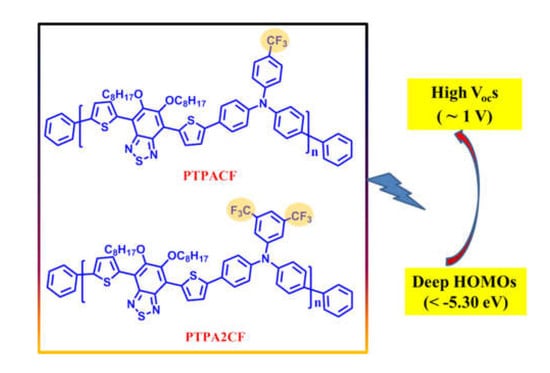
Trifluoromethyl-Substituted Large Band-Gap Polytriphenylamines for Polymer Solar Cells with High Open-Circuit Voltages
Abstract
:1. Introduction
2. Experimental Section
2.1. Characterization and Instrumentation
2.2. Device Fabrication
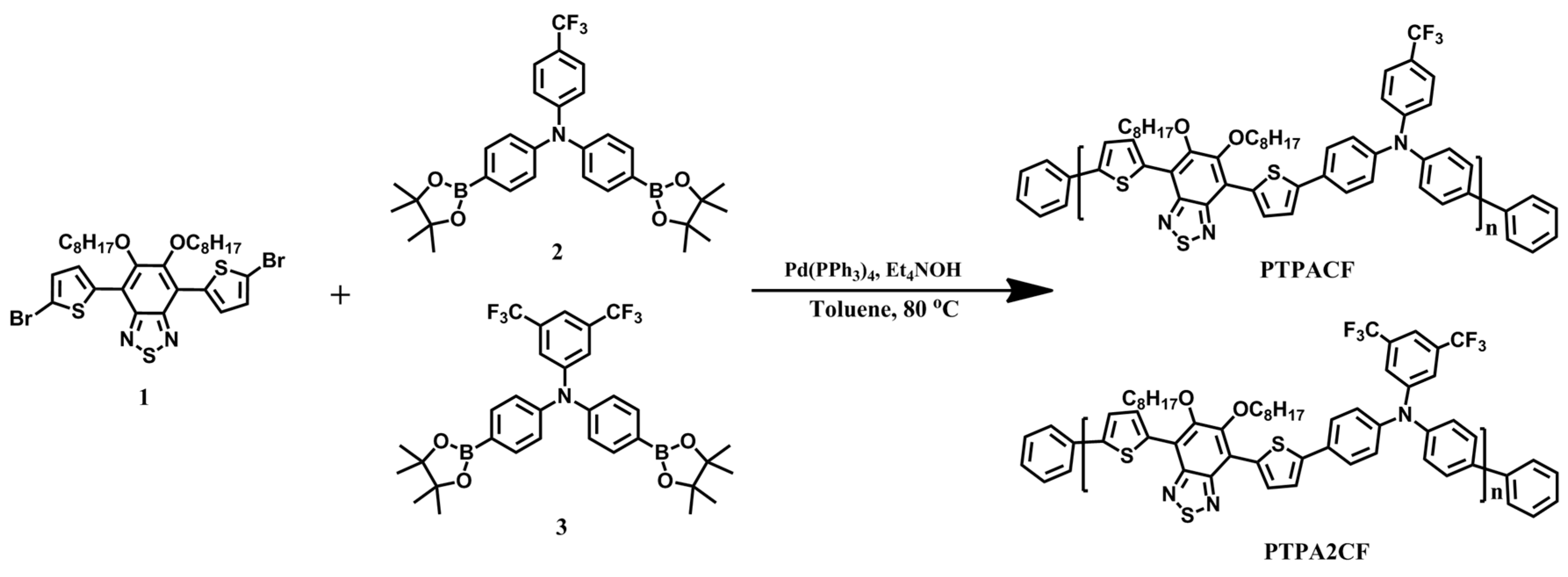
2.3. Synthesis of Monomers and Polymers
2.3.1. General Procedure for Preparing Polymers
2.3.2. Poly(N,N-diphenyl-4-(trifluoromethyl)aniline-alt-4,7di(thiophen-2-yl)-5,6-bis(octyloxy)benzothiadiazole) PTPACF
2.3.3. Poly(N,N-diphenyl-3,5-bis(trifluoromethyl)aniline-alt-4,7di(thiophen-2-yl)-5,6-bis(octyloxy)benzothiadiazole) PTPA2CF
3. Results and Discussion
3.1. Synthesis
3.2. Thermal Stability
3.3. UV-vis Absorption and Electrochemical Properties
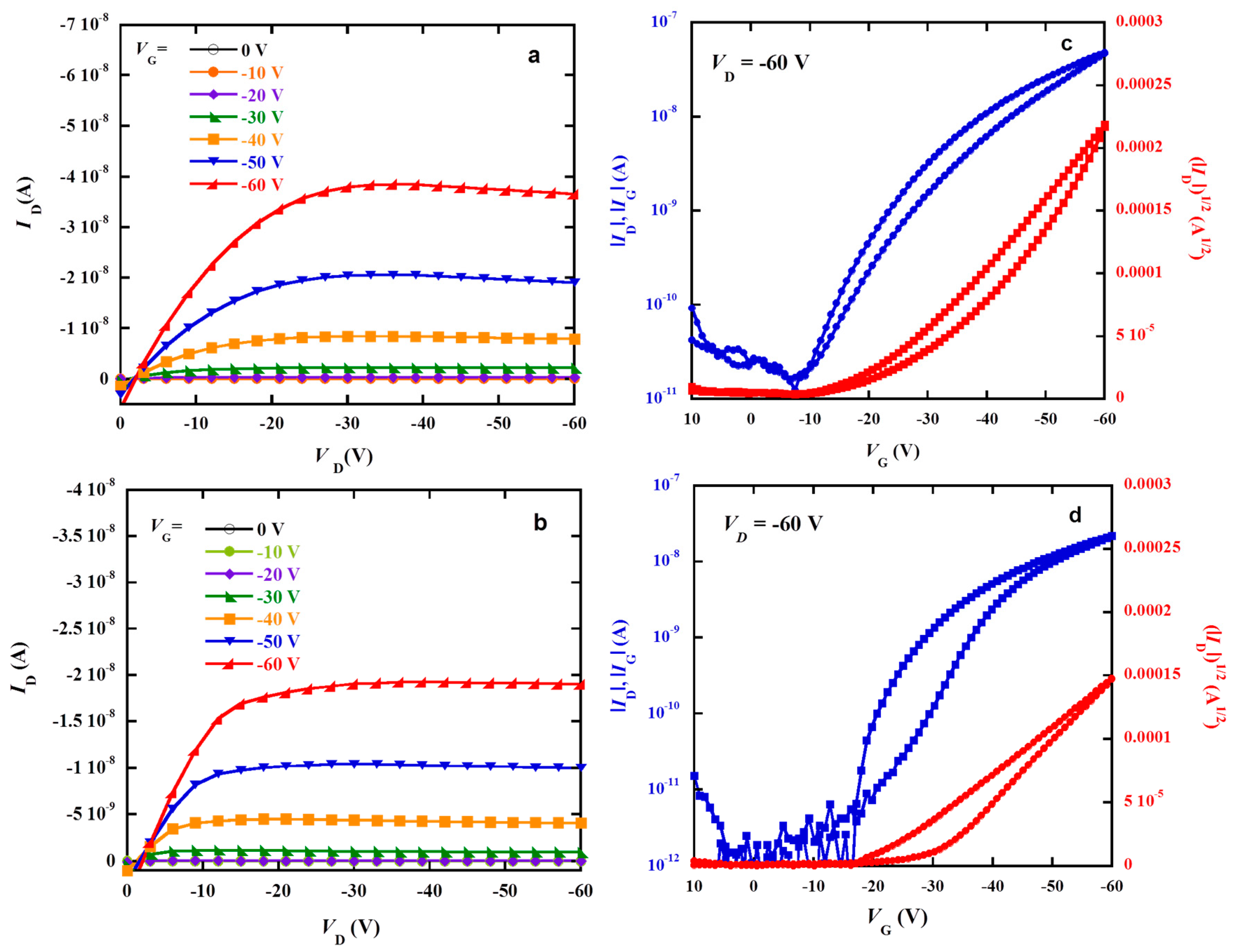
3.4. Hole Mobility
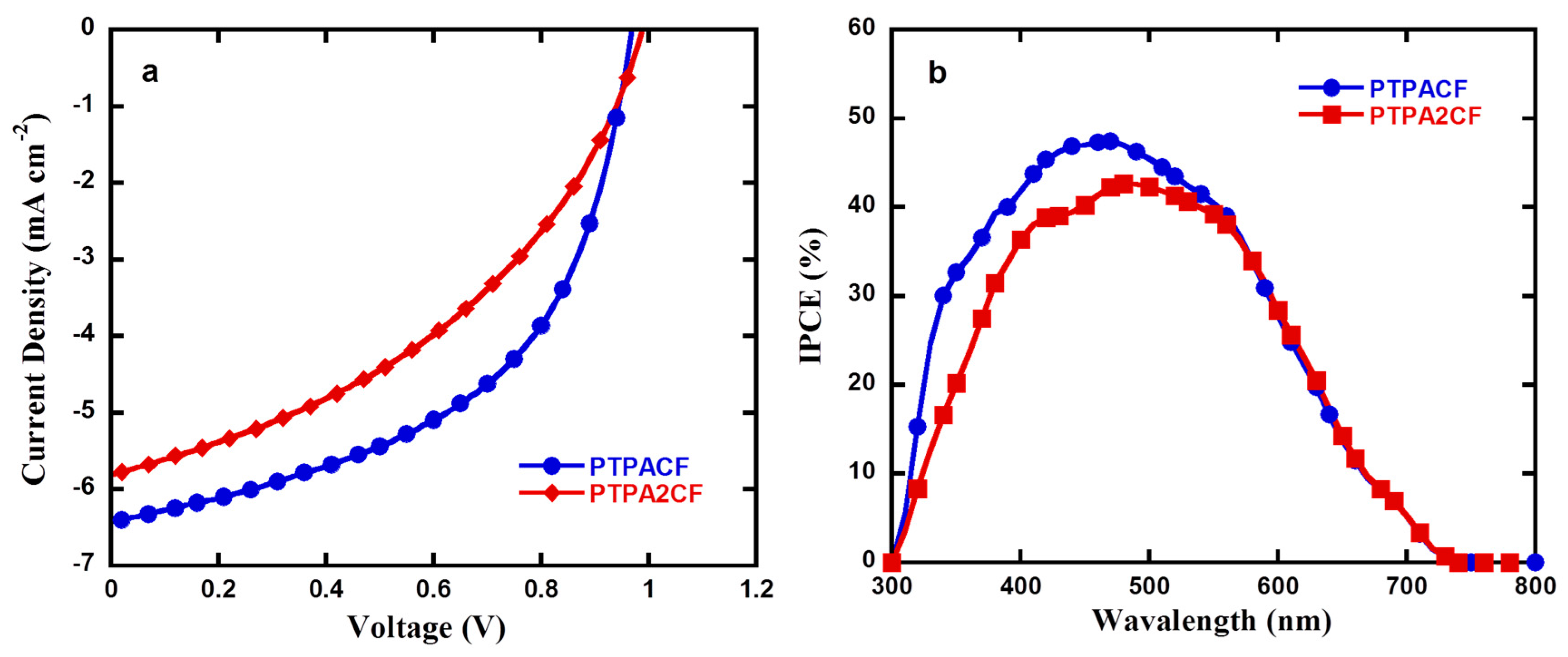
3.5. Photovoltaic Performance
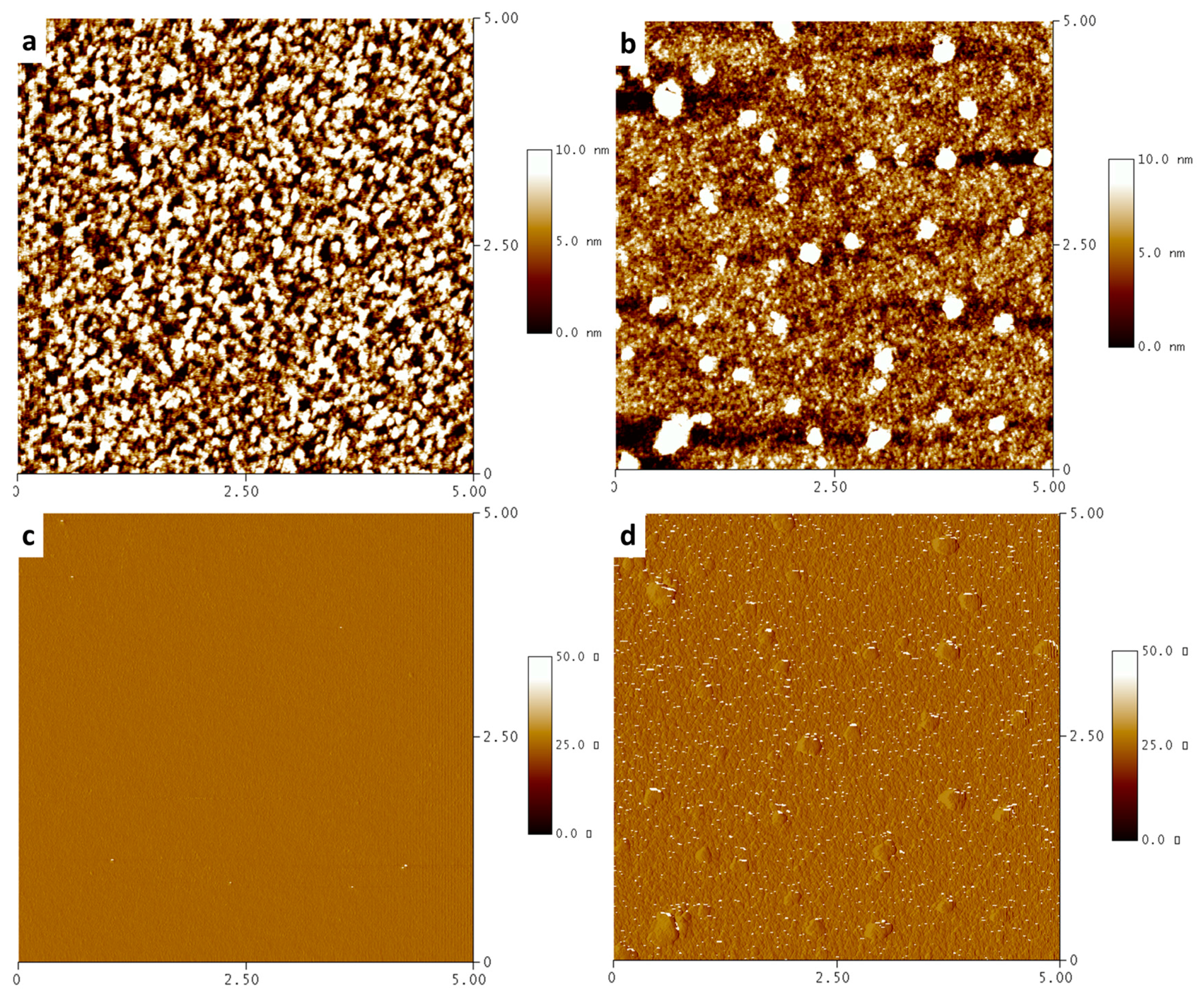
3.6. Atomic Force Microscopy (AFM) Morphologies
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sivakov, V.; Andrä, G.; Gawlik, A.; Berger, A.; Plentz, J.; Falk, F.; Christiansen, S.H. Silicon nanowire-based solar cells on glass: Synthesis, optical properties, and cell parameters. Nano Lett. 2009, 9, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Dou, L.; You, J.; Hong, Z.; Xu, Z.; Li, G.; Street, R.A.; Yang, Y. 25th anniversary article: A decade of organic/polymeric photovoltaic research. Adv. Mater. 2013, 25, 6642–6671. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Yang, S.; Hsu, C. Synthesis of conjugated polymers for organic solar cell applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, R.; Yang, Y. Polymer solar cells. Nat. Photonics 2012, 6, 153–161. [Google Scholar] [CrossRef]
- Helgesen, M.; Søndergaard, R.; Krebs, F.C. Advanced materials and processes for polymer solar cell devices. J. Mater. Chem. 2010, 20, 36–60. [Google Scholar] [CrossRef]
- Wong, W.; Ho, C. Organometallic photovoltaics: A new and versatile approach for harvesting solar energy using conjugated polymetallaynes. Acc. Chem. Res. 2010, 43, 1246–1256. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Jia, X.; Ding, L. Ternary organic solar cells offer 14% power conversion efficiency. Sci. Bull. 2017, 62, 1562–1564. [Google Scholar] [CrossRef]
- Dennler, G.; Scharber, M.C.; Brabec, C.J. Polymer-fullerene bulk-heterojunction solar cells. Adv. Mater. 2009, 21, 1323–1338. [Google Scholar] [CrossRef]
- Soci, C.; Hwang, I.W.; Moses, D.; Zhu, Z.; Waller, D.; Gaudiana, R.; Brabec, C.J.; Heeger, A.J. Photoconductivity of a low-bandgap conjugated polymer. Adv. Funct. Mater. 2007, 17, 632–636. [Google Scholar] [CrossRef]
- Wang, Q.; Li, Y.; Song, P.; Su, R.; Ma, F.; Yang, Y. Non-fullerene acceptor-based solar cells: From structural design to interface charge separation and charge transport. Polymers 2017, 9, 692. [Google Scholar] [CrossRef]
- Gao, Y.; Liu, M.; Zhang, Y.; Liu, Z.; Yang, Y.; Zhao, L. Recent development on narrow bandgap conjugated polymers for polymer solar cells. Polymers 2017, 9, 39. [Google Scholar] [CrossRef]
- Zhang, B.; Hu, X.; Wang, M.; Xiao, H.; Gong, X.; Yang, W.; Cao, Y. Highly efficient polymer solar cells based on poly(carbazole-alt-thiophene-benzofurazan). New J. Chem. 2012, 36, 242–247. [Google Scholar] [CrossRef]
- Guo, X.; Baumgarten, M.; Müllen, K. Designing π-conjugated polymers for organic electronics. Prog. Polym. Sci. 2013, 38, 1832–1908. [Google Scholar] [CrossRef]
- Zhang, B.; Yu, L.; Fan, L.; Wang, N.; Hu, L.W.; Yang, W. Indolo[3,2-b]carbazole and benzofurazan based narrow band-gap polymers for photovoltaic cells. New J. Chem. 2014, 38, 4587–4593. [Google Scholar] [CrossRef]
- Liang, Y.; Xu, Z.; Xia, J.; Tsai, S.; Wu, Y.; Li, G.; Ray, C.; Yu, L. For the bright future-bulk heterojunction polymer solar cells with power conversion efficiency of 7.4%. Adv. Mater. 2010, 22, E135–E138. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Jhuo, H.; Cheng, Y.; Chen, S. Fullerene derivative-doped zinc oxide nanofilm as the cathode of inverted polymer solar cells with low-bandgap polymer (PTB7-Th) for high performance. Adv. Mater. 2013, 25, 4766–4771. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Xiao, B.; Liu, F.; Wu, H.; Yang, Y.; Xiao, S.; Wang, C.; Russell, T.P.; Cao, Y. Single-junction polymer solar cells with high efficiency and photovoltage. Nat. Photonics 2015, 9, 174–179. [Google Scholar] [CrossRef]
- Lin, Y.; Wang, J.; Zhang, Z.; Bai, H.; Li, Y.; Zhu, D.; Zhan, X. An electron acceptor challenging fullerenes for efficient polymer solar cells. Adv. Mater. 2015, 27, 1170–1174. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; He, Q.; Zhao, F.; Huo, L.; Mai, J.; Lu, X.; Su, C.; Li, T.; Wang, J.; Zhu, J.; et al. A facile planar fused-ring electron acceptor for as-cast polymer solar cells with 8.71% efficiency. J. Am. Chem. Soc. 2016, 138, 2973–2976. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Zhao, F.; Wu, Y.; Chen, K.; Xia, Y.; Li, G.; Prasad, S.K.K.; Zhu, J.; Huo, L.; Bin, H.; et al. Mapping polymer donors toward high-efficiency fullerene free organic solar cells. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [PubMed]
- Bin, H.; Gao, L.; Zhang, Z.; Yang, Y.; Zhang, Y.; Zhang, C.; Chen, S.; Xue, L.; Yang, C.; Xiao, M.; et al. 11.4% Efficiency non-fullerene polymer solar cells with trialkylsilyl substituted 2D-conjugated polymer as donor. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Qian, D.; Zhang, S.; Li, S.; Inganäs, O.; Gao, F.; Hou, J. Fullerene-free polymer solar cells with over 11% efficiency and excellent thermal stability. Adv. Mater. 2016, 28, 4734–4739. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ye, L.; Zhao, W.; Zhang, S.; Mukherjee, S.; Ade, H.; Hou, J. Energy-level modulation of small-molecule electron acceptors to achieve over 12% efficiency in polymer solar cells. Adv. Mater. 2016, 28, 9423–9429. [Google Scholar] [CrossRef] [PubMed]
- Roquet, S.; Cravino, A.; Leriche, P.; Alévêque, O.; Frère, P.; Roncali, J. Triphenylamine-thienylenevinylene hybrid systems with internal charge transfer as donor materials for heterojunction solar cells. J. Am. Chem. Soc. 2006, 128, 3459–3466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Cai, W.; Niu, H.; Wang, W.; Bai, X.; Hou, Y. Novel polyamides with 5H-dibenzo[b,f]azepin-5-yl-substituted triphenylamine: Synthesis and visible-NIR electrochromic properties. Polymers 2017, 9, 542. [Google Scholar] [CrossRef]
- Hancock, J.M.; Gifford, A.P.; Zhu, Y.; Lou, Y.; Jenekhe, S.A. n-Type conjugated oligoquinoline and oligoquinoxaline with triphenylamine endgroups: Efficient ambipolar light emitters for device applications. Chem. Mater. 2006, 18, 4924–4932. [Google Scholar] [CrossRef]
- Zhang, B.; Liang, J.; Hu, L.; Peng, F.; Chen, G.; Yang, W. Triphenylamine-based broad band-gap polymers for bulk-heterojunction polymer solar cells. J. Mater. Sci. 2015, 50, 5609–5619. [Google Scholar] [CrossRef]
- Luo, Z.; Xiong, W.; Liu, T.; Cheng, W.; Wu, K.; Sun, Y.; Yang, C. Triphenylamine-cored star-shape compounds as non-fullerene acceptor for high-efficiency organic solar cells: Tuning the optoelectronic properties by S/Se-annulated perylene diimide. Org. Electron. 2017, 41, 166–172. [Google Scholar] [CrossRef]
- Zhang, B.; Chen, G.; Xu, J.; Hu, L.; Yang, W. Feasible energy level tuning in polymer solar cells based on broad band-gap polytriphenylamine derivatives. New J. Chem. 2016, 4, 402–412. [Google Scholar] [CrossRef]
- Chen, G.; Zhang, F.; Liu, M.; Song, J.; Lian, J.; Zeng, P.; Yip, H.; Yang, W.; Zhang, B.; Cao, Y. Fabrication of high-performance and low-hysteresis lead halide perovskite solar cells by utilizing a versatile alcohol-soluble bispyridinium salt as an efficient cathode modifier. J. Mater. Chem. A 2017, 5, 17943–17953. [Google Scholar] [CrossRef]
- Griffini, G.; Douglas, J.D.; Piliego, C.; Holcombe, T.W.; Turri, S.; Fréchet, J.M.J.; Mynar, J.L. Long-term thermal stability of high-efficiency polymer solar cells based on photocrosslinkable donor-acceptor conjugated polymers. Adv. Mater. 2011, 23, 1660–1664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Müller, C. On the glass transition of polymer semiconductors and its impact on polymer solar cell stability. Chem. Mater. 2015, 27, 2740–2754. [Google Scholar] [CrossRef]
- Holmes, N.P.; Vaughan, B.; Williams, E.L.; Kroon, R.; Anderrson, M.R.; Kilcoyne, A.L.D.; Sonar, P.; Zhou, X.J.; Dastoor, P.C.; Belcher, W.J. Diketopyrrolopyrrole-based polymer:fullerene nanoparticle films with thermally stable morphology for organic photovoltaic applications. MRS Commun. 2017, 7, 67–73. [Google Scholar] [CrossRef]
- Hedström, S.; Henriksson, P.; Wang, E.; Andersson, M.R.; Persson, P. Light-harvesting capabilities of low band gap donor-acceptor polymers. Phys. Chem. Chem. Phys. 2014, 16, 24853–24865. [Google Scholar] [CrossRef] [PubMed]
- Surin, M.; Hennebicq, E.; Ego, C.; Marsitzky, D.; Grimsdale, A.C.; Müllen, K.; Brédas, J.; Lazzaroni, R.; Leclère, P. Correlation between the microscopic morphology and the solid-state photoluminescence properties in fluorene-based polymers and copolymers. Chem. Mater. 2004, 16, 994–1001. [Google Scholar] [CrossRef]
- Cardona, C.M.; Li, W.; Kaifer, A.E.; Stockdale, D.; Bazan, G.C. Electrochemical considerations for determining absolute frontier orbital energy levels of conjugated polymers for solar cell applications. Adv. Mater. 2011, 23, 2367–2371. [Google Scholar] [CrossRef] [PubMed]
- Cravino, A.; Roquet, S.; Alévêque, O.; Leriche, P.; Frère, P.; Roncali, J. Triphenylamine-oligothiophene conjugated systems as organic semiconductors for opto-electronics. Chem. Mater. 2006, 18, 2584–2590. [Google Scholar] [CrossRef] [Green Version]
- He, Z.; Wu, H.; Cao, Y. Recent advances in polymer solar cells: Realization of high device performance by incorporating water/alcohol-soluble conjugated polymers as electrode buffer layer. Adv. Mater. 2014, 26, 1006–1024. [Google Scholar] [CrossRef] [PubMed]
- Xia, R.; Leem, D.; Kirchartz, T.; Spencer, S.; Murphy, C.; He, Z.; Wu, H.; Su, S.; Cao, Y.; Kim, J.S.; et al. Investigation of a conjugated polyelectrolyte interlayer for inverted polymer:fullerene solar cells. Adv. Energy Mater. 2013, 3, 718–723. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Su, S.; Xu, M.; Wu, H.; Cao, Y. Enhanced power-conversion efficiency in polymer solar cells using an inverted device structure. Nat. Photonics 2012, 6, 593–597. [Google Scholar] [CrossRef]



| Polymers | Mn | Mw | PDI | Td (°C) |
|---|---|---|---|---|
| PTPACF | 9100 | 15,700 | 1.73 | 335 |
| PTPA2CF | 8400 | 12,900 | 1.54 | 338 |
| Polymers | λmax, in chloroform (nm) | λmax, in film (nm) | λonset (nm) | Eg, optical (eV) | Eox (V) | EHOMO (eV) | ELUMO (eV) |
|---|---|---|---|---|---|---|---|
| PTPACF | 363, 498 | 375, 516 | 616 | 2.01 | 0.92 | −5.33 | −3.32 |
| PTPA2CF | 365, 495 | 373, 505 | 600 | 2.07 | 0.97 | −5.38 | −3.31 |
| Polymers | μ (×10−3, cm2 V−1 S−1) | Jsc (mA cm−2) | Voc (V) | FF (%) | PCE (%) |
|---|---|---|---|---|---|
| PTPACF | 2.5 | 6.42 | 0.98 | 52.0 | 3.24 |
| PTPA2CF | 1.1 | 5.81 | 0.99 | 41.8 | 2.40 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, S.; Deng, W.; Sun, S.; Lan, L.; He, Z.; Yang, W.; Zhang, B. Trifluoromethyl-Substituted Large Band-Gap Polytriphenylamines for Polymer Solar Cells with High Open-Circuit Voltages. Polymers 2018, 10, 52. https://doi.org/10.3390/polym10010052
Yi S, Deng W, Sun S, Lan L, He Z, Yang W, Zhang B. Trifluoromethyl-Substituted Large Band-Gap Polytriphenylamines for Polymer Solar Cells with High Open-Circuit Voltages. Polymers. 2018; 10(1):52. https://doi.org/10.3390/polym10010052
Chicago/Turabian StyleYi, Shuwang, Wanyuan Deng, Sheng Sun, Linfeng Lan, Zhicai He, Wei Yang, and Bin Zhang. 2018. "Trifluoromethyl-Substituted Large Band-Gap Polytriphenylamines for Polymer Solar Cells with High Open-Circuit Voltages" Polymers 10, no. 1: 52. https://doi.org/10.3390/polym10010052