2Cu7(SeO3)4O2Br4 | P-1 | 4 | 7.7657(3) | 8.3750(3) | 9.2626(4) | α = 110.227(2) | [64] |
| β = 104.897(2) | ||||||||
| γ = 107.195(3) | ||||||||
| 4 | K[Cu3O](SeO3)2Cl | P-1 | 2 | 7.6821(5) | 8.1179(5) | 8.7836(6) | α = 113.193(3) | [65] |
| β = 108.735(4) | ||||||||
| γ = 98.245(4) | ||||||||
| 5 | Cu3(SeO3)2Cl2 | C2/m | 2 | 8.9333(12) | 6.2164(7) | 7.5815(12) | β = 110.238(13) | [66] |
| 6 | Cu3(SeO3)2Cl2 | P-1 | 2 | 6.1240(4) | 7.7880(5) | 8.5170(6) | α = 92.755(4) | [15] |
| β = 95.735(4) | ||||||||
| γ = 92.853(4) | ||||||||
| 7 | Cu5O2(SeO3)2Cl2 | P21/c | 2 | 6.030(1) | 13.744(3) | 5.562(1) | β = 95.75(1) | [67,68] |
| 8 | β-Cu5O2(SeO3)2Cl2 | P21/c | 2 | 5.3982(5) | 8.0543(8) | 11.1277(10) | β = 99.258(2) | [69] |
| 9 | Cu7O2(SeO3)2Cl6 | P21/c | 4 | 10.958(9) | 14.483(5) | 10.494(14) | β = 113.61(7) | [70] |
| 10 | Cu9O2(SeO3)4Cl6 | I2/m | 2 | 14.170(3) | 6.262(1) | 12.999(3) | β = 113.05(1) | [71,72] |
| 11 | Cu9O2(SeO3)4CI6 | P21/n | 2 | 12.922(3) | 6.262(2) | 14.042(4) | β = 112.88(2) | [73] |
| 12 | CuICuII4O(SeO3)Cl5 | P21/m | 2 | 9.203(3) | 6.232(2) | 9.557(3) | β = 91.970(8) | [74] |
| 13 | Co4(SeO3)3Cl2 | Pnma | 4 | 7.9751(1) | 14.4048(2) | 9.7103(2) | [75] | |
| 14 | Co3(Se2O5)2Cl2 | C2/m | 2 | 7.1973(10) | 13.9961(19) | 5.8334(9) | β = 107.524(16) | [75] |
| 15 | Ba2Co(SeO3)2Cl2 | Pnnm | 2 | 6.7635(4) | 12.6454(7) | 5.3866(3) | [76] | |
| 16 | Fe6Ca2(SeO3)9Cl4 | P63/m | 2 | 12.118(2) | 12.703(4) | [77] | ||
| 17 | CoNd10(SeO3)12Cl8 | P2/c | 4 | 15.699(2) | 15.7002(2) | 19.171(2) | β = 113,995(5) | [78] |
| 18 | CoSm(SeO3)2Cl | P-1 | 4 | 7.123(1) | 8.8895(2) | 12.162(2) | α = 72.25(1) | [79] |
| β = 71.27(1) | ||||||||
| γ = 72.08(1) | ||||||||
| 19 | CuGd(SeO3)2Cl | P-1 | 4 | 7.043(4) | 9.096(4) | 12.010(7) | α = 70.84(4) | [79] |
| β = 73.01(4) | ||||||||
| γ = 70.69(4) | ||||||||
| 20 | MnSm(SeO3)2Cl | P-1 | 2 | 7.008(2) | 7.241(2) | 8.034(2) | α = 86.90(3) | [79] |
| β = 71.57(3) | ||||||||
| γ = 64.33(3) | ||||||||
| 21 | CuICuII5PbO2(SeO3)2Cl5 | C2/m | 4 | 18.468(2) | 6.1475(8) | 15.314(2) | β = 119.284(2) | [80] |
| 22 | KCdCu7O2(SeO3)2Cl9 | P63/mmc | 2 | 8.7805(8) | 15.521(2) | [81] | ||
| 23 | KPb1.5Cu6Zn(SeO3)2O2Cl10 | Pnnm | 4 | 9.132(2) | 19.415(4) | 13.213(3) | [82] | |
| 24 | MnBi(SeO3)2Cl | P-1 | 2 | 7.0926(8) | 7.2695(6) | 8.0160(8) | α = 88.226(4) | [83,84] |
| β = 72.005(3) | ||||||||
| γ = 64.560(4) | ||||||||
| 25 | (Pb2Cu2+9O4)(SeO3)4(Cu+Cl2)Cl5 | C2/m | 2 | 18.605(17) | 6.204(6) | 12.673(12) | β = 109.869(17) | [85] |
| 26 | (PbCu2+5O2)(SeO3)2(Cu+Cl2)Cl3 | C2/m | 2 | 18.4956(4) | 6.14540(10) | 15.2985(4) | β = 119.3111(10) | [85] |
| 27 | (PbxCu2+(6−x)O2)(SeO3)2(Cu+Cl2)K(1−x)Cl(4−x) | C2/m | 1 | 15.1158(11) | 6.1853(4) | 9.2672(9) | β = 95.965(5) | [85] |
| 28 | Pb2VO2(SeO3)2Cl | P21 | 2 | 8.333(3) | 5.3171(16) | 10.710(4) | β = 111.701(5) | [86] |
| 29 | Pb5Cu(SeO3)4Cl4 | C2/c | 4 | 24.917(3) | 5.5060(10) | 14.242(2) | β = 101.770(10) | [87] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berdonosov, P.S.; Kuznetsova, E.S.; Dolgikh, V.A. Transition Metal Selenite Halides: A Fascinating Family of Magnetic Compounds. Crystals 2018, 8, 159. https://doi.org/10.3390/cryst8040159
Berdonosov PS, Kuznetsova ES, Dolgikh VA. Transition Metal Selenite Halides: A Fascinating Family of Magnetic Compounds. Crystals. 2018; 8(4):159. https://doi.org/10.3390/cryst8040159
Chicago/Turabian StyleBerdonosov, Peter S., Elena S. Kuznetsova, and Valery A. Dolgikh. 2018. "Transition Metal Selenite Halides: A Fascinating Family of Magnetic Compounds" Crystals 8, no. 4: 159. https://doi.org/10.3390/cryst8040159





