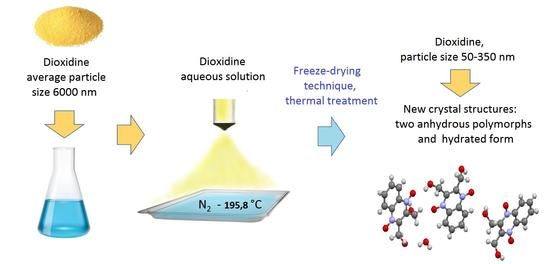
Cryochemically Obtained Nanoforms of Antimicrobial Drug Substance Dioxidine and Their Physico-chemical and Structural Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Characterization Techniques
2.3.1. UV-visible
2.3.2. 1H-NMR
2.3.3. FTIR Spectra
2.3.4. Thermoanalytical Investigations
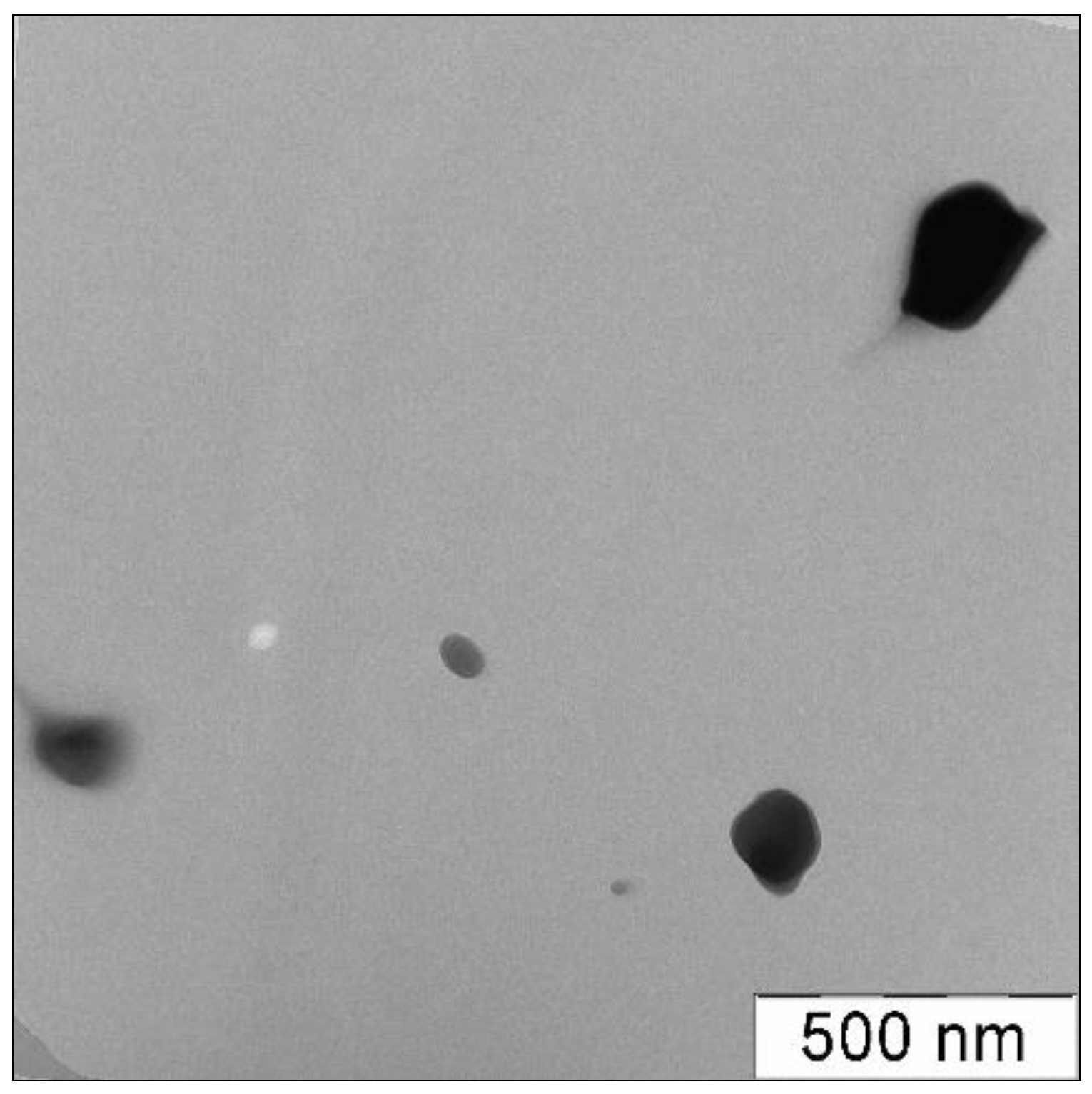
2.3.5. Transmission Electron Microscopy
2.3.6. BET-surface Area Measurements
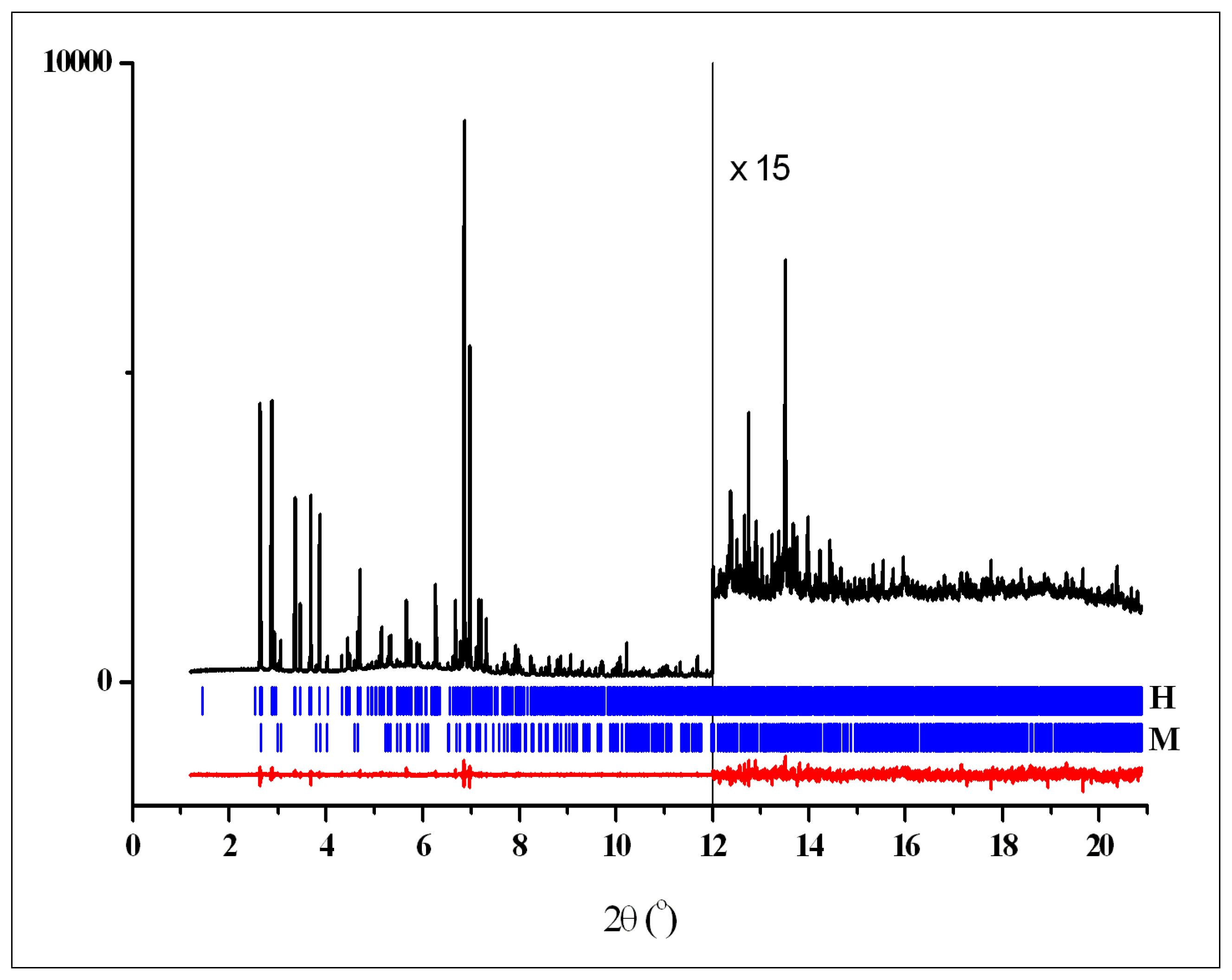
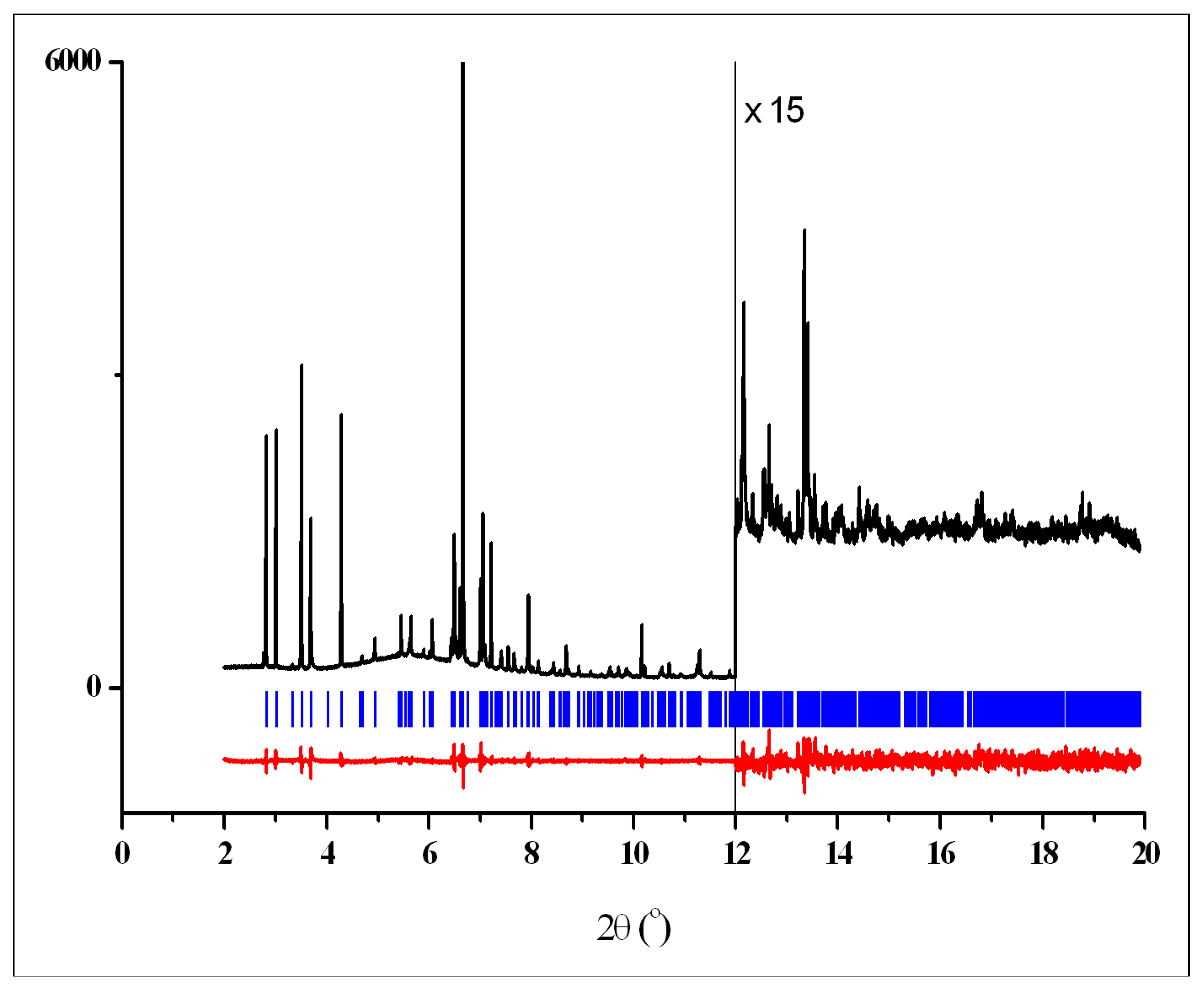
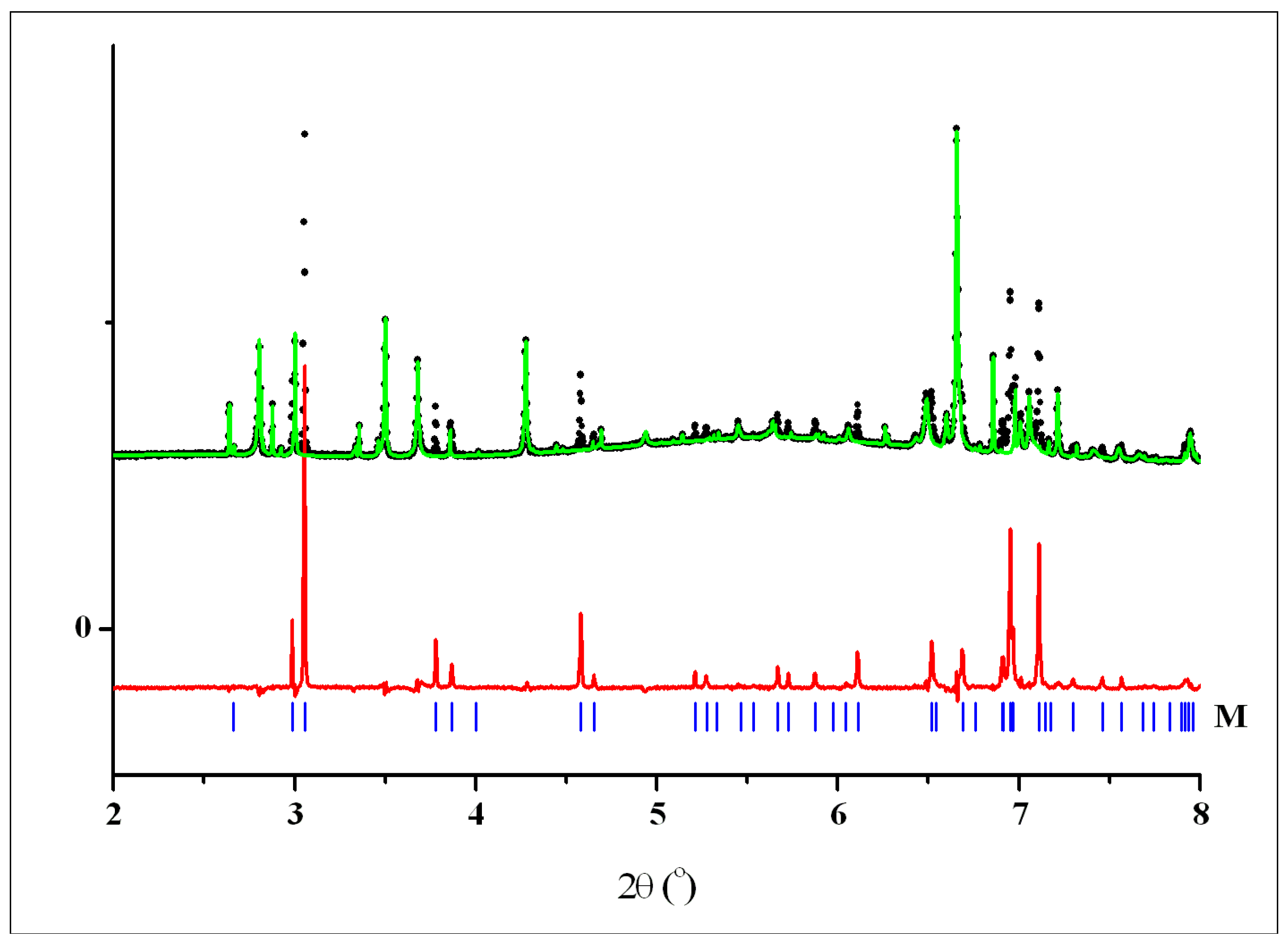
2.3.7. X-ray Powder Diffraction
2.3.8. Indexing
2.3.9. Structure Determination
2.3.10. Structure Refinement
2.4. Determination of Dissolution Rate of Dioxidine Forms
3. Results and Discussion
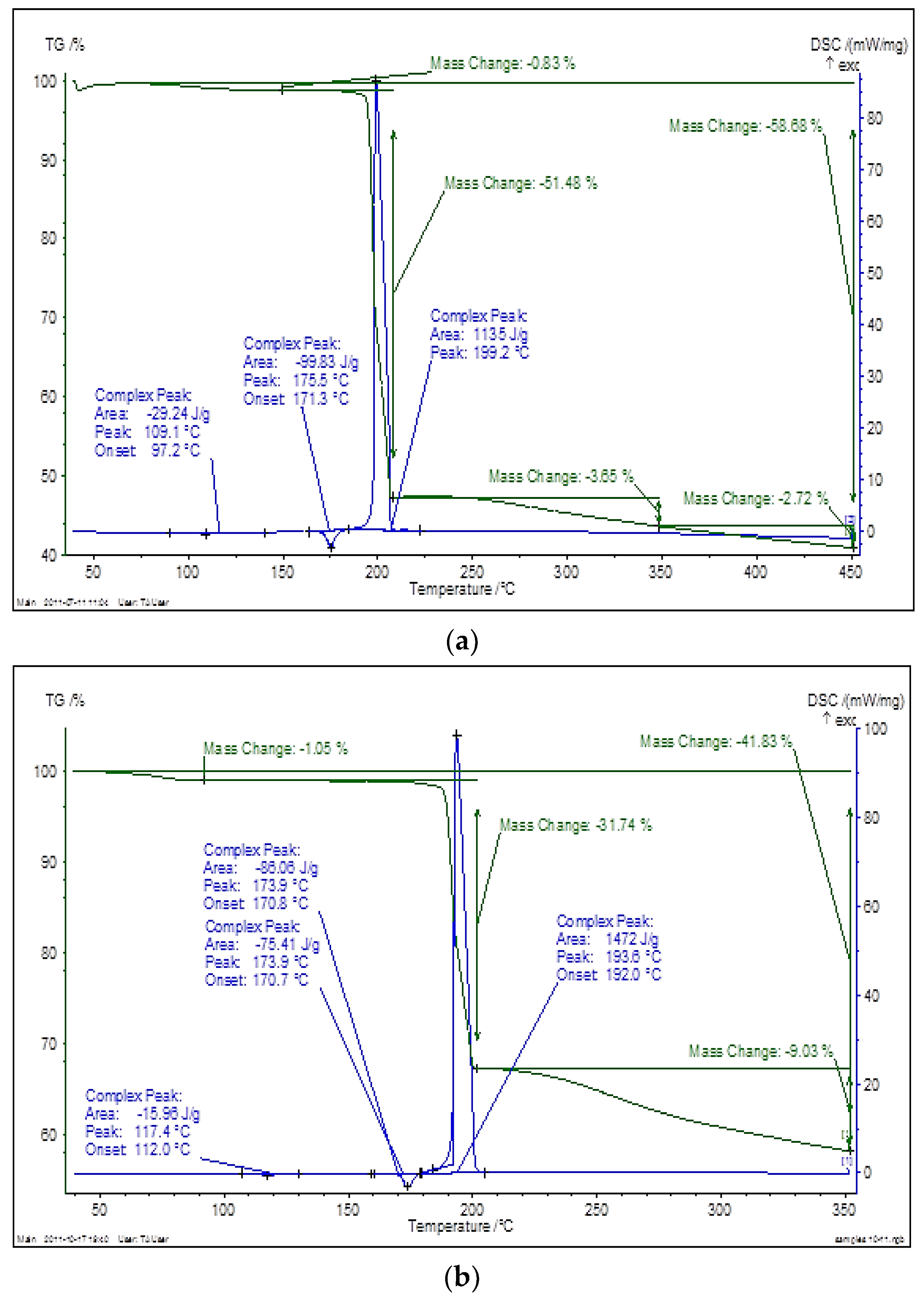
3.1. Physicochemical Characterization of Cryomodified Dioxidine
3.2. Crystal Structures
4. Conclusions
5. Patents
Supporting Materials
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peltonen, L.; Hirvonen, J. Pharmaceutical Nanocrystals by Nanomilling: Critical process parameters, particle fracturing and stabilization methods. J. Pharm. Pharmacol. 2010, 62, 1569–1579. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Yeo, Y. Nanocrystals for the parenteral delivery of poorly water-soluble drugs. Curr. Opin. Solid State Mater. Sci. 2012, 16, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keck, C.M.; Muller, R.H. Drug nanocrystals of poorly soluble drugs produced by high pressure homogenization. Eur. J. Pharm. Biopharm. 2006, 62, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, D.; Chen, M. Drug nanocrystals for the formulation of poorly soluble drugs and its application as a potential drug delivery system. J. Nanopart. Res. 2008, 10, 845–862. [Google Scholar] [CrossRef]
- Junyaprasert, V.B.; Morakul, B. Nanocrystals for enhancement of oral bioavailability of poorly water-soluble drugs. Asian J. Pharm. Sci. 2015, 10, 13–23. [Google Scholar] [CrossRef]
- Sarnes, A.; Kovalainen, M.; Häkkinen, M.R.; Laaksonen, T.; Laru, J.; Kiesvaara, J.; Ilkka, J.; Oksala, O.; Rönkkö, S.; Järvinen, K.; et al. Nanocrystal-based peroral itraconazole delivery: superior in vitro dissolution enhancement versus sporanox® is not realized in In Vivo drug absorption. J. Control. Release 2014, 180, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Sarma, B.; Chen, J.; His, H.-Y.; Myerson, A.S. Solid forms of pharmaceuticals: polymorphs, salts and cocrystals. Korean J. Chem. Eng. 2011, 28, 315–322. [Google Scholar] [CrossRef]
- Lee, E.H. A practical guide to pharmaceutical polymorph screening & selection. Asian J. Pharm. Sci. 2014, 9, 163–175. [Google Scholar]
- Yu, L.X.; Furness, M.S.; Raw, A.; Woodland, O.K.P.; Nashed, N.E.; Ramos, E.; Miller, S.P.F.; Adams, R.C.; Fang, F.; Patel, R.M.; et al. Scientific considerations of pharmaceutical solid polymorphism in abbreviated new drug applications. Pharm. Res. 2003, 20, 531–536. [Google Scholar] [CrossRef] [PubMed]
- Guranda, D.T.; Gil’deeva, G.N. Preparation of drug polymorphs. Pharm. Chem. J. 2010, 44, 254–260. [Google Scholar] [CrossRef]
- Blagden, N.; de Matas, M.; Gavan, P.T.; York, P. Crystal engineering of active pharmaceutical ingredients to improve solubility and dissolution rates. Adv. Drug Deliv. Rev. 2007, 59, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Bak, A.; Gore, A.; Yanez, E.; Stanton, M.; Tufekcic, S.; Syed, R.; Akrami, A.; Rose, M.; Surapaneni, S.; Bostick, T.; et al. The co-crystal approach to improve the exposure of a water-insoluble compound: amg 517 sorbic acid co-crystal characterization and pharmacokinetics. J. Pharm. Sci. 2008, 97, 3942–3956. [Google Scholar] [CrossRef] [PubMed]
- Kang, B.K.; Lee, J.S.; Chon, S.K.; Jeong, S.Y.; Yuk, S.H.; Khang, G.; Lee, H.B.; Cho, S.H. Development of self-microemulsifying drug delivery systems (SMEDDS) for oral bioavailability enhancement of simvastatin in beagle dogs. Int. J. Pharm. 2004, 274, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, C.; Kawabata, Y.; Wada, K.; Yamada, S.; Onoue, S. Microenvironmental pH-modification to improve dissolution behavior and oral absorption for drugs with pH-dependent solubility. Expert. Opin. Drug. Deliv. 2014, 11, 505–516. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Brewster, M.E. Pharmaceutical applications of cyclodextrins. 1. Drug solubilization and stabilization. J. Pharm. Sci. 1996, 85, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Muller, R.H.; Gohla, S.; Keck, C.M. State of the art of nanocrystals—special features, production, nanotoxicology aspects and intracellular delivery. Eur. J. Pharm. Biopharm. 2011, 78, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Henwood, S.Q.; Liebenberg, W.; Tiedt, L.R. Characterization of the solubility and dissolution properties of several new rifampicin polymorphs, solvates, and hydrates. Drug Dev. Ind. Pharm. 2001, 27, 1017–1030. [Google Scholar] [CrossRef] [PubMed]
- Braun, D.E.; Gelbrich, T.; Kahlenberg, V.; Tessadri, R.; Wieser, J.; Griesser, U.J. Characterization of the solubility and dissolution properties of several new rifampicin polymorphs, solvates, and hydrates. J. Pharm. Sci. 2008, 98, 2010–2026. [Google Scholar] [CrossRef] [PubMed]
- Velaga, S.P.; Berger, R.; Carlfors, J. Supercritical fluids crystallization of budesonide and flunisolide. Pharm. Res. 2002, 19, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Pasquali, I.; Bettini, R.; Giordano, F. Supercritical fluid technologies: an innovative approach for manipulating the solid-state of pharmaceuticals. Adv. Drug Deliv. Rev. 2008, 60, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Sergeev, G.B.; Komarov, V.S.; Shabatin, V.P. Method of Obtaining Powders of Drugs. Patent RU 2195264, 25 December 2002. [Google Scholar]
- Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Obtaining ultradispersed dioxidine powder modified via cryochemical synthesis and determining its antibacterial activity. Mosc. Univ. Chem. Bull. 2016, 71, 295–298. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Semenov, A.M.; Shabatina, T.I. Cryochemical synthesis and antibacterial activity of a hybrid composition based on ag nanoparticles and dioxidine. Mosc. Univ. Chem. Bull. 2017, 72, 6–9. [Google Scholar] [CrossRef]
- Chakkittakandy, R.; Corver, J.A.W.M.; Planken, P.C.M. Terahertz Spectroscopy to Identify the Polymorphs in Freeze-Dried Mannitol. J. Pharm. Sci. 2010, 99, 932–940. [Google Scholar] [CrossRef] [PubMed]
- Morozov, Y.N.; Utekhina, A.Y.; Shabatin, V.P.; Chernyshev, V.V.; Sergeev, G.B. Cryosynthesis of nanosized drug substances. Russ. J. Gen. Chem. 2014, 84, 1010–1017. [Google Scholar] [CrossRef]
- Morozov, Y.N.; Fedorov, V.V.; Shabatin, V.P.; Vernaya, O.I.; Chernyshev, V.V.; Abel, A.S.; Arhangel’skii, I.V.; Shabatina, T.I.; Sergeev, G.B. cryochemical modification of drugs: nanosized form iii piroxicam and its physical and chemical properties. Mosc. Univ. Chem. Bull. 2016, 71, 287–294. [Google Scholar] [CrossRef]
- Chernyshev, V.V.; Morozov, Y.N.; Bushmarinov, I.S.; Makoed, A.A.; Sergeev, G.B. New polymorph of dehydroepiandrosterone (dhea) obtained via cryomodification. Cryst. Growth Des. 2016, 16, 1088–1095. [Google Scholar] [CrossRef]
- Vernaya, O.I.; Shabatin, V.P.; Shabatina Khvatov, D.I.; Semenov, A.M.; Yudina, T.P.; Danilov, V.S. Cryochemical modification, activity, and toxicity of dioxidine. Russ. J. Phys. Chem. A 2017, 91, 229–232. [Google Scholar] [CrossRef]
- Shabatin, V.P.; Vernaya, O.I.; Semenov, A.M.; Shabatina, T.I. Crystalline β—Modification Of 2,3-Bis-(Hydroxymethyl)-Quinoxaline-N,N′-Dioxide, Method for its Preparation and Pharmaceutical Composition on Its Basis. Patent RU2563256, 11 April 2014. [Google Scholar]
- Padeiskaya, E.N.; Pershin, G.N.; Kostyuchenok, B.M. Dioxidine, a new antibacterial drug for the treatment of suppurative infections. Pharm. Chem. J. 1977, 11, 1148–1154. [Google Scholar] [CrossRef]
- Padeiskaya, E.N.; Shipilova, L.D.; Budanova, L.I.; Sizova, T.N.; Ovdeenko, N.I.; Sedov, V.V. Pharmacokinetics of dioxidine: penetration of the drug into organs and tissues after single and repeated introductions. Pharm. Chem. J. 1983, 17, 391–396. [Google Scholar] [CrossRef]
- Werner, P.-E.; Eriksson, L.; Westdahl, M. TREOR: A semi-exhaustive trial-and-error powder indexing program for all symmetries. J. Appl. Crystallogr. 1985, 18, 367–370. [Google Scholar] [CrossRef]
- Visser, J.W. A fully automatic program for finding the unit cell from powder data. J. Appl. Crystallogr. 1969, 2, 89–95. [Google Scholar] [CrossRef] [Green Version]
- Zlokazov, V.B. MRIAAU a program for autoindexing multiphase polycrystals. J. Appl. Crystallogr. 1992, 25, 69–72. [Google Scholar] [CrossRef]
- Zlokazov, V.B. AUTOX—A program for autoindexing reflections from multiphase polycrystals. Comput. Phys. Commun. 1995, 85, 415–422. [Google Scholar] [CrossRef]
- Pawley, G.S. Unit-Cell Refinement from Powder Diffraction Scans. J. Appl. Crystallogr. 1981, 14, 357–361. [Google Scholar] [CrossRef]
- Zhukov, S.G.; Chernyshev, V.V.; Babaev, E.V.; Sonneveld, E.J.; Schenk, H. Application of simulated annealing approach for structure solution of molecular crystals from x-ray laboratory powder data. Z. Kristallogr. 2001, 216, 5–9. [Google Scholar] [CrossRef]
- Chernyshev, V.V.; Schenk, H. A grid search procedure of positioning a known molecule in an unknown crystal structure with the use of powder diffraction data. Z. Kristallogr. 1998, 213, 1–3. [Google Scholar] [CrossRef]
- Zlokazov, V.B.; Chernyshev, V.V. mria—A program for a full profile analysis of powder multiphase neutron-diffraction time-of-flight (direct and fourier) spectra. J. Appl. Crystallogr. 1992, 25, 447–451. [Google Scholar] [CrossRef]
- Dvoryantseva, G.G.; Lindeman, S.V.; Aleksanyan, S.V.; Struchkov, Y.T.; Teten’chuk, K.P.; Khabarova, L.S.; Elina, A.S. Connection Between the structure and the antibacterial activity of the n-oxides of quinoxalines. molecular structure of dioxidine and quinoxidine. Pharm. Chem. J. 1990, 24, 80–84. [Google Scholar] [CrossRef]
- Isaeva, V.I.; Belyaeva, E.V.; Fitch, A.N.; Chernyshev, V.V.; Klyamkin, S.N.; Kustov, L.M. synthesis and structural characterization of a series of novel zn(ii)-based mofs with pyridine-2,5-dicarboxylate linkers. Cryst. Growth Des. 2013, 13, 5305–5315. [Google Scholar] [CrossRef]
- Popa, N.C. The (hkl) dependence of diffraction-line broadening caused by strain and size for all laue groups in rietveld refinement. J. Appl. Crystallogr. 1998, 31, 176–180. [Google Scholar] [CrossRef]
- Ahtee, M.; Nurmela, M.; Suortti, P.; Järvinen, M. correction for preferred orientation in rietveld refinement. J. Appl. Crystallogr. 1989, 22, 261–268. [Google Scholar] [CrossRef]
- Järvinen, M. Application of symmetrized harmonics expansion to correction of the preferred orientation effect. J. Appl. Crystallogr. 1993, 26, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. D 2009, D65, 148–155. [Google Scholar] [CrossRef] [PubMed]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—new features for the visualization and investigation of crystal structures. J. Appl. Crystallogr. 2008, 41, 466–470. [Google Scholar] [CrossRef]






| Crystal Polymorph Forms | T | M | H |
|---|---|---|---|
| Empirical formula | C10H10N2O4 | C10H10N2O4 | C10H10N2O4·0.33H2O |
| Formula weight | 222.20 | 222.20 | 228.21 |
| Crystal system | triclinic | monoclinic | orthorhombic |
| Space group | P-1 | P21/c | Pbca |
| a, Å | 7.3194 (7) | 9.1709 (9) | 18.0035 (15) |
| b, Å | 8.0774 (8) | 15.3486 (14) | 31.8369 (19) |
| c, Å | 8.9872 (8) | 7.0367 (7) | 10.2555 (12) |
| θ, deg | 71.516 (11) | 90 | 90 |
| β, deg | 70.815 (9) | 110.316 (12) | 90 |
| θ, deg | 79.317 (12) | 90 | 90 |
| V, Å3 | 473.98 (8) | 928.87 (16) | 5878.2 (9) |
| M20 | 260 | 154 | 144 |
| F30 | 583 (0.0014, 38) | 421 (0.0023, 43) | 397 (0.0021, 68) |
| Z | 2 | 4 | 24 |
| Diffractometer | ID22, ESRF | ID22, ESRF | ID22, ESRF |
| Wavelength, Å | 0.399996 (3) | 0.399996 (3) | 0.399996 (3) |
| ρcalc, g/cm3 | 1.557 | 1.589 | 1.547 |
| μ, mm−1 | 0.027 | 0.028 | 0.027 |
| 2θmin–2θmax, increment, deg | 2.001–19.902, 0.003 | 1.200–19.995, 0.003 | 1.200–20.877, 0.003 |
| Number of parameters/restraints | 124/56 | 118/56 | 235/177 |
| Rp/Rwp/Rexp | 0.0316/0.0438/0.0168 | 0.0235/0.0317/0.0178 | 0.0243/0.0325/0.0165 |
| goodness-of-fit | 2.608 | 1.764 | 1.947 |
| D–H···A | D–H | H···A | D···A | ∠O–H···A | |
| T | |||||
| O14−H14···O11i | 0.82 | 1.95 | 2.721(3) | 156 | |
| O16−H16···O12ii | 0.82 | 1.92 | 2.733(3) | 171 | |
| C13−H13B···O16iii | 0.97 | 2.42 | 3.205(5) | 138 | |
| C15−H15A···O11iv | 0.97 | 2.52 | 3.241(5) | 131 | |
| C8−H8···O12v | 0.93 | 2.54 | 3.382(5) | 150 | |
| M | |||||
| O14−H14···O11vi | 0.82 | 2.21 | 3.001(6) | 162 | |
| O16−H16···O14vii | 0.82 | 1.86 | 2.588(6) | 148 | |
| C13−H13B···O16viii | 0.93 | 2.28 | 3.106(8) | 147 | |
| C15−H15A···O14vii | 0.97 | 2.37 | 2.922(8) | 115 | |
| H | |||||
| O14A−H14A···O11C | 0.82 | 2.13 | 2.741(8) | 131 | |
| O14B−H14B···O12Cix | 0.82 | 1.93 | 2.692(8) | 154 | |
| O14C−H14C···O16Bx | 0.82 | 1.92 | 2.710(8) | 163 | |
| O16A−H16A···O12A | 0.82 | 2.37 | 2.884(8) | 122 | |
| O16A−H16A···O12Axi | 0.82 | 2.03 | 2.755(8) | 147 | |
| O16B−H16B···O16Axi | 0.82 | 2 | 2.769(8) | 157 | |
| O16C−H16C···O11Bxii | 0.82 | 2.07 | 2.827(8) | 153 | |
| O1W−H1A···O16C | 0.85 | 2.02 | 2.828(8) | 157 | |
| O1W−H1B···O14Bxiii | 0.85 | 2.07 | 2.815(8) | 147 | |
| C5A−H5A···O16B | 0.93 | 2.51 | 3.287(11) | 141 | |
| C5B−H5B···O14Axi | 0.93 | 2.57 | 3.490(9) | 171 | |
| C6A−H6A···O16Cxiv | 0.93 | 2.56 | 3.289(9) | 136 | |
| C7B−H7B···O11Axi | 0.93 | 2.28 | 3.180(11) | 161 | |
| C13C−H13E···O11A | 0.97 | 2.5 | 3.306(11) | 140 | |
| C15A−H15B···O11Cxvi | 0.97 | 2.37 | 3.138(11) | 135 | |
| C15C−H15E···O11Bxvii | 0.97 | 2.36 | 3.222(10) | 148 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shabatina, T.I.; Vernaya, O.I.; Shabatin, V.P.; Evseeva, I.V.; Melnikov, M.Y.; Fitch, A.N.; Chernyshev, V.V. Cryochemically Obtained Nanoforms of Antimicrobial Drug Substance Dioxidine and Their Physico-chemical and Structural Properties. Crystals 2018, 8, 298. https://doi.org/10.3390/cryst8070298
Shabatina TI, Vernaya OI, Shabatin VP, Evseeva IV, Melnikov MY, Fitch AN, Chernyshev VV. Cryochemically Obtained Nanoforms of Antimicrobial Drug Substance Dioxidine and Their Physico-chemical and Structural Properties. Crystals. 2018; 8(7):298. https://doi.org/10.3390/cryst8070298
Chicago/Turabian StyleShabatina, Tatyana I., Olga I. Vernaya, Vladimir P. Shabatin, Iuliia V. Evseeva, Michail Ya Melnikov, Andrew N. Fitch, and Vladimir V. Chernyshev. 2018. "Cryochemically Obtained Nanoforms of Antimicrobial Drug Substance Dioxidine and Their Physico-chemical and Structural Properties" Crystals 8, no. 7: 298. https://doi.org/10.3390/cryst8070298