Sucrose-Assisted Synthesis of Layered Lithium-Rich Oxide Li[Li0.2Mn0.56Ni0.16Co0.08]O2 as a Cathode of Lithium-Ion Battery
Abstract
:1. Introduction
2. Experimental
2.1. The Preparation of Li[Li0.2Mn0.56Ni0.16Co0.08]O2
2.2. Electrochemical Measurements
2.3. Material Characterizations
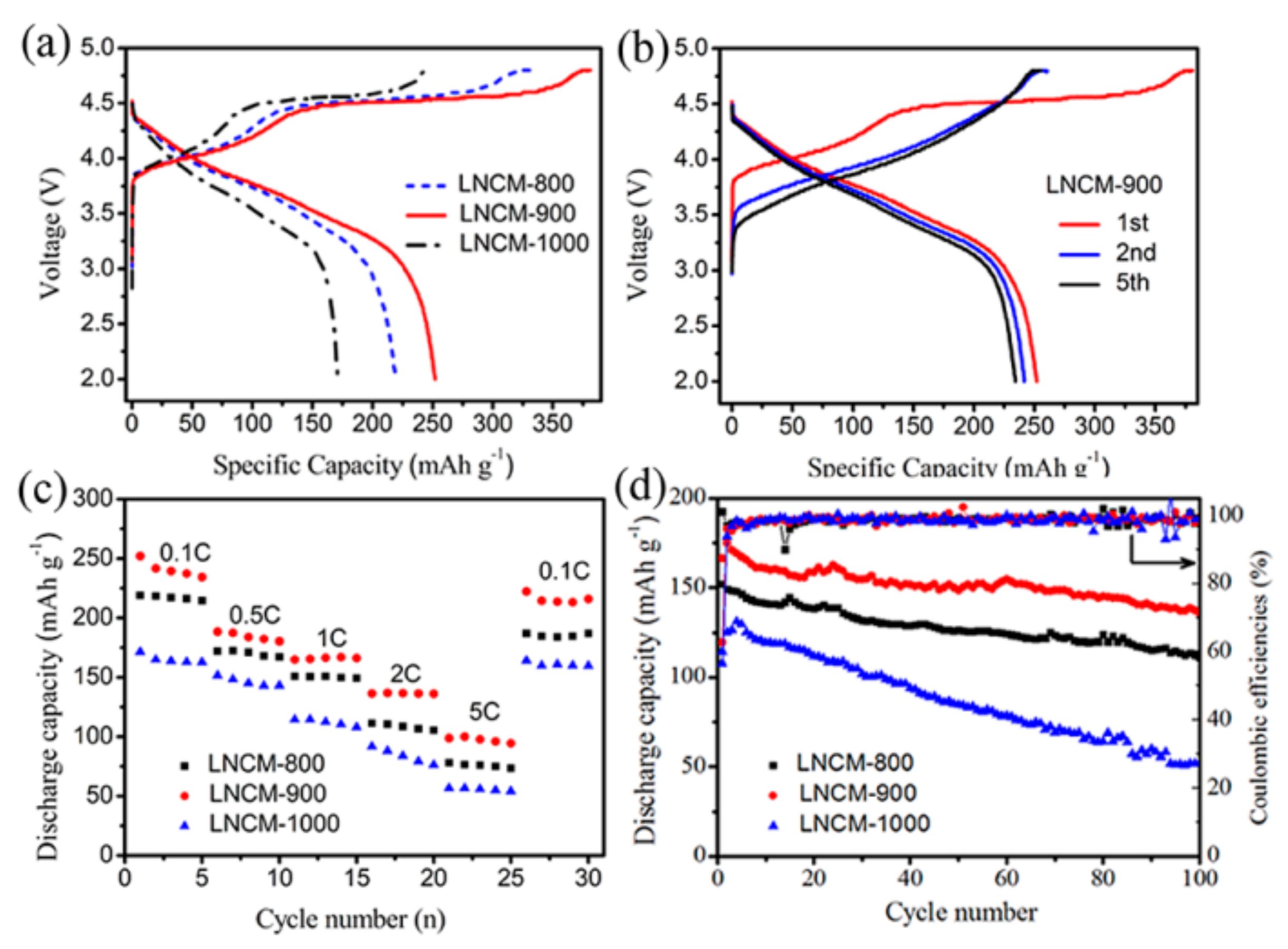
3. Results and Discussion
3.1. Analysis of Structure and Morphology
3.2. Electrochemical Properties
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef] [PubMed]
- Larcher, D.; Tarascon, J.M. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 2015, 7, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical energy storage for the grid: a battery of choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed]
- Goodenough, J.B.; Park, K.S. The Li-ion rechargeable battery: a perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Thackeray, M.M.; Wolverton, C.; Isaacs, E.D.; Environ, E. Electrical energy storage for transportation—Approaching the limits of, and going beyond, lithium-ion batteries. Energy Environ. Sci. 2012, 5, 7854–7863. [Google Scholar] [CrossRef]
- Park, J.H.; Cho, J.H.; Lee, E.H.; Kim, J.M.; Lee, S.Y. Thickness-tunable polyimide nanoencapsulating layers and their influence on cell performance/thermal stability of high-voltage LiCoO2 cathode materials for lithium-ion batteries. J. Power Sources 2013, 244, 442–449. [Google Scholar] [CrossRef]
- Wang, G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P.; Ahn, H. Mesoporous LiFePO4/C Nanocomposite Cathode Materials for High Power Lithium Ion Batteries with Superior Performance. Adv. Mater. 2010, 22, 4944–4948. [Google Scholar] [CrossRef]
- Lee, M.-J.; Lee, S.; Oh, P.; Kim, Y.; Cho, J. High Performance LiMn2O4 Cathode Materials Grown with Epitaxial Layered Nanostructure for Li-Ion Batteries. Nano Lett. 2014, 14, 993–999. [Google Scholar] [CrossRef]
- Yoshio, M.; Wang, H.; Fukuda, K.; Umeno, T.; Abe, T.; Ogumi, Z. Improvement of natural graphite as a lithium-ion battery anode material, from raw flake to carbon-coated sphere. J. Mater. Chem. 2004, 14, 1754–1758. [Google Scholar] [CrossRef]
- Han, Z.J.; Yabuuchi, N.; Shimomura, K.; Murase, M.; Yui, H.; Komaba, S. High-capacity Si–graphite composite electrodes with a self-formed porous structure by a partially neutralized polyacrylate for Li-ion batteries. Energy Environ. Sci. 2012, 5, 9014–9020. [Google Scholar] [CrossRef]
- Xiang, H.F.; Zhang, X.; Jin, Q.Y.; Zhang, C.P.; Chen, C.H.; Ge, X.W. Effect of capacity matchup in the LiNi0.5Mn1.5O4 /Li4Ti5O12 cells. J. Power Sources 2018, 183, 355–360. [Google Scholar] [CrossRef]
- Ellis, B.L.; Lee, K.T.; Nazar, F.L. Positive Electrode Materials for Li-Ion and Li-Batteries. Chem. Mater. 2010, 22, 691–714. [Google Scholar] [CrossRef]
- Zhu, Y.; You, J.; Huang, H.; Li, G.; Zhou, W.; Guo, J. Facile synthesis and electrochemical properties of layered Li[Ni1/3Mn1/3Co1/3]O2 as cathode materials for lithium-ion batteries. Front. Mater. Sci. 2017, 11, 155–161. [Google Scholar] [CrossRef]
- Xie, Y.; Saubanere, M.; Doublet, M.L. Requirements for reversible extra-capacity in Li-rich layered oxides for Li-ion batteries. Energy Env. Sci. 2017, 10, 266–274. [Google Scholar] [CrossRef]
- Hy, S.; Liu, H.; Zhang, M.; Qian, D.; Hwang, B.J.; Meng, Y.S. Performance and design considerations for lithium excess layered oxide positive electrode materials for lithium ion batteries. Energy Environ. Sci. 2016, 9, 1931–1954. [Google Scholar] [CrossRef]
- Jarvis, K.A.; Deng, Z.; Allard, L.F.; Manthiram, A.; Ferreira, P.J. Atomic Structure of a Lithium-Rich Layered Oxide Material for Lithium-Ion Batteries: Evidence of a Solid Solution. Chem. Mater. 2011, 23, 3614–3621. [Google Scholar] [CrossRef]
- Rozier, P.; Tarascon, J.M. Review—Li-Rich Layered Oxide Cathodes for Next-Generation Li-Ion Batteries: Chances and Challenges. J. Electrochem. Soc. 2015, 162, A2490–A2499. [Google Scholar] [CrossRef]
- Kobayashi, G.; Irii, Y.; Matsumoto, F.; Ito, A.; Ohsawa, Y.; Yamamoto, S.; Cui, Y.; Son, J.Y.; Sato, Y. Improving cycling performance of Li-rich layered cathode materials through combination of Al2O3 -based surface modification and stepwise precycling. J. Power Sources 2016, 303, 250–256. [Google Scholar] [CrossRef]
- Zhao, T.; Li, L.; Chen, R.; Wu, H.; Zhang, X.; Chen, S.; Xie, M.; Wu, F.; Lu, J.; Amine, K. Design of surface protective layer of LiF/FeF3 nanoparticles in Li-rich cathode for high-capacity Li-ion batteries. Nano Energy 2015, 15, 164–176. [Google Scholar] [CrossRef]
- Chen, D.; Tu, W.; Chen, M.; Hong, P.; Zhong, X.; Zhu, Y.; Yu, Q.; Li, W. Synthesis and performances of Li-Rich@AlF3 @Graphene as cathode of lithium ion battery. Electrochim. Acta 2016, 193, 45–53. [Google Scholar] [CrossRef]
- Chen, D.; Yu, Q.; Xiang, X.; Chen, M.; Chen, Z.; Song, S.; Xiong, L.; Liao, Y.; Xing, L.; Li, W. Porous layered lithium-rich oxide nanorods: Synthesis and performances as cathode of lithium ion battery. Electrochim. Acta 2015, 154, 83–93. [Google Scholar] [CrossRef]
- Penki, T.R.; Shanmughasundaram, D.; Munichandraiah, N. Porous lithium rich Li1.2Mn0.54Ni0.22Fe0.04O2 prepared by microemulsion route as a high capacity and high rate capability positive electrode material. Electrochim. Acta 2014, 143, 152–160. [Google Scholar] [CrossRef]
- Qian, Y.; Deng, Y.; Shi, Z.; Zhou, Y.; Zhuang, Q.; Chen, G. Sub-micrometer-sized LiMn1.5Ni0.5O4 spheres as high rate cathode materials for long-life lithium ion batteries. Electrochem. Commun. 2018, 27, 92–95. [Google Scholar] [CrossRef]
- Xu, B.; Fell, C.R.; Chi, M.; Meng, Y.S. Identifying surface structural changes in layered Li-excess nickel manganese oxides in high voltage lithium ion batteries: A joint experimental and theoretical study. Energy Environ. Sci. 2011, 4, 2223–2233. [Google Scholar] [CrossRef]
- Xiang, X.; Li, W. Significant influence of insufficient lithium on electrochemical performance of lithium-rich layered oxide cathodes for lithium ion batteries. Electrochim. Acta 2014, 133, 422–427. [Google Scholar] [CrossRef]
- Shi, S.J.; Tu, J.P.; Tang, Y.Y.; Yu, Y.X.; Zhang, Y.Q.; Wang, X.L.; Gu, C.D. Combustion synthesis and electrochemical performance of Li[Li0.2Mn0.54Ni0.13Co0.13]O2 with improved rate capability. J. Power Sources 2013, 228, 14–23. [Google Scholar] [CrossRef]
- Ohzuku, T. Electrochemistry and Structural Chemistry of LiNiO2 (R3m) for 4 Volt Secondary Lithium Cells. J. Electrochem. Soc. 1993, 140, 1862–1870. [Google Scholar] [CrossRef]
- Zhu, Z.; Zhu, L. Synthesis of layered cathode material 0.5Li2MnO3·0.5LiMn1/3Ni1/3Co1/3O2 by an improved co-precipitation method for lithium-ion battery. J. Power Source 2014, 256, 178–182. [Google Scholar] [CrossRef]
- Qiao, Q.Q.; Li, G.R.; Wang, Y.L.; Gao, X.P. To enhance the capacity of Li-rich layered oxides by surface modification with metal–organic frameworks (MOFs) as cathodes for advanced lithium-ion batteries. J. Mater. Chem. A 2016, 4, 4440–4447. [Google Scholar] [CrossRef]
- Xia, Q.; Zhao, X.; Xu, M.; Ding, Z.; Liu, J.; Chen, L.; Ivey, D.; Wei, W. A Li-rich Layered@Spinel@Carbon heterostructured cathode material for high capacity and high rate lithium-ion batteries fabricated via an in situ synchronous carbonization-reduction method. J. Mater. Chem. A 2015, 3, 3995–4003. [Google Scholar] [CrossRef]
- Martha, S.K.; Nanda, J.; Veith, G.M.; Dudney, N.J. Electrochemical and rate performance study of high-voltage lithium-rich composition: Li1.2M 0.525Ni0.175Co0.1O2. J. Power Sources 2012, 199, 220–226. [Google Scholar] [CrossRef]
- Hy, S.; Felix, F.; Rick, J.; Su, W.-N.; Hwang, B.J. Direct In situ Observation of Li2O Evolution on Li-Rich High-Capacity Cathode Material, Li [NixLi(1–2x)/3Mn(2–x)/3] O2 (0 ≤ x ≤ 0.5). J. Am. Chem. Soc. 2014, 136, 999–1007. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiang, X.; Chen, D.; Liao, Y.; Huang, Q.; Li, W. Polyethylene glycol-assisted synthesis of hierarchically porous layered lithium-rich oxide as cathode of lithium ion battery. J. Power Sources 2015, 279, 197–204. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Huang, H.; Zhong, W. Sucrose-Assisted Synthesis of Layered Lithium-Rich Oxide Li[Li0.2Mn0.56Ni0.16Co0.08]O2 as a Cathode of Lithium-Ion Battery. Crystals 2019, 9, 436. https://doi.org/10.3390/cryst9090436
Song X, Huang H, Zhong W. Sucrose-Assisted Synthesis of Layered Lithium-Rich Oxide Li[Li0.2Mn0.56Ni0.16Co0.08]O2 as a Cathode of Lithium-Ion Battery. Crystals. 2019; 9(9):436. https://doi.org/10.3390/cryst9090436
Chicago/Turabian StyleSong, Xueyin, Haifu Huang, and Wei Zhong. 2019. "Sucrose-Assisted Synthesis of Layered Lithium-Rich Oxide Li[Li0.2Mn0.56Ni0.16Co0.08]O2 as a Cathode of Lithium-Ion Battery" Crystals 9, no. 9: 436. https://doi.org/10.3390/cryst9090436



