CuZnSn(SxSe1-x)4 Solar Cell Prepared by the Sol-Gel Method Following a Modified Three-Step Selenization Process
Abstract
:1. Introduction
2. Experimental Details
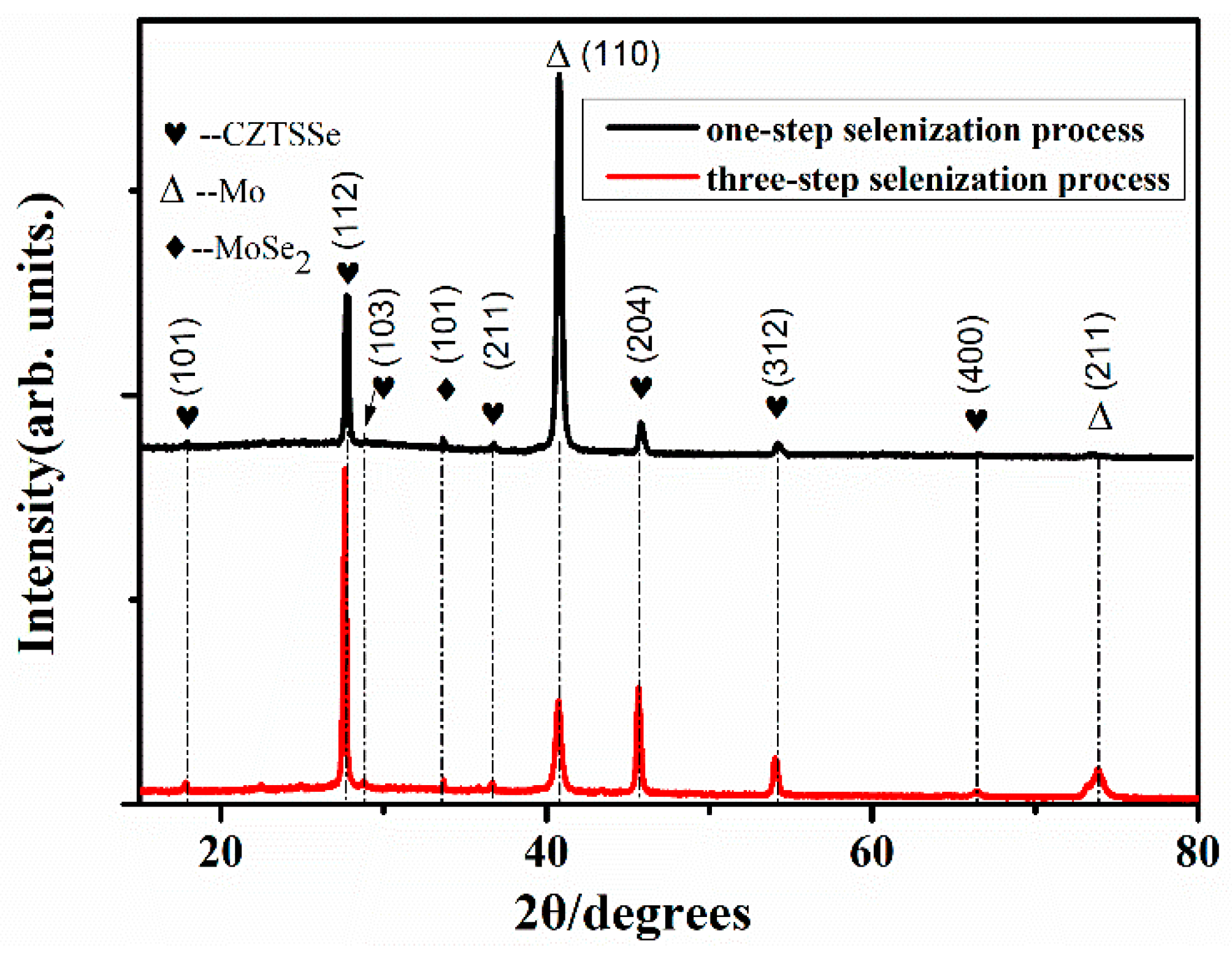
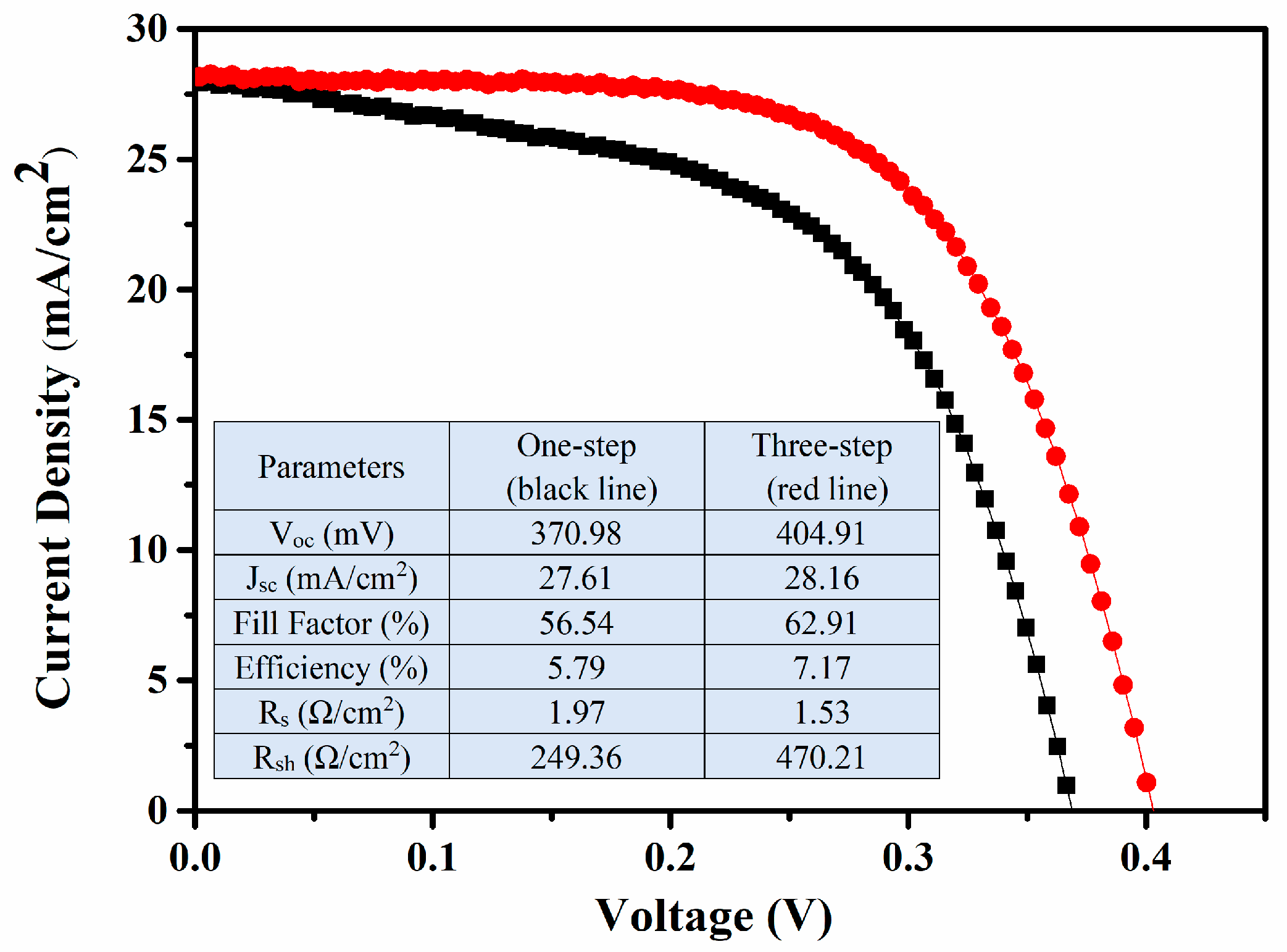
3. Results and Discussions
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burst, J.M.; Duenow, J.N.; Albin, D.S.; Colegrove, E.; Reese, M.O.; Aguiar, J.A.; Jiang, C.S.; Patel, M.K.; Al-Jassim, M.M.; Kuciauskas, D.; et al. CdTe solar cells with open-circuit voltage breaking the 1 V barrier. Nat. Energy 2016, 1, 16015. [Google Scholar] [CrossRef]
- Reinhard, P.; Buecheler, S.; Tiwari, A.N. Technological status of Cu(In,Ga)(Se,S)2-based photovoltaics. Sol. Energy Mater. Sol. Cells 2013, 119, 287–290. [Google Scholar] [CrossRef]
- Wadia, C.; Alivisatos, A.P.; Kammen, D.M. Materials availability expands the opportunity for large-scale photovoltaics deployment. Environ. Sci. Technol. 2009, 43, 2072–2077. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, G.; Tian, Q.; Huang, L.; Gao, S.; Pan, D. Solution-processed Cu2CdSn(S,Se)4 thin film solar cells. Sol. Energy Mater. Sol. Cells 2015, 133, 15–20. [Google Scholar] [CrossRef]
- Pal, K.; Singh, P.; Bhaduri, A.; Thapa, K.B. Current challenges and future prospects for a highly efficient (>20%) kesterite CZTS solar cell: A review. Sol. Energy Mater. Sol. Cells 2019, 196, 138–156. [Google Scholar] [CrossRef]
- Li, J.; Wang, H.; Luo, M.; Tang, J.; Chen, C.; Liu, W.; Liu, F.; Sun, Y.; Han, J.; Zhang, Y. 10% Efficiency Cu2ZnSn(S,Se)4 thin film solar cells fabricated by magnetron sputtering with enlarged depletion region width. Sol. Energy Mater. Sol. Cells 2016, 149, 242–249. [Google Scholar] [CrossRef]
- Lee, Y.S.; Gershon, T.; Gunawan, O.; Todorov, T.K.; Gokmen, T.; Virgus, Y.; Guha, S. Cu2ZnSnSe4 Thin-Film Solar Cells by Thermal Co-evaporation with 11.6% Efficiency and Improved Minority Carrier Diffusion Length. Adv. Energy Mater. 2015, 5, 1401372. [Google Scholar] [CrossRef]
- Yao, L.; Ao, J.; Jeng, M.-J.; Bi, J.; Gao, S.; Sun, G.; He, Q.; Zhou, Z.; Zhang, Y.; Sun, Y.; et al. Reactive Mechanism of Cu2ZnSnSe4 Thin Films Prepared by Reactive Annealing of the Cu/Zn Metal Layer in a SnSex + Se Atmosphere. Crystals 2018, 9, 10. [Google Scholar] [CrossRef]
- Moholkar, A.V.; Shinde, S.S.; Babar, A.R.; Sim, K.-U.; Lee, H.K.; Rajpure, K.Y.; Patil, P.S.; Bhosale, C.H.; Kim, J.H. Synthesis and characterization of Cu2ZnSnS4 thin films grown by PLD: solar cells. J. Alloy. Compd. 2011, 509, 7439–7446. [Google Scholar] [CrossRef]
- Lin, X.; Klenk, R.; Wang, L.; Köhler, T.; Albert, J.; Fiechter, S.; Ennaoui, A.; Lux-Steiner, M.C. 11.3% efficiency Cu(In,Ga)(S,Se)2 thin film solar cells via drop-on-demand inkjet printing. Energy Environ. Sci. 2016, 9, 2037–2043. [Google Scholar] [CrossRef]
- Xin, H.; Katahara, J.K.; Braly, I.L.; Hillhouse, H.W. 8% Efficient Cu2ZnSn(S,Se)4 Solar Cells from Redox Equilibrated Simple Precursors in DMSO. Adv. Energy Mater. 2014, 4, 1301823. [Google Scholar] [CrossRef]
- Haass, S.G.; Diethelm, M.; Werner, M.; Bissig, B.; Romanyuk, Y.E.; Tiwari, A.N. 11.2% Efficient Solution Processed Kesterite Solar Cell with a Low Voltage Deficit. Adv. Energy Mater. 2015, 5, 1500712. [Google Scholar] [CrossRef]
- Wang, W.; Winkler, M.T.; Gunawan, O.; Gokmen, T.; Todorov, T.K.; Zhu, Y.; Mitzi, D.B. Device Characteristics of CZTSSe Thin-Film Solar Cells with 12.6% Efficiency. Adv. Energy Mater. 2014, 4, 1301465. [Google Scholar] [CrossRef]
- Schnabel, T.; Abzieher, T.; Friedlmeier, T.M.; Ahlswede, E. Solution-Based Preparation of Cu2ZnSn(S,Se)4 for Solar Cells-Comparison of SnSe2 and Elemental Se as Chalcogen Source. IEEE J. Photovolt. 2015, 5, 670–675. [Google Scholar] [CrossRef]
- Stanchik, A.V.; Gremenok, V.F.; Juskenas, R.; Tyukhov, I.I.; Tivanov, M.S.; Fettkenhauer, C.; Shvartsman, V.V.; Giraitis, R.; Hagemann, U.; Lupascu, D.C. Effects of selenization time and temperature on the growth of Cu2ZnSnSe4 thin films on a metal substrate for flexible solar cells. Sol. Energy 2019, 178, 142–149. [Google Scholar] [CrossRef]
- Zhao, Y.; Han, X.; Chang, L.; Dong, C.; Li, J.; Yan, X. Effects of selenization conditions on microstructure evolution in solution processed Cu2ZnSn(S,Se)4 solar cells. Sol. Energy Mater. Sol. Cells 2019, 195, 274–279. [Google Scholar] [CrossRef]
- Yao, L.; Ao, J.; Jeng, M.J.; Bi, J.; Gao, S.; He, Q.; Zhou, Z.; Sun, G.; Sun, Y.; Chang, L.B.; et al. CZTSe solar cells prepared by electrodeposition of Cu-Sn-Zn stack layer followed by selenization at low Se pressure. Nanoscale Res. Lett. 2014, 9, 678. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yu, S.; Gong, Y.; Yan, W.; Zhang, R.; Liu, S.; Huang, W.; Xin, H. 10.3% Efficient CuIn(S,Se)2 Solar Cells from DMF Molecular Solution with the Absorber Selenized under High Argon Pressure. Sol. RRL 2018, 2, 1800044. [Google Scholar] [CrossRef]
- Clark, J.A.; Uhl, A.R.; Martin, T.R.; Hillhouse, H.W. Evolution of Morphology and Composition during Annealing and Selenization in Solution-Processed Cu2ZnSn(S,Se)4. Chem. Mater. 2017, 29, 9328–9339. [Google Scholar] [CrossRef]
- Scragg, J.J.; Watjen, J.T.; Edoff, M.; Ericson, T.; Kubart, T.; Platzer-Bjorkman, C. A detrimental reaction at the molybdenum back contact in Cu2ZnSn(S,Se)4 thin-film solar cells. J. Am. Chem. Soc. 2012, 134, 19330–19333. [Google Scholar] [CrossRef]
- Jeon, J.O.; Lee, K.D.; Seul Oh, L.; Seo, S.W.; Lee, D.K.; Kim, H.; Jeong, J.H.; Ko, M.J.; Kim, B.; Son, H.J.; et al. Highly efficient copper-zinc-tin-selenide (CZTSe) solar cells by electrodeposition. ChemSusChem 2014, 7, 1073–1077. [Google Scholar] [CrossRef] [PubMed]
- Shin, B.; Bojarczuk, N.A.; Guha, S. On the kinetics of MoSe2 interfacial layer formation in chalcogen-based thin film solar cells with a molybdenum back contact. Appl. Phys. Lett. 2013, 102, 091907. [Google Scholar] [CrossRef]
- Bucherl, C.N.; Oleson, K.R.; Hillhouse, H.W. Thin film solar cells from sintered nanocrystals. Curr. Opin. Chem. Eng. 2013, 2, 168–177. [Google Scholar] [CrossRef]
- Weber, A.; Mainz, R.; Schock, H.W. On the Sn loss from thin films of the material system Cu–Zn–Sn–S in high vacuum. J. Appl. Phys. 2010, 107, 013516. [Google Scholar] [CrossRef]
- Bree, G.; Coughlan, C.; Geaney, H.; Ryan, K.M. Investigation into the Selenization Mechanisms of Wurtzite CZTS Nanorods. ACS Appl. Mater. Interfaces 2018, 10, 7117–7125. [Google Scholar] [CrossRef] [PubMed]
- Holzwarth, U.; Gibson, N. The Scherrer equation versus the ‘Debye-Scherrer equation’. Nat. Nanotechnol. 2011, 6, 534. [Google Scholar] [CrossRef] [PubMed]
- Gokmen, T.; Gunawan, O.; Todorov, T.K.; Mitzi, D.B. Band tailing and efficiency limitation in kesterite solar cells. Appl. Phys. Lett. 2013, 103, 103506. [Google Scholar] [CrossRef]
- Temgoua, S.; Bodeux, R.; Naghavi, N. Influence of the annealing atmosphere and precursor’s thickness on the properties of CZTSSe based solar cells. Sol. Energy Mater. Sol. Cells 2019, 191, 123–132. [Google Scholar] [CrossRef]




| Sample | Element Compositions (%) | Composition Ratios | ||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Zn | Sn | S | Se | Cu/(Zn + Sn) | Zn/Sn | ||
| Precursor film | 23.81 | 15.55 | 14.86 | 45.78 | - | 0.78 | 1.05 | |
| Selenide films | One-step | 20.94 | 13.92 | 11.71 | 5.13 | 48.31 | 0.82 | 1.19 |
| Three-step | 21.07 | 13.97 | 12.03 | 5.79 | 47.15 | 0.81 | 1.16 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Tang, C.; Zhang, C.; Li, G.; Zhao, Y.; Li, W.; Chen, G.; Yang, T. CuZnSn(SxSe1-x)4 Solar Cell Prepared by the Sol-Gel Method Following a Modified Three-Step Selenization Process. Crystals 2019, 9, 474. https://doi.org/10.3390/cryst9090474
Shen X, Tang C, Zhang C, Li G, Zhao Y, Li W, Chen G, Yang T. CuZnSn(SxSe1-x)4 Solar Cell Prepared by the Sol-Gel Method Following a Modified Three-Step Selenization Process. Crystals. 2019; 9(9):474. https://doi.org/10.3390/cryst9090474
Chicago/Turabian StyleShen, Xunan, Chengchun Tang, Chao Zhang, Guanda Li, Yue Zhao, Wei Li, Guifeng Chen, and Tie Yang. 2019. "CuZnSn(SxSe1-x)4 Solar Cell Prepared by the Sol-Gel Method Following a Modified Three-Step Selenization Process" Crystals 9, no. 9: 474. https://doi.org/10.3390/cryst9090474





