Theoretical Model and Experimental Investigations on Solution-Mediated Polymorphic Transformation of Theophylline: From Polymorph I to Polymorph II
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Characterization Methods
2.2.1. PXRD
2.2.2. SEM
2.2.3. TGA
2.2.4. Raman Spectroscopy
2.2.5. FTIR
2.3. Solution Concentration Measurements
2.4. Polymorphic Transformation Experiments
2.5. Theoretical Model
3. Results and Discussion
3.1. Characterizations
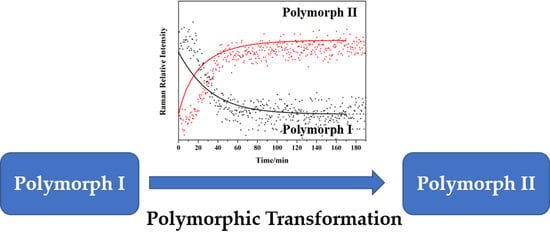
3.2. Solution-Mediated Transformation
3.2.1. Influence of System Temperature
3.2.2. Influence of Solvent
3.3. Transformation Process Models and Simulation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Du, W.; Yin, Q.; Hao, H.; Bao, Y.; Zhang, X.; Huang, J.; Li, X.; Xie, C.; Gong, J. Solution-Mediated Polymorphic Transformation of Prasugrel Hydrochloride from Form II to Form I. Ind. Eng. Chem. Res. 2014, 53, 5652–5659. [Google Scholar] [CrossRef]
- Guo, N.; Hou, B.; Wang, N.; Xiao, Y.; Huang, J.; Guo, Y.; Zong, S.; Hao, H. In Situ Monitoring and Modeling of the Solution-Mediated Polymorphic Transformation of Rifampicin: From Form II to Form I. J. Pharm. Sci. 2018, 107, 344–352. [Google Scholar] [CrossRef]
- Xu, Y.; Wu, S.-P.; Liu, X.-J.; Zhang, L.-J.; Lu, J. Crystal characterization and transformation of the forms I and II of anticoagulant drug rivaroxaban. Cryst. Res. Technol. 2017, 52, 1600379. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, S.; Liu, Y.; Chen, M.; Chen, Y.; Du, S.; Wang, Y.; Sun, P.; Sun, M.; Shi, P.; et al. Seed-Assisted Effects on Solution-Mediated Phase Transformation: A Case Study of l-Histidine in Antisolvent Crystallization. Ind. Eng. Chem. Res. 2018, 57, 784–793. [Google Scholar] [CrossRef]
- Wang, J.-R.; Zhu, B.; Zhang, Z.; Bao, J.; Deng, G.; Ding, Q.; Mei, X. Polymorphism of Triamcinolone Acetonide Acetate and Its Implication for the Morphology Stability of the Finished Drug Product. Cryst. Growth Des. 2017, 17, 3482–3490. [Google Scholar] [CrossRef]
- Jiang, S.; Jansens, P.J.; ter Horst, J.H. Control over Polymorph Formation of o-Aminobenzoic Acid. Cryst. Growth Des. 2010, 10, 2541–2547. [Google Scholar] [CrossRef]
- Liu, W.; Wei, H.; Black, S. An Investigation of the Transformation of Carbamazepine from Anhydrate to Hydrate Using in Situ FBRM and PVM. Org. Process Res. Dev. 2009, 13, 494–500. [Google Scholar] [CrossRef]
- Maher, A.; Rasmuson, Å.C.; Croker, D.M.; Hodnett, B.K. Solubility of the Metastable Polymorph of Piracetam (Form II) in a Range of Solvents. J. Chem. Eng. Data 2012, 57, 3525–3531. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, H.; Xu, H.; Ren, F.; Ren, G. Influence of Temperature, Solvents, and Excipients on Crystal Transformation of Agomelatine. Org. Process Res. Dev. 2016, 20, 1559–1565. [Google Scholar] [CrossRef]
- Maher, A.; Croker, D.M.; Seaton, C.C.; Rasmuson, Å.C.; Hodnett, B.K. Solution-Mediated Polymorphic Transformation: Form II to Form III Piracetam in Organic Solvents. Cryst. Growth Des. 2014, 14, 3967–3974. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Wang, L.-Y.; Sha, Z.-L.; Wang, Y.-F.; Yang, L.-B.; Zhao, X.-Y.; Du, W. Interplay between Thermodynamics and Kinetics on Polymorphic Appearance in the Solution Crystallization of an Enantiotropic System, Gestodene. Cryst. Growth Des. 2017, 17, 4582–4595. [Google Scholar] [CrossRef]
- Flood, A.E.; Wantha, L. Population balance modeling of the solution mediated transformation of polymorphs: Limitations and future trends. J. Cryst. Growth 2013, 373, 7–12. [Google Scholar] [CrossRef]
- Rodríguez-Hornedo, N.; Murphy, D. Surfactant-facilitated crystallization of dihydrate carbamazepine during dissolution of anhydrous polymorph. J. Pharm. Sci. 2004, 93, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Wang, M.; Dong, B.; Feng, Q.; Xu, C. Monitoring the Polymorphic Transformation of Imidacloprid Using in Situ FBRM and PVM. Org. Process Res. 2013, 17, 375–381. [Google Scholar] [CrossRef]
- Seton, L.; Khamar, D.; Bradshaw, I.; Hutcheon, G. Processing induced transformations: Phase impurities introduced during hydration/dehydration. Chem. Eng. Sci. 2012, 77, 57–64. [Google Scholar] [CrossRef]
- Fucke, K.; McIntyre, G.J.; Wilkinson, C.; Henry, M.; Howard, J.A.K.; Steed, J.W. New Insights into an Old Molecule: Interaction Energies of Theophylline Crystal Forms. Cryst. Growth Des. 2012, 12, 1395–1401. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dai, L. A Method for the Quantitative Analysis of a Key Component in Complex Mixtures Using Raman Spectroscopy Based on Peak Decomposition. Anal. Sci. 2019, 35, 511–515. [Google Scholar] [CrossRef]
- Weinstein, R.D.; Hanlon, W.H.; Donohue, J.P.; Simeone, M.; Rozich, A.; Muske, K.R. Solubility of Felodipine and Nitrendipine in Liquid and Supercritical Carbon Dioxide by Cloud Point and UV Spectroscopy. J. Chem. Eng. Data 2007, 52, 256–260. [Google Scholar] [CrossRef]
- Seton, L.; Khamar, D.; Bradshaw, I.J.; Hutcheon, G.A. Solid State Forms of Theophylline: Presenting a New Anhydrous Polymorph. Cryst. Growth Des. 2010, 10, 3879–3886. [Google Scholar] [CrossRef]
- Wu, S.; Du, S.; Chen, M.; Li, K.; Jia, L.; Zhang, D.; Macaringue, E.G.J.; Hou, B.; Gong, J. Crystal Structures and Phase Behavior of Sulfadiazine and a Method for the Preparation of Aggregates with Good Performance. Chem. Eng. Technol. 2018, 41, 532–540. [Google Scholar] [CrossRef]
- Fagerberg, J.H.; Sjogren, E.; Bergstrom, C.A.S. Concomitant intake of alcohol may increase the absorption of poorly soluble drugs. Eur. J. Pharm. Sci. 2015, 67, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, R.; Sugano, K.; Higashida, A.; Hayashi, Y.; Machida, M.; Aso, Y.; Yamashita, S. Oral absorption of poorly water-soluble drugs: Computer simulation of fraction absorbed in humans from a miniscale dissolution test. Pharm. Res. 2006, 23, 1144–1156. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, P.; Wagner, B.; Grimm, H.P.; Petrig-Schaffland, J.; Schuler, F.; Alvarez-Sánchez, R. Development of a Unified Dissolution and Precipitation Model and Its Use for the Prediction of Oral Drug Absorption. Mol. Pharm. 2016, 13, 586–598. [Google Scholar] [CrossRef] [PubMed]
- Khamar, D.; Bradshaw, I.J.; Hutcheon, G.A.; Seton, L. Solid State Transformations Mediated by a Kinetically Stable Form. Cryst. Growth Des. 2012, 12, 109–118. [Google Scholar] [CrossRef]
- Li, R.; Zhan, S.; Chen, G.; Jin, Y.; Yu, B.; Zhao, J.; Han, D.; Fan, H. Equilibrium Solubility, Model Correlation, and Solvent Effect of Indole-3-acetic Acid in Twelve Pure Solvents. J. Chem. Eng. Data 2019, 64, 1802–1808. [Google Scholar] [CrossRef]











| Temperatures | Solid Forms | Kdiss (min−1) | Csol (mol/L) | Kgrowth (L/[mol·min]) | knuc (min−1·[L/mol]α−1) | α |
|---|---|---|---|---|---|---|
| 25 °C | Polymorph I | 1.38e−2 | 1.12e−1 | 1.23e−1 | 1.98e−1 | 5.01 |
| Polymorph II | 1.28e−2 | 9.41e−2 | 1.36e−1 | 1.12e−1 | 1.48 | |
| 30 °C | Polymorph I | 4.04e−2 | 2.02e−1 | 2.00e−1 | 8.96e−2 | 3.31 |
| Polymorph II | 3.06e−2 | 1.85e−1 | 1.65e−1 | 1.36e−1 | 1.30 | |
| 35 °C | Polymorph I | 7.72e−2 | 3.84e−1 | 2.01e−1 | 9.73e−2 | 3.20 |
| Polymorph II | 3.45e−2 | 2.09e−1 | 1.65e−1 | 1.19e−1 | 1.32 |
| Temperatures | 102ARD | 103RMSD |
|---|---|---|
| 25 °C | 5.48 | 2.19 |
| 30 °C | 5.52 | 3.89 |
| 35 °C | 2.22 | 1.78 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, M.; Wang, Y.; Li, F.; Bao, Y.; Huang, X.; Shi, H.; Hao, H. Theoretical Model and Experimental Investigations on Solution-Mediated Polymorphic Transformation of Theophylline: From Polymorph I to Polymorph II. Crystals 2019, 9, 260. https://doi.org/10.3390/cryst9050260
Zhu M, Wang Y, Li F, Bao Y, Huang X, Shi H, Hao H. Theoretical Model and Experimental Investigations on Solution-Mediated Polymorphic Transformation of Theophylline: From Polymorph I to Polymorph II. Crystals. 2019; 9(5):260. https://doi.org/10.3390/cryst9050260
Chicago/Turabian StyleZhu, Manli, Yongli Wang, Fei Li, Ying Bao, Xin Huang, Huanhuan Shi, and Hongxun Hao. 2019. "Theoretical Model and Experimental Investigations on Solution-Mediated Polymorphic Transformation of Theophylline: From Polymorph I to Polymorph II" Crystals 9, no. 5: 260. https://doi.org/10.3390/cryst9050260