High-Temperature Cooperative Spin Crossover Transitions and Single-Crystal Reflection Spectra of [FeIII(qsal)2](CH3OSO3) and Related Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Compounds 1–3
2.2. Physical Measurements
2.3. Crystal Structure Determinations of 1–3
2.4. Density Functional Theory (DFT) Calculations
3. Results and Discussion
3.1. Synthesis of Compounds 1–3
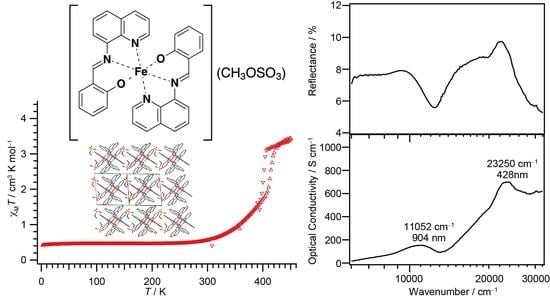
3.2. Magnetic Susceptibility for 1–3
3.3. Mössbauer Spectroscopy for 1 and 3
3.4. Optical Properties of the [Fe(qsal)2] Compounds
3.4.1. Reflection and Optical Conductivity Spectra of Single Crystals for 1 and 3
3.4.2. Time-Dependent Density Functional Theory (TD-DFT) Calculations for the LS and HS [Fe(qsal)2] Cations
3.5. Crystal Structures of 1–3
3.5.1. Molecular Structure Description of the [Fe(qsal)2] Cation in 1–3
3.5.2. Molecular Arrangement of 1
3.5.3. Molecular Arrangement of 2
3.5.4. Molecular Arrangement of 3 at 293 K
3.5.5. Correlation between the Crystal Structures and Magnetic Behaviors for the [Fe(qsal)2] Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gütlich, P.; Goodwin, H.A. (Eds.) Spin Crossover in Transition Metal Compounds I−III; Springer: Berlin/Heidelberg, Germany, 2004; ISBN 3-540-40394-9, 3-540-40396-5 and 3-540-40395-7. [Google Scholar]
- Halcrow, M.A. (Ed.) Spin-Crossover Materials; John Wiley & Sons, Ltd.: Oxford, UK, 2013; ISBN 978-1-119-99867-9. [Google Scholar]
- Takahashi, K. (Ed.) Spin-Crossover Complexes; MDPI: Basel, Switzerland, 2018; ISBN 978-3-03842-825-1. [Google Scholar]
- Bousseksou, A.; Molnár, G.; Salmon, L.; Nicolazzi, W. Molecular spin crossover phenomenon: Recent achievements and prospects. Chem. Soc. Rev. 2011, 40, 3313–3335. [Google Scholar] [CrossRef] [PubMed]
- Gütlich, P.; Gaspar, A.B.; Garcia, Y. Spin state switching in iron coordination compounds. Beilstein J. Org. Chem. 2013, 9, 342–391. [Google Scholar] [CrossRef] [PubMed]
- Dahl, B.M.; Dahl, O. Studies of Chelates with Heterocyclic Ligands IV. Transition Metal Complexes with N-(8-quinolyl)-salicylaldimine. Acta Chem. Scand. 1969, 23, 1503–1513. [Google Scholar] [CrossRef]
- Dickinson, R.C.; Baker, W.A.; Collins, R.L. The Magnetic Properties of bis[N-(8-quinolyl)-salicylaldimine]halogenoiron(III)·X hydrate, Fe(8-QS)2X·xH2O: A Reexamination. J. Inorg. Nucl. Chem. 1977, 39, 1531–1533. [Google Scholar] [CrossRef]
- Oshio, H.; Kitazaki, K.; Mishiro, J.; Kato, N.; Maeda, Y.; Takashima, Y. New Spin-crossover Iron(III) Complexes with Large Hysteresis Effects and Time Dependence of their Magnetism. J. Chem. Soc. Dalton Trans. 1987, 1341–1347. [Google Scholar] [CrossRef]
- Hayami, S.; Gu, Z.; Yoshiki, H.; Fujishima, A.; Sato, O. Iron(III) Spin-Crossover Compounds with a Wide Apparent Thermal Hysteresis around Room Temperature. J. Am. Chem. Soc. 2001, 123, 11644–11650. [Google Scholar] [CrossRef] [PubMed]
- Hayami, S.; Kawahara, T.; Juhasz, G.; Kawamura, K.; Uehashi, K.; Sato, O.; Maeda, Y. Iron(III) Spin Transition Compound with a Large Thermal Hysteresis. J. Radioanal. Nucl. Chem. 2003, 255, 443–447. [Google Scholar] [CrossRef]
- Hayami, S.; Hiki, K.; Kawahara, T.; Maeda, Y.; Urakami, D.; Inoue, K.; Ohama, M.; Kawata, S.; Sato, O. Photo-Induced Spin Transition of Iron(III) Compounds with π-π Intermolecular Interactions. Chem. A Eur. J. 2009, 15, 3497–3508. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, T.A.; Ovchinnikov, I.V.; Garipov, R.R.; Ivanova, G.I. Spin Crossover [Fe(qsal)2]X (X = Cl, SCN, CF3SO3) Complexes: EPR and DFT Study. Appl. Magn. Reson. 2011, 40, 1–10. [Google Scholar] [CrossRef]
- Takahashi, K.; Sato, T.; Mori, H.; Tajima, H.; Sato, O. Correlation between the Magnetic Behaviors and Dimensionality of Intermolecular Interactions in Fe(III) Spin Crossover Compounds. Phys. B Condens. Matter 2010, 405, S65–S68. [Google Scholar] [CrossRef]
- Takahashi, K.; Sato, T.; Mori, H.; Tajima, H.; Einaga, Y.; Sato, O. Cooperative Spin Transition and Thermally Quenched High-Spin State in New Polymorph of [Fe(qsal)2]I3. Hyperfine Interact. 2012, 206, 1–5. [Google Scholar] [CrossRef]
- Djukic, B.; Jenkins, H.A.; Seda, T.; Lemaire, M.T. Structural and Magnetic Properties of Homoleptic Iron(III) Complexes Containing N-(8-Quinolyl)-Salicylaldimine [Fe(Qsal)2]+X− {X = I or (Qsal)FeCl3}. Transit. Metal Chem. 2013, 38, 207–212. [Google Scholar] [CrossRef]
- Tsukiashi, A.; Nakaya, M.; Kobayashi, F.; Ohtani, R.; Nakamura, M.; Harrowfield, J.M.; Kim, Y.; Hayami, S. Intermolecular Interaction Tuning of Spin-Crossover Iron(III) Complexes with Aromatic Counteranions. Inorg. Chem. 2018, 57, 2834–2842. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.J.; Phonsri, W.; Harding, P.; Gass, I.A.; Murray, K.S.; Moubaraki, B.; Cashion, J.D.; Liu, L.; Telfer, S.G. Abrupt Spin Crossover in an Iron(III) Quinolylsalicylaldimine Complex: Structural Insights and Solvent Effects. Chem. Commun. 2013, 49, 6340–6342. [Google Scholar] [CrossRef] [PubMed]
- Harding, D.J.; Sertphon, D.; Harding, P.; Murray, K.S.; Moubaraki, B.; Cashion, J.D.; Adams, H. FeIII Quinolylsalicylaldimine Complexes: A Rare Mixed-Spin-State Complex and Abrupt Spin Crossover. Chem. A Eur. J. 2013, 19, 1082–1090. [Google Scholar] [CrossRef]
- Harding, D.J.; Phonsri, W.; Harding, P.; Murray, K.S.; Moubaraki, B.; Jameson, G.N.L. Abrupt Two-Step and Symmetry Breaking Spin Crossover in an Iron(III) Complex: An Exceptionally Wide [LS-HS] Plateau. Dalton Trans. 2014, 44, 15079–15082. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Harding, D.J.; Harding, P.; Murray, K.S.; Moubaraki, B.; Gass, I.A.; Cashion, J.D.; Jameson, G.N.L.; Adams, H. Stepped Spin Crossover in Fe(III) Halogen Substituted Quinolylsalicylaldimine Complexes. Dalton Trans. 2014, 43, 17509–17518. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Harding, P.; Liu, L.; Telfer, S.G.; Murray, K.S.; Moubaraki, B.; Ross, T.M.; Jameson, G.N.L.; Harding, D.J. Solvent Modified Spin Crossover in an Iron(III) Complex: Phase Changes and an Exceptionally Wide Hysteresis. Chem. Sci. 2017, 8, 3949–3959. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Cui, H.; Kobayashi, H.; Einaga, Y.; Sato, O. The Light-Induced Excited Spin State Trapping Effect on Ni(dmit)2 Salt with an Fe(III) Spin-Crossover Cation: [Fe(qsal)2][Ni(dmit)2]·2CH3CN. Chem. Lett. 2005, 34, 1240–1241. [Google Scholar] [CrossRef]
- Takahashi, K.; Cui, H.-B.; Okano, Y.; Kobayashi, H.; Einaga, Y.; Sato, O. Electrical Conductivity Modulation Coupled to a High-Spin-Low-Spin Conversion in the Molecular System [FeIII(qsal)2][Ni(dmit)2]3·CH3CN·H2O. Inorg. Chem. 2006, 45, 5739–5741. [Google Scholar] [CrossRef]
- Faulmann, C.; Dorbes, S.; Lampert, S.; Jacob, K.; Garreau de Bonneval, B.; Molnár, G.; Bousseksou, A.; Real, J.A.; Valade, L. Crystal Structure, Magnetic Properties and Mössbauer Studies of [Fe(qsal)2][Ni(dmit)2]. Inorg. Chim. Acta 2007, 360, 3870–3878. [Google Scholar] [CrossRef]
- Takahashi, K.; Cui, H.-B.; Okano, Y.; Kobayashi, H.; Mori, H.; Tajima, H.; Einaga, Y.; Sato, O. Evidence of the Chemical Uniaxial Strain Effect on Electrical Conductivity in the Spin-Crossover Conducting Molecular System: [FeIII(qnal)2][Pd(dmit)2]5·Acetone. J. Am. Chem. Soc. 2008, 130, 6688–6689. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Mori, H.; Kobayashi, H.; Sato, O. Mechanism of Reversible Spin Transition with a Thermal Hysteresis Loop in [FeIII(qsal)2][Ni(dmise)2]·2CH3CN: Selenium Analogue of the Precursor of an Fe(III) Spin-Crossover Molecular Conducting System. Polyhedron 2009, 28, 1776–1781. [Google Scholar] [CrossRef]
- Neves, A.I.S.; Dias, J.C.; Vieira, B.J.C.; Santos, I.C.; Castelo Branco, M.B.; Pereira, L.C.J.; Waerenborgh, J.C.; Almeida, M.; Belo, D.; da Gama, V.A. New Hybrid Material Exhibiting Room Temperature Spin-Crossover and Ferromagnetic Cluster-Glass Behavior. CrystEngComm 2009, 11, 2160–2168. [Google Scholar] [CrossRef]
- Faulmann, C.; Chahine, J.; Valade, L.; Chastanet, G.; Létard, J.F.; De Caro, D. Photomagnetic Studies of Spin-Crossover-and Photochromic-Based Complexes. Eur. J. Inorg. Chem. 2013, 5, 1058–1067. [Google Scholar] [CrossRef]
- Togo, T.; Amolegbe, S.A.; Yamaguchi, R.; Kuroda-Sowa, T.; Nakaya, M.; Shimayama, K.; Nakamura, M.; Hayami, S. Crystal Structure and Spin-Crossover Behavior of Iron(III) Complex with Nitroprusside. Chem. Lett. 2013, 42, 1542–1544. [Google Scholar] [CrossRef]
- Fukuroi, K.; Takahashi, K.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Synergistic Spin Transition between Spin Crossover and Spin-Peierls-like Singlet Formation in the Halogen-Bonded Molecular Hybrid System: [Fe(Iqsal)2][Ni(dmit)2]⋅CH3CN⋅H2O. Angew. Chem. Int. Ed. 2014, 53, 1983–1986. [Google Scholar] [CrossRef]
- Vieira, B.J.C.; Dias, J.C.; Santos, I.C.; Pereira, L.C.J.; da Gama, V.; Waerenborgh, J.C. Thermal Hysteresis in a Spin-Crossover FeIII Quinolylsalicylaldimine Complex, FeIII(5-Br-qsal)2Ni(dmit)2·solv: Solvent Effects. Inorg. Chem. 2015, 54, 1354–1362. [Google Scholar] [CrossRef]
- Vieira, B.J.C.; Coutinho, J.T.; Dias, J.C.; Nunes, J.C.; Santos, I.C.; Pereira, L.C.J.; Da Gama, V.; Waerenborgh, J.C. Crystal Structure and Spin Crossover Behavior of the [Fe(5-Cl-Qsal)2][Ni(Dmit)2]·2CH3CN Complex. Polyhedron 2015, 85, 643–651. [Google Scholar] [CrossRef]
- Takahashi, K.; Sakurai, T.; Zhang, W.-M.; Okubo, S.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Mori, H. Spin-Singlet Transition in the Magnetic Hybrid Compound from a Spin-Crossover Fe(III) Cation and π-Radical Anion. Inorganics 2017, 5, 54. [Google Scholar] [CrossRef]
- Phonsri, W.; Davies, C.G.; Jameson, G.N.L.; Moubaraki, B.; Murray, K.S. Spin Crossover, Polymorphism and Porosity to Liquid Solvent in Heteroleptic Iron(III) {Quinolylsalicylaldimine/Thiosemicarbazone-Salicylaldimine} Complexes. Chem. A Eur. J. 2016, 22, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Macedo, D.; Moubaraki, B.; Cashion, J.; Murray, K. Heteroleptic Iron(III) Spin Crossover Complexes; Ligand Substitution Effects. Magnetochemistry 2016, 2, 3. [Google Scholar] [CrossRef]
- Murata, S.; Takahashi, K.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y. Cooperative Spin-Crossover Transition from Three-Dimensional Purely π-Stacking Interactions in a Neutral Heteroleptic Azobisphenolate FeIII Complex with a N3O3 Coordination Sphere. Dalton Trans. 2017, 46, 5786–5789. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Macedo, D.S.; Davies, C.G.; Jameson, G.N.L.; Moubaraki, B.; Murray, K.S. Heteroleptic Iron(III) Schiff Base Spin Crossover Complexes: Halogen Substitution, Solvent Loss and Crystallite Size Effects. Dalton Trans. 2017, 46, 7020–7029. [Google Scholar] [CrossRef] [PubMed]
- Kuroda-Sowa, T.; Yu, Z.; Senzaki, Y.; Sugimoto, K.; Maekawa, M.; Munakata, M.; Hayami, S.; Maeda, Y. Abrupt Spin Transitions and LIESST Effects Observed in FeII Spin-Crossover Complexes with Extended π-Conjugated Schiff-Base Ligands Having N4O2 Donor Sets. Chem. Lett. 2008, 37, 1216–1217. [Google Scholar] [CrossRef]
- Yu, Z.; Kuroda-Sowa, T.; Kume, H.; Okubo, T.; Maekawa, M.; Munakata, M. Effects of Metal Doping on the Spin-Crossover Properties of an Iron(II) Complex with Extended π-Conjugated Schiff-Base Ligand Having an N4O2 Donor Set. Bull. Chem. Soc. Jpn. 2009, 82, 333–337. [Google Scholar] [CrossRef]
- Kuroda-Sowa, T.; Kimura, K.; Kawasaki, J.; Okubo, T.; Maekawa, M. Effects of Weak Interactions on Spin Crossover Properties of Iron(II) Complexes with Extended π-Conjugated Schiff-Base Ligands. Polyhedron 2011, 30, 3189–3192. [Google Scholar] [CrossRef]
- Iasco, O.; Rivière, E.; Guillot, R.; Buron-Le Cointe, M.; Meunier, J.-F.; Bousseksou, A.; Boillot, M.-L. FeII(pap-5NO2)2 and FeII(qsal-5NO2)2 Schiff-Base Spin-Crossover Complexes: A Rare Example with Photomagnetism and Room-Temperature Bistability. Inorg. Chem. 2015, 54, 1791–1799. [Google Scholar] [CrossRef] [PubMed]
- Phonsri, W.; Macedo, D.S.; Vignesh, K.R.; Rajaraman, G.; Davies, C.G.; Jameson, G.N.L.; Moubaraki, B.; Ward, J.S.; Kruger, P.E.; Chastanet, G.; et al. Halogen Substitution Effects on N2O Schiff Base Ligands in Unprecedented Abrupt FeII Spin Crossover Complexes. Chem. A Eur. J. 2017, 23, 7052–7065. [Google Scholar] [CrossRef] [PubMed]
- Kuroda-Sowa, T.; Isobe, R.; Yamao, N.; Fukumasu, T.; Okubo, T.; Maekawa, M. Variety of Spin Transition Temperatures of Iron(II) Spin Crossover Complexes with Halogen Substituted Schiff-Base Ligands, HqsalX(X = F, Cl, Br, and I). Polyhedron 2017, 136, 74–78. [Google Scholar] [CrossRef]
- Atzori, M.; Poggini, L.; Squillantini, L.; Cortigiani, B.; Gonidec, M.; Bencok, P.; Sessoli, R.; Mannini, M. Thermal and Light-Induced Spin Transition in a Nanometric Film of a New High-Vacuum Processable Spin Crossover Complex. J. Mater. Chem. C 2018, 6, 8885–8889. [Google Scholar] [CrossRef]
- König, E. Landolt-Börnstein Neue Serie Gruppe II, Vol. 2; Hellwege, K.-H., Hellwege, A.M., Eds.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1966; pp. 16–18. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision D.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. A New Mixing of Hartree-Fock and Local Density-Functional Theories. J. Chem. Phys. 1993, 98, 1372–1377. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Wachters, A.J.H. Gaussian Basis Set for Molecular Wavefunctions Containing Third-Row Atoms. J. Chem. Phys. 1970, 52, 1033–1036. [Google Scholar] [CrossRef]
- Hay, P.J. Gaussian Basis Sets for Molecular Calculations. The Representation of 3d Orbitals in Transition-Metal Atoms. J. Chem. Phys. 1977, 66, 4377–4384. [Google Scholar] [CrossRef]
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular excitation energies to high-lying bound states from time-dependent density-functional response theory: Characterization and correction of the time-dependent local density approximation ionization threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Yanai, T.; Tew, D.P.; Handy, N.C. A new hybrid exchange-correlation functional using the Coulomb-attenuating method (CAM-B3LYP). Chem. Phys. Lett. 2004, 393, 51–57. [Google Scholar] [CrossRef]
- Halcrow, M.A. Iron(II) Complexes of 2,6-di(pyrazol-1-yl)pyridines—A Versatile System for Spin-Crossover Research. Coord. Chem. Rev. 2009, 253, 2493–2514. [Google Scholar] [CrossRef]
- Takahashi, K.; Kawamukai, K.; Okai, M.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K. A new family of anionic FeIII spin crossover complexes featuring a weak-field N2O4 coordination octahedron. Chem. Eur. J. 2016, 22, 1253–1257. [Google Scholar] [CrossRef]
- Bondi, A. Van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Murata, S.; Takahashi, K.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K. The Role of Coulomb Interactions for Spin Crossover Behaviors and Crystal Structural Transformation in Novel Anionic Fe(III) Complexes from a π-Extended ONO Ligand. Crystals 2016, 6, 49. [Google Scholar] [CrossRef]
- Takahashi, K.; Okai, M.; Mochida, T.; Sakurai, T.; Ohta, H.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K.; Konaka, H.; et al. Contribution of Coulomb Interactions to a Two-Step Crystal Structure Phase Transformation Coupled with a Significant Change in Spin Crossover Behavior for a Series of Charged FeII Complexes from 2,6-bis(2-methylthiazol-4-yl)pyridine. Inorg. Chem. 2018, 57, 1277–1287. [Google Scholar] [CrossRef] [PubMed]









| Compound | T/K | Spin-State 1 | IS 2 | QS 3 | LW 4 | Ref. | |
| X | Solv | ||||||
| CH3OSO3 (1) | − | 293 | LS (100%) | 0.0040(18) | 2.689(4) | 0.342(5) | this work |
| CH3SO3 (3) | CH3OH | 293 | HS (100%) | 0.376(14) | 1.11(2) | 0.98(3) | this work |
| Cl | 1.5H2O | 298 | HS (100%) | 0.28 | 0.68 | − | [7] |
| 77 | HS | 0.36 | 0.70 | − | |||
| LS | 0.04 | 2.44 | − | ||||
| 4.3 | HS | 0.40 | 0.66 | − | |||
| LS | 0.14 | 2.63 | − | ||||
| NCS | − | 288 | HS (100%) | 0.253 | 0 | 1.737 | [8] |
| 78 | HS (6.5%) | 0.360 | 0 | 1.075 | |||
| LS (93.5%) | 0.191 | 2.660 | 0.288 0.326 | ||||
| NCSe | CH3OH | 293 | LS (100%) | 0.07 | 2.52 | − | [9] |
| NCSe | CH2Cl2 | 293 | LS (100%) | 0.11 | 2.47 | − | [9] |
| I3 | − | 293 | HS (100%) | 0.232 | 0.616 | − | [14] |
| 15 | LS (100%) | 0.075 | 2.878 | − | |||
| [Ni(dmit)2] | 2CH3CN | 293 | HS (100%) | 0.276 | 0.745 | − | [22] |
| 9 | HS (13%) | 0.32 | 0.68 | − | |||
| LS (87%) | 0.07 | 2.74 | − | ||||
| [Ni(mnt)2] | − | 293 | HS (100%) | 0.307(16) | 0.91(2) | 0.79(4) | [33] |
| 100 | HS (100%) | 0.48(3) | 1.07(3) | 1.54(5) | |||
| LS | HS | ||||
| Wavelength/nm | f | Assignment | Wavelength/nm | f | Assignment |
| 2138.01 | 0.0000 | d-d, LMCT | 656.61 | 0.0138 | LMCT |
| 1837.95 | 0.0001 | d-d, LMCT | 628.25 | 0.0000 | LMCT |
| 842.64 | 0.0000 | d-d, LMCT | 579.97 | 0.0099 | LMCT |
| 802.03 | 0.0000 | d-d, LMCT | 569.29 | 0.0020 | LMCT |
| 653.38 | 0.0001 | d-d, LMCT | 559.22 | 0.0004 | LMCT |
| 636.23 | 0.0000 | d-d, LMCT | 546.71 | 0.0056 | LMCT |
| 619.84 | 0.0000 | π-π* | 525.34 | 0.0304 | LMCT |
| 615.97 | 0.0000 | π-π*, d-d | 488.80 | 0.0007 | LMCT |
| 515.63 | 0.0005 | d-d, LMCT | 453.77 | 0.0053 | π-π* |
| 500.30 | 0.0196 | LMCT, π-π* | 452.76 | 0.0101 | π-π* |
| 490.33 | 0.0007 | π-π*, d-d | 425.61 | 0.0144 | LMCT |
| 487.20 | 0.0059 | LMCT, π-π* | 416.98 | 0.0001 | LMCT |
| 481.57 | 0.0018 | LMCT, d-d | 385.54 | 0.0580 | LMCT, d-d |
| 479.60 | 0.0001 | d-d, LMCT | 382.47 | 0.0805 | π-π* |
| 464.66 | 0.0021 | LMCT, π-π* | 375.07 | 0.1551 | LMCT, π-π* |
| 413.29 | 0.0474 | LMCT, π-π* | 363.99 | 0.0556 | LMCT |
| 411.68 | 0.0559 | π-π*, LMCT | 363.21 | 0.0017 | LMCT |
| 403.64 | 0.1539 | π-π*, LMCT | 357.28 | 0.0305 | LMCT |
| 400.94 | 0.0967 | π-π* | 353.41 | 0.0003 | LMCT, d-d |
| 383.76 | 0.0044 | LMCT, d-d | 350.25 | 0.0033 | LMCT |
| Formula | C33H25FeN4O6S | |||
| Formula Weight | 661.48 | |||
| Color | black | |||
| Dimension/mm | 0.30 × 0.30 × 0.15 | |||
| T/K | 293 | 360 | 400 | 425 |
| Crystal System | triclinic | triclinic | triclinic | monoclinic |
| Space Group | P | P | P | P2/n |
| a/Å | 9.7672(6) | 9.7725(7) | 9.743(2) | 33.110(8) |
| b/Å | 11.8138(7) | 11.9003(8) | 12.031(3) | 9.886(3) |
| c/Å | 12.9116(8) | 12.9660(9) | 13.023(3) | 34.385(9) |
| α/° | 70.6370(10) | 70.5486(8) | 70.377(2) | 90 |
| β/° | 85.4390(10) | 85.6245(9) | 85.801(3) | 115.807(3) |
| γ/° | 85.5930(10) | 85.5470(8) | 85.532(2) | 90 |
| V/Å3 | 1399.10(15) | 1415.49(17) | 1431.7(5) | 10133(4) |
| Z | 2 | 2 | 2 | 14 |
| ρcalcd/g cm−3 | 1.570 | 1.552 | 1.534 | 1.518 |
| μ (Mo-Kα) | 0.671 | 0.663 | 0.655 | 0.648 |
| 2θmax/° | 51.36 | 50.06 | 50.04 | 52.74 |
| No. Reflections (Rint) | 7116 (0.0145) | 6907 (0.0158) | 6956 (0.0184) | 52039 (0.0361) |
| No. Observations (I > 2.00σ(I)) | 5158 (4887) | 4922 (4577) | 4961 (4483) | 20655 (14398) |
| No. Variables | 407 | 407 | 407 | 1423 |
| R1 (I > 2.00σ(I)) | 0.0282 | 0.0305 | 0.0338 | 0.0747 |
| R (all data) | 0.0298 | 0.0329 | 0.0374 | 0.1033 |
| wR2 (all data) | 0.0782 | 0.0867 | 0.0982 | 0.2109 |
| Residual Electron Density / e Å−3 | 0.270 −0.413 | 0.297 −0.362 | 0.282 −0.280 | 1.263 −0.597 |
| Goodness of Fit | 1.067 | 1.061 | 1.047 | 1.009 |
| Compound | 2 | 3 | |
| Formula | C33H22F3FeN4O5S | C34H29FeN4O6S | |
| Formula Weight | 699.45 | 677.52 | |
| Color | black | black | |
| Dimension/mm | 0.30 × 0.125 × 0.03 | 0.40 × 0.15 × 0.065 | |
| T/K | 293 | 400 | 293 |
| Crystal System | monoclinic | monoclinic | triclinic |
| Space Group | P21/n | P21/n | P |
| a/Å | 9.8302(15) | 9.934(4) | 8.6110(8) |
| b/Å | 26.985(4) | 26.659(11) | 12.6981(12) |
| c/Å | 11.6733(17) | 12.366(5) | 15.1480(14) |
| α/° | 90 | 90 | 114.7000(10) |
| β/° | 110.038(2) | 111.436(4) | 94.1660(10) |
| γ/° | 90 | 90 | 92.3320(10) |
| V/Å3 | 2909.1(8) | 3048(2) | 1496.2(2) |
| Z | 4 | 4 | 2 |
| ρcalcd/g cm–3 | 1.597 | 1.524 | 1.504 |
| μ (Mo-Kα) | 0.661 | 0.631 | 0.629 |
| 2θmax/° | 54.96 | 53.19 | 50.04 |
| No. Reflections (Rint) | 16774 (0.0188) | 16916 (0.0202) | 7231 (0.0152) |
| No. Observations (I > 2.00σ(I)) | 6634 (5595) | 6710 (4931) | 5166 (4942) |
| No. Variables | 424 | 424 | 417 |
| R1 (I > 2.00σ(I)) | 0.0358 | 0.0620 | 0.0347 |
| R (all data) | 0.0445 | 0.0839 | 0.0361 |
| wR2 (all data) | 0.0966 | 0.1976 | 0.0991 |
| Residual Electron Density/e Å–3 | 0.396 −0.251 | 0.762 −0.554 | 0.962 −0.388 |
| Goodness of Fit | 1.022 | 1.044 | 1.049 |
| Compound | 1 | 1 | 1 | 1A | 1B | 1C | 1D | 2 | 2 | 3 | [Ni(dmit)2] 4 | NCSe 5 | [Ni(mnt)2] 6 |
| T/K | 293 | 360 | 400 | 425 | 425 | 425 | 425 | 293 | 400 | 293 | 273 | 230 | 293 |
| Fe1-O1/Å | 1.8741(11) | 1.8692(13) | 1.8715(16) | 1.880(3) | 1.881(4) | 1.903(3) | 1.892(4) | 1.8707(12) | 1.900(3) | 1.9158(15) | 1.913(2) | 1.868(2) | 1.9278(19) |
| Fe1-O2/Å | 1.8779(12) | 1.8830(13) | 1.8880(15) | 1.877(4) | 1.900(3) | 1.911(3) | 1.892(4) | 1.8693(12) | 1.894(2) | 1.9085(15) | 1.914(3) | 1.874(2) | 1.908(2) |
| Fe1-N1/Å | 1.9434(13) | 1.9674(14) | 2.0091(16) | 2.019(3) | 2.096(4) | 2.141(4) | 2.062(4) | 1.9500(14) | 2.107(3) | 2.1250(17) | 2.125(3) | 1.936(3) | 2.097(2) |
| Fe1-N2/Å | 1.9804(13) | 2.0083(14) | 2.0497(16) | 2.066(4) | 2.156(4) | 2.151(4) | 2.091(4) | 1.9802(15) | 2.148(3) | 2.1722(18) | 2.150(2) | 1.969(3) | 2.195(2) |
| Fe1-N3/Å | 1.9438(13) | 1.9681(14) | 2.0105(17) | 2.032(3) | 2.101(3) | 2.143(4) | 2.062(4) | 1.9460(15) | 2.103(3) | 2.1291(17) | 2.138(3) | 1.945(3) | 2.131(2) |
| Fe1-N4/Å | 1.9782(14) | 2.0049(16) | 2.0483(18) | 2.068(4) | 2.133(4) | 2.164(4) | 2.091(4) | 1.9842(16) | 2.148(3) | 2.1595(17) | 2.151(3) | 1.986(3) | 2.136(2) |
| Σ1/° | 51.13(17) | 49.6(2) | 50.6(2) | 52.1(5) | 65.9(5) | 74.5(5) | 52.7(6) | 52.4(2) | 73.6(4) | 77.7(2) | 72.5(3) | 52.1(4) | 83.1(3) |
| Θ2/° | 61.8(2) | 61.9(2) | 66.2(3) | 71.3(6) | 104.1(6) | 143.4(6) | 84.6(7) | 71.0(3) | 114.9(5) | 126.4(3) | 124.2(4) | 69.4(5) | 149.1(4) |
| Φ3/° | 2.50(5) | 3.74(6) | 5.77(6) | 7.54(15) | 12.20(15) | 15.33(14) | 10.8(2) | 1.86(6) | 9.42(11) | 15.80(7) | 13.79(9) | 1.50(14) | 17.83(8) |
| CCDC No. | 1891471 | 1891472 | 1891473 | 1891474 | 1891475 | 1891476 | 1891477 | 289952 | 194657 | 1559560 | |||
| Position a | 293 K | 360 K | 400 K | |
| p | Plane(C1−C6)···C10 b | 3.243 | 3.252 | 3.261 |
| Plane(C8−C16,N2)···C5 b | 3.264 | 3.288 | 3.320 | |
| C4···C11 b | 3.267(3) | 3.279(3) | 3.293(4) | |
| C6···C9 b | 3.245(3) | 3.270(3) | 3.297(3) | |
| q | C23···C26 c | 3.375(3) | 3.403(3) | 3.436(4) |
| r | Plane(C17−C22)···Plane(C17−C22) d | 3.535 | 3.561 | 3.581 |
| C18···C20 d | 3.553(3) | 3.576(3) | 3.594(4) | |
| s | Plane(C8−C16,N2)···Plane(C8−C16,N2) e | 3.266 | 3.276 | 3.281 |
| C10···C11 e | 3.377(3) | 3.381(3) | 3.388(3) | |
| C10···C12 e | 3.370(3) | 3.383(3) | 3.384(4) |
| Position a | 293 K | 400 K | |
| t | Plane(C1−C6)···C25 b | 3.503 | 3.617 |
| Plane(C8−C16,N2)···C20 b | 3.282 | 3.428 | |
| Plane(C8−C16,N2)···C21 b | 3.263 | 3.415 | |
| Plane(C17−C22)···C9 c | 3.327 | 3.465 | |
| Plane(C17−C22)···C10 c | 3.328 | 3.457 | |
| Plane(C24−C32,N4)···C4 c | 3.580 | 3.578 | |
| Plane(C24−C32,N4)···C5 c | 3.302 | 3.359 | |
| C5···C24 b | 3.331(3) | 3.394(6) | |
| C5···C25 b | 3.515(3) | 3.687(7) | |
| C7···C23 b | 3.255(3) | 3.375(5) | |
| C8···C21 b | 3.289(3) | 3.440(6) | |
| C9···C21 b | 3.442(3) | 3.709(7) | |
| C9···C22 b | 3.476(3) | 3.630(6) | |
| C10···C19 b | 3.368(3) | 3.485(8) | |
| C10···C20 b | 3.470(3) | 3.747(8) | |
| C11···C20 b | 3.430(3) | 3.650(7) | |
| u | Plane(C1−C6)···C19 d | 3.529 | 3.638 |
| Plane(C17−C22)···C2 d | 3.421 | 3.546 | |
| Plane(C17−C22)···C3 d | 3.511 | 3.690 | |
| C1···C19 d | 3.592(3) | 3.686(7) | |
| C2···C20 d | 3.463(3) | 3.585(7) |
| Position a | 293 K | |
| v | Plane(C17−C22)···C27 b | 3.427 |
| Plane(C24−C32,N4)···C19 c | 3.353 | |
| C18···C28 b | 3.379(4) | |
| C20···C26 b | 3.427(4) | |
| C2···C30 b (T-shape) | 3.440(4) | |
| C1···C30 b (T-shape) | 3.451(3) | |
| w | Plane(C24−C32,N4)···Plane(C24−C32,N4) d | 3.493 |
| C25···C30 d | 3.525(4) | |
| C27···C29 d | 3.464(4) | |
| x | Plane(C8−C16,N2)···C7 e | 3.439 |
| C7···C10 e | 3.475(3) | |
| y | Plane(C8−C16,N2)···Plane(C8−C16,N2) f | 3.652 |
| C10···C11 f | 3.679(4) |
| Compound | X | CH3OSO3 (1) | CF3SO3 (2) | NCS | NCSe | ||
| Solv | − | − | − | − | |||
| Magnetic behavior 1 | Heating Process | gSCO 290 → 410 K aSCO T1/2↑ = 414 K gSCO 420 → 450 K | gSCO 290 → 360 K aSCO T1/2↑ = 365 K gSCO 370 → 450 K | gSCO 200 → 270 K aSCO T1/2↑ = 289 K | aSCO T1/2↑ = 215 K gSCO 230 → 270 K aSCO T1/2↑ = 282 K | ||
| Cooling Process | gSCO 450 → 405 K aSCO T1/2↓ = 402 K gSCO 400 → 290 K | gSCO 450 → 370 K aSCO T1/2↓ = 361 K gSCO 355 → 290 K | gSCO 350 → 220 K aSCO T1/2↓ = 205 K | aSCO T1/2↓ = 212 K | |||
| Short distances/ Å 2 | Temp. | 293 K | 425 K | 293 K | 400 K | 293 K | 296 K |
| Spin-State | LS | mHS | LS | mHS | mHS | HS | |
| 1D | 3.245 3.267 3.375 | 3.276 3.309 3.352 | 3.255 3.289 3.331 | 3.375 3.440 3.394 | 3.466 3.527 3.593 | 3.491 3.533 3.545 | |
| B1D(1) | 3.370 | 3.407 | 3.463 | 3.585 | 3.710 | 3.538 | |
| B1D(2) | 3.553 | 3.638 | 3.886 | 3.943 | 4.218 | 3.680 | |
| CCDC No. | 1891471 | 1891474 | 1891475 | 1891476 | 717842 | 717844 | |
| Ref. | this work | this work | [11] | [11] | |||
| Compound | X | I | NCS | NCSe | I3 | CH3SO3 (3) | |
| Solv | − | CH2Cl2 | CH2Cl2 | − | CH3OH | ||
| Magnetic behavior 1 | LS 2 − 300 K | LS 113 K | LS 150 − 360 K | gSCO 200 − 260 K | HS 2 − 300 K | ||
| Short Distances/Å 2 | Temp. | 296 K | 113 K | 230 K | 50 K | 293 K | 293 K |
| Spin-State | LS | LS | LS | mLS | mHS | HS | |
| 1D | 3.375 3.409 3.424 | 3.229 3.384 3.388 | 3.317 3.360 3.383 | 3.226 3.266 3.269 | 3.371 3.376 3.395 | 3.379 | |
| B1D(1) | 3.487 | 3.494 | 3.455 | 3.456 | 3.579 | 3.464 3.475 | |
| B1D(2) | 3.910 | 3.865 | 3.956 | 3.527 (CH···π) | 3.688 (CH···π) | 3.620 | |
| CCDC No. | 902864 | 717843 | 194657 | 749309 | 749310 | 1891477 | |
| Ref. | [15] | [11] | [9] | [13] | this work | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takahashi, K.; Yamamoto, K.; Yamamoto, T.; Einaga, Y.; Shiota, Y.; Yoshizawa, K.; Mori, H. High-Temperature Cooperative Spin Crossover Transitions and Single-Crystal Reflection Spectra of [FeIII(qsal)2](CH3OSO3) and Related Compounds. Crystals 2019, 9, 81. https://doi.org/10.3390/cryst9020081
Takahashi K, Yamamoto K, Yamamoto T, Einaga Y, Shiota Y, Yoshizawa K, Mori H. High-Temperature Cooperative Spin Crossover Transitions and Single-Crystal Reflection Spectra of [FeIII(qsal)2](CH3OSO3) and Related Compounds. Crystals. 2019; 9(2):81. https://doi.org/10.3390/cryst9020081
Chicago/Turabian StyleTakahashi, Kazuyuki, Kaoru Yamamoto, Takashi Yamamoto, Yasuaki Einaga, Yoshihito Shiota, Kazunari Yoshizawa, and Hatsumi Mori. 2019. "High-Temperature Cooperative Spin Crossover Transitions and Single-Crystal Reflection Spectra of [FeIII(qsal)2](CH3OSO3) and Related Compounds" Crystals 9, no. 2: 81. https://doi.org/10.3390/cryst9020081