Graphene Adhesion Mechanics on Iron Substrates: Insight from Molecular Dynamic Simulations
Abstract
:1. Introduction
2. Molecular Dynamic Setup and Interatomic Potentials
2.1. Molecular Dynamic Setup
2.2. Interatomic Potentials for Graphene and Iron System
3. Results and Discussion
3.1. Adhesion Features of Monolayer Graphene Nanosheet and Iron Surfaces
3.2. Adhesion Features of Bilayer or Trilayer Graphene Nanosheet and Iron Surfaces
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lee, C.; Wei, X.; Kysar, J.W.; Hone, J. Measurement of the Elastic Properties and Intrinsic Strength of Monolayer Graphene. Science 2008, 321, 385–388. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Liang, C.; Ji, W.; De, S. A theoretical analysis of the effect of the hydrogenation of graphene to graphane on its mechanical properties. Phys. Chem. Chem. Phys. 2013, 15, 2003–2011. [Google Scholar] [CrossRef] [PubMed]
- Cadelano, E.; Colombo, L. Effect of hydrogen coverage on the Young’s modulus of graphene. Phys. Rev. B 2012, 85, 245434. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Fal’ko, V.I.; Colombo, L.; Gellert, P.R.; Schwab, M.G.; Kim, K. A roadmap for graphene. Nature 2012, 490, 192–200. [Google Scholar] [CrossRef]
- Hou, J.; Deng, B.H.; Zhu, H.X.; Lan, Y.C.; Shi, Y.F.; De, S.; Liu, L.; Chakraborty, P.; Gao, F.; Peng, Q. Magic auxeticity angle of graphene. Carbon 2019, 149, 350–354. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Geng, X.; Wang, H.; Wang, P.; Liu, A.; Lan, Y.; Peng, Q. A Review of Current Development of Graphene Mechanics. Crystals 2018, 8, 357. [Google Scholar] [CrossRef]
- Avouris, P.; Dimitrakopoulos, C. Graphene: Synthesis and applications. Mater. Today 2012, 15, 86–97. [Google Scholar] [CrossRef]
- Robertson, A.W.; Warner, J.H. Hexagonal Single Crystal Domains of Few-Layer Graphene on Copper Foils. Nano Lett. 2011, 11, 1182–1189. [Google Scholar] [CrossRef]
- Wang, W.H.; Peng, Q.; Dai, Y.Q.; Qian, Z.F.; Liu, S. Temperature dependence of raman spectra of graphene on copper foil substrate. J. Mater. Sci. 2016, 27, 3888–3893. [Google Scholar] [CrossRef]
- Zou, Z.; Carnevali, V.; Jugovac, M.; Patera, L.L.; Sala, A.; Panighel, M.; Cepek, C.; Soldano, G.; Mariscal, M.M.; Peressi, M.; et al. Graphene on nickel (100) micro-grains: Modulating the interface interaction by extended moiré superstructures. Carbon 2018, 130, 441–447. [Google Scholar] [CrossRef]
- You, Y.; Yoshimura, M.; Cholake, S.; Lee, G.H.; Sahajwalla, V.; Joshi, R. A Controlled Carburization Process to Obtain Graphene-Fe3C-Fe Composites. Adv. Mater. Interfaces 2018, 5, 1800599. [Google Scholar] [CrossRef]
- Wintterlin, J.; Bocquet, M.L. Graphene on metal surfaces. Surf. Sci. 2009, 603, 1841–1852. [Google Scholar] [CrossRef]
- Liu, P.; Cottrill, A.L.; Kozawa, D.; Koman, V.B.; Parviz, D.; Liu, A.T.; Yang, J.; Tran, T.Q.; Wong, M.H.; Wang, S.; et al. Emerging trends in 2D nanotechnology that are redefining our understanding of “Nanocomposites”. Nano Today 2018, 21, 18–40. [Google Scholar] [CrossRef]
- Bonaccorso, F.; Colombo, L.; Yu, G.; Stoller, M.; Tozzini, V.; Ferrari, A.C.; Ruoff, R.S.; Pellegrini, V. Graphene, related two-dimensional crystals, and hybrid systems for energy conversion and storage. Science 2015, 347, 1246501. [Google Scholar] [CrossRef]
- Fasolino, A.; Los, J.H.; Katsnelson, M.I. Intrinsic ripples in graphene. Nat. Mater. 2007, 6, 858–861. [Google Scholar] [CrossRef] [Green Version]
- Dai, J.F.; Wang, G.J.; Ma, L.; Wu, C.K. Surface properties of graphene: Relationship to graphene-polymer composites. Rev. Adv. Mater. Sci. 2015, 40, 60–71. [Google Scholar]
- Zheng, J.; Wang, Y.; Wang, L.; Quhe, R.; Ni, Z.; Mei, W.N.; Gao, Z.; Yu, D.; Shi, J.; Lu, J. Interfacial Properties of Bilayer and Trilayer Graphene on Metal Substrates. Sci. Rep. 2013, 3, 2081. [Google Scholar] [CrossRef]
- Gong, L.; Kinloch, I.A.; Young, R.J.; Riaz, I.; Jalil, R.; Novoselov, K.S. Interfacial Stress Transfer in a Graphene Monolayer Nanocomposite. Adv. Mater. 2010, 22, 2694–2697. [Google Scholar] [CrossRef] [Green Version]
- Su, B.; Han, Z.; Liu, B. Cellular Automaton Modeling of Austenite Nucleation and Growth in Hypoeutectoid Steel during Heating Process. ISIJ Int. 2013, 53, 527–534. [Google Scholar] [CrossRef] [Green Version]
- Han, Z.; Han, G.; Luo, A.A.; Liu, B. Large-scale three-dimensional phase-field simulation of multi-variant β-Mg17Al12 in Mg–Al-based alloys. Comput. Mater. Sci. 2015, 101, 248–254. [Google Scholar] [CrossRef]
- Cao, J.; Jin, J.; Wang, L.; Li, S.; Zong, Y. Modeling for onset strain of deformation-induced martensite transformation in Q&P steel by a mean-field Eshelby approach. Model. Simul. Mater. Sci. Eng. 2019, 27, 085002. [Google Scholar]
- Vinogradov, N.A.; Zakharov, A.A.; Kocevski, V.; Rusz, J.; Simonov, K.A.; Eriksson, O.; Mikkelsen, A.; Lundgren, E.; Vinogradov, A.S.; Mårtensson, N.; et al. Formation and Structure of Graphene Waves on Fe (110). Phys. Rev. Lett. 2012, 109, 026101. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, A.F.; Liang, T.; Zhang, D.; Choudhary, K.; Phillpot, S.R.; Sinnott, S.B. Graphene–Titanium Interfaces from Molecular Dynamics Simulations. ACS Appl. Mater. Interfaces 2017, 9, 33288–33297. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.; Shin, W.C.; Kim, T.Y.; Mun, J.H.; Kim, T.S.; Cho, B.J. Direct Measurement of Adhesion Energy of Monolayer Graphene As-Grown on Copper and Its Application to Renewable Transfer Process. Nano Lett. 2012, 12, 1448–1452. [Google Scholar] [CrossRef]
- Das, S.; Lahiri, D.; Lee, D.Y.; Agarwal, A.; Choi, W. Measurements of the adhesion energy of graphene to metallic substrates. Carbon 2013, 59, 121–129. [Google Scholar] [CrossRef]
- Xu, Z.; Buehler, M.J. Interface structure and mechanics between graphene and metal substrates: A first-principles study. J. Phys. Condens. Matter 2010, 22, 485301. [Google Scholar] [CrossRef]
- Süle, P.; Szendrő, M. Time-lapsed graphene moiré superlattices on Cu (111). Model. Simul. Mat. Sci. Eng. 2015, 23, 29–33. [Google Scholar] [CrossRef]
- Chang, W.; Rajan, S.; Peng, B.; Ren, C.; Sutton, M.; Li, C. Adhesion energy of as-grown graphene on nickel substrates via StereoDIC-based blister experiments. Carbon 2019, 153, 699–706. [Google Scholar] [CrossRef]
- Restuccia, P.; Righi, M.C. Tribochemistry of graphene on iron and its possible role in lubrication of steel. Carbon 2016, 106, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Jin, J.; Cao, J.; Yang, P.; Peng, Q. Interaction of Edge Dislocations with Graphene Nanosheets in Graphene/Fe Composites. Crystals 2018, 8, 160. [Google Scholar] [CrossRef]
- Li, D.; Müller, M.B.; Gilje, S.; Kaner, R.B.; Wallace, G.G. Processable aqueous dispersions of graphene nanosheets. Nat. Nanotechnol. 2008, 3, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Meng, F.; Yang, Y.; Lu, C.; Deng, H.; Wang, L.; De, S.; Gao, F. Shockwave generates<100>dislocation loops in bcc iron. Nat. Commun. 2018, 9, 4880. [Google Scholar] [CrossRef] [PubMed]
- Plimpton, S. Fast Parallel Algorithms for Short-Range Molecular Dynamics. J. Comput. Phys. 1995, 117, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Hoover, W.G. Canonical dynamics: Equilibrium phase-space distributions. Phys. Rev. A 1985, 31, 1695–1697. [Google Scholar] [CrossRef] [Green Version]
- He, X.; Bai, Q.; Shen, R. Atomistic perspective of how graphene protects metal substrate from surface damage in rough contacts. Carbon 2018, 130, 672–679. [Google Scholar] [CrossRef]
- Shi, X.; Yin, Q.; Wei, Y. A theoretical analysis of the surface dependent binding, peeling and folding of graphene on single crystal copper. Carbon 2012, 50, 3055–3063. [Google Scholar] [CrossRef] [Green Version]
- Sidorenkov, A.V.; Kolesnikov, S.V.; Saletsky, A.M. Molecular dynamics simulation of graphene on Cu (111) with different Lennard-Jones parameters. Eur. Phys. J. B 2016, 89, 220. [Google Scholar] [CrossRef]
- Lee, B.J. A modified embedded-atom method interatomic potential for the Fe-C system. Acta Mater. 2006, 54, 701–711. [Google Scholar] [CrossRef]
- Masoud, A.; Van Duin, A.C.; Kubicki, J.D. Development of a reactive force field for iron-oxyhydroxide systems. J. Phys. Chem. A 2010, 114, 6298–6307. [Google Scholar]
- Becquart, C.; Raulot, J.; Bencteux, G.; Domain, C.; Perez, M.; Garruchet, S.; Nguyen, H. Atomistic modeling of an Fe system with a small concentration of C. Comput. Mater. Sci. 2007, 40, 119–129. [Google Scholar] [CrossRef]
- Vinogradov, N.; Simonov, K.; Generalov, A.; Drnec, J.; Carlà, F.; Vinogradov, A.; Preobrajenski, A.; Mårtensson, N.; Felici, R.; Vinogradov, N. The structural evolution of graphene/Fe (110) systems upon annealing. Carbon 2017, 111, 113–120. [Google Scholar] [CrossRef]
- Hepburn, D.J.; Ackland, G.J. Metallic-covalent interatomic potential for carbon in iron. Phys. Rev. B 2008, 78, 165115. [Google Scholar] [CrossRef]
- Zan, R.; Bangert, U.; Ramasse, Q.; Novoselov, K.S. Interaction of Metals with Suspended Graphene Observed by Transmission Electron Microscopy. J. Phys. Chem. Lett. 2012, 3, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Hupalo, M.; Liu, X.; Wang, C.Z.; Lu, W.C.; Yao, Y.X.; Ho, K.M.; Tringides, M.C. Metal Nanostructure Formation on Graphene: Weak versus Strong Bonding. Adv. Mater. 2011, 23, 2082–2087. [Google Scholar] [CrossRef]
- Zan, R.; Bangert, U.; Ramasse, Q.; Novoselov, K.S. Metal−Graphene Interaction Studied via Atomic Resolution Scanning Transmission Electron Microscopy. Nano Lett. 2011, 11, 1087–1092. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, C.; Cheng, H.P. Metal-terminated graphene nanoribbons. Phys. Rev. B 2010, 82, 205429. [Google Scholar] [CrossRef]
- Hupalo, M.; Binz, S.; Tringides, M.C. Strong metal adatom–substrate interaction of Gd and Fe with graphene. J. Phys. Condens. Matter 2011, 23, 045005. [Google Scholar] [CrossRef]
- Mendelev, M.I.; Han, S.; Srolovitz, D.J.; Ackland, G.; Sun, D.Y.; Asta, M. Development of new interatomic potentials appropriate for crystalline and liquid iron. Philos. Mag. 2003, 83, 3977–3994. [Google Scholar] [CrossRef]
- Donald, W.B.; Olga, A.S.; Judith, A.H.; Steven, J.S.; Boris, N.; Susan, B.S. A second-generation reactive empirical bond order (REBO) potential energy expression for hydrocarbons. J. Phys. Condens. Matter 2002, 14, 783. [Google Scholar]
- O’Connor, T.C.; Andzelm, J.; Robbins, M.O. AIREBO-M: A reactive model for hydrocarbons at extreme pressures. J. Chem. Phys. 2015, 142, 024903. [Google Scholar] [CrossRef]
- Zhang, J.M.; Wang, D.D.; Xu, K.W. Calculation of the surface energy of bcc transition metals by using the second nearest–neighbor modified embedded atom method. Appl. Surf. Sci. 2006, 252, 8217–8222. [Google Scholar] [CrossRef]
- Elettro, H.; Melo, F. Ripples and wrinkles in graphene: Beyond continuum mechanics. In Wrinkled Polymer Surfaces; Springer: Cham, Switzerland, 2019; pp. 229–252. [Google Scholar]
- Kroeger, D.; Cisternas, E.; Correa, J. Bilayer graphene films over Ru (0001) surface: Ab-initio calculations and STM images simulation. Surf. Sci. 2015, 634, 31–36. [Google Scholar] [CrossRef]
- Hu, X.; Meng, F. First-Principle Study on the Interaction between Fe and Trivacancy in Graphene. J. Nanomater. 2016, 2016, 2. [Google Scholar] [CrossRef]
- Gass, M.H.; Bangert, U.; Bleloch, A.L.; Wang, P.; Nair, R.R.; Geim, A.K. Free-standing graphene at atomic resolution. Nat. Nanotechnol. 2008, 3, 676–681. [Google Scholar] [CrossRef] [PubMed]




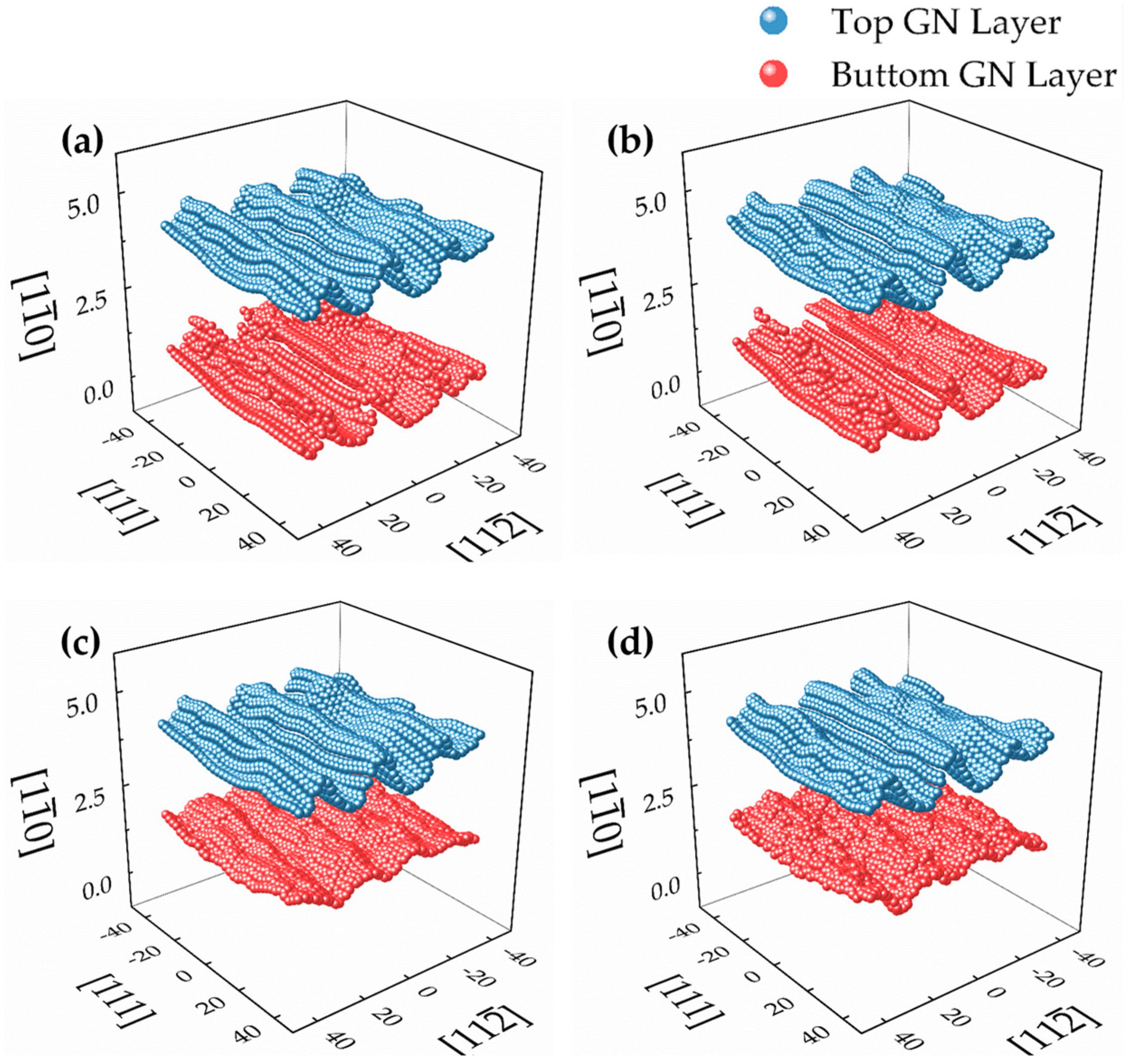

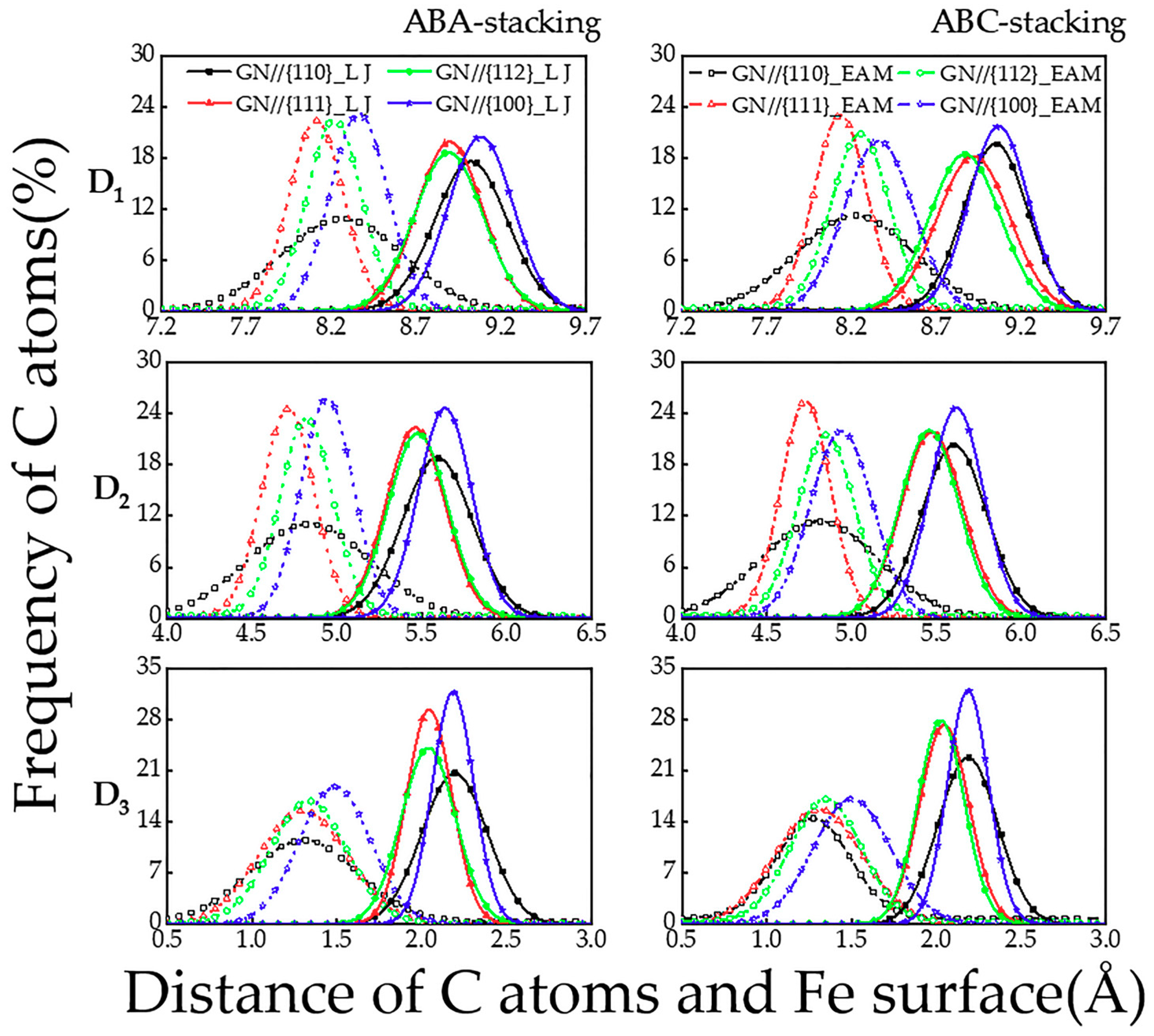
| Gr/Metal | Graphene/Fe (110) | Graphene/Cu (111) | Graphene/Ni (111) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FP [29] | MD [35] | FP [26] | MD-LJ [36] | MD-LJ [37] | EXP [24] | EXP [25] | FP [26] | EXP [28] | EXP [25] | ||||
| Adhesion | LJ [35] | MEAM [38] | ReaxFF [39] | ||||||||||
| E (J/m2) | 0.89 | 0.94 | 40.09 | 15.04 | 0.40 | 0.40 | 0.05–0.2 | 0.72 | 12.75 | 1.46 | 6.76 | 72.7 | |
| GN/Fe | {110} | {111} | {112} | {100} | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adhesion | LJ | EAM | LJ | EAM | LJ | EAM | LJ | EAM | |
| E (J/m2) | 0.47 | 26.83 | 0.62 | 24.87 | 0.70 | 25.13 | 0.74 | 25.01 | |
| 1 (Å) | 2.2 | 1.4 | 2.0 | 1.3 | 2.0 | 1.4 | 2.2 | 1.4 | |
| GN/Fe | {110} | {111} | {112} | {100} | |||||
|---|---|---|---|---|---|---|---|---|---|
| Adhesion | LJ | EAM | LJ | EAM | LJ | EAM | LJ | EAM | |
| Bilayer GN AB-stack | E (J/m2) | 0.80 | 26.93 | 0.22 | 25.00 | 0.49 | 25.08 | 0.15 | 25.29 |
| 1 (Å) | 2.2 | 1.4 | 2.0 | 1.3 | 2.0 | 1.4 | 2.2 | 1.4 | |
| 2 (Å) | 5.6 | 4.9 | 5.5 | 4.8 | 5.5 | 4.9 | 5.6 | 4.9 | |
| AA-stack | E (J/m2) | −1.22 | 27.00 | −0.62 | 24.91 | −0.56 | 25.26 | 0.47 | 26.33 |
| 1 (Å) | 2.2 | 1.4 | 2.0 | 1.2 | 2.0 | 1.4 | 2.2 | 1.4 | |
| 2 (Å) | 5.6 | 4.9 | 5.5 | 4.9 | 5.5 | 4.9 | 5.6 | 4.9 | |
| Trilayer GN ABA-stack | E (J/m2) | 0.12 | 28.41 | −1.03 | 25.02 | −0.86 | 25.70 | 0.28 | 25.03 |
| 1 (Å) | 2.2 | 1.4 | 2.0 | 1.3 | 2.0 | 1.4 | 2.2 | 1.5 | |
| 2 (Å) | 5.6 | 4.9 | 5.5 | 4.7 | 5.5 | 4.8 | 5.6 | 5.6 | |
| 3 (Å) | 9.0 | 8.4 | 8.9 | 8.2 | 8.9 | 8.2 | 9.1 | 8.4 | |
| ABC-stack | E (J/m2) | −0.1 | 27.36 | 0.48 | 25.08 | −0.18 | 25.76 | −0.22 | 24.99 |
| 1 (Å) | 2.2 | 1.4 | 2.0 | 1.3 | 2.0 | 1.4 | 2.2 | 1.5 | |
| 2 (Å) | 5.6 | 4.9 | 5.5 | 4.7 | 5.5 | 4.9 | 5.6 | 5.0 | |
| 3 (Å) | 9.1 | 8.3 | 8.9 | 8.2 | 8.9 | 8.3 | 9.1 | 8.4 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Jin, J.; Yang, P.; Zong, Y.; Peng, Q. Graphene Adhesion Mechanics on Iron Substrates: Insight from Molecular Dynamic Simulations. Crystals 2019, 9, 579. https://doi.org/10.3390/cryst9110579
Wang L, Jin J, Yang P, Zong Y, Peng Q. Graphene Adhesion Mechanics on Iron Substrates: Insight from Molecular Dynamic Simulations. Crystals. 2019; 9(11):579. https://doi.org/10.3390/cryst9110579
Chicago/Turabian StyleWang, Lu, Jianfeng Jin, Peijun Yang, Yaping Zong, and Qing Peng. 2019. "Graphene Adhesion Mechanics on Iron Substrates: Insight from Molecular Dynamic Simulations" Crystals 9, no. 11: 579. https://doi.org/10.3390/cryst9110579






