Facile and Rapid Formation of Giant Vesicles from Glass Beads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
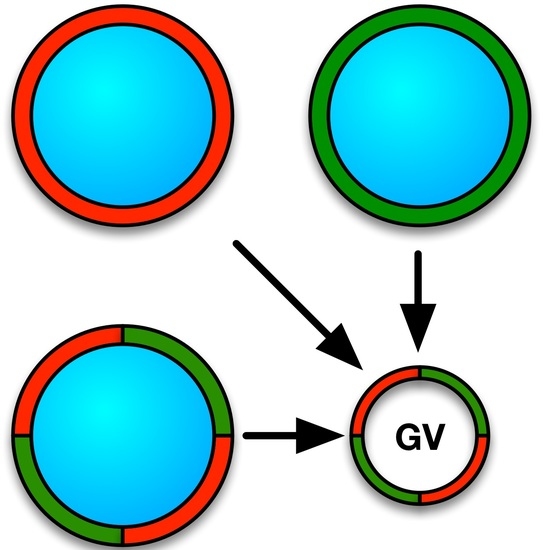
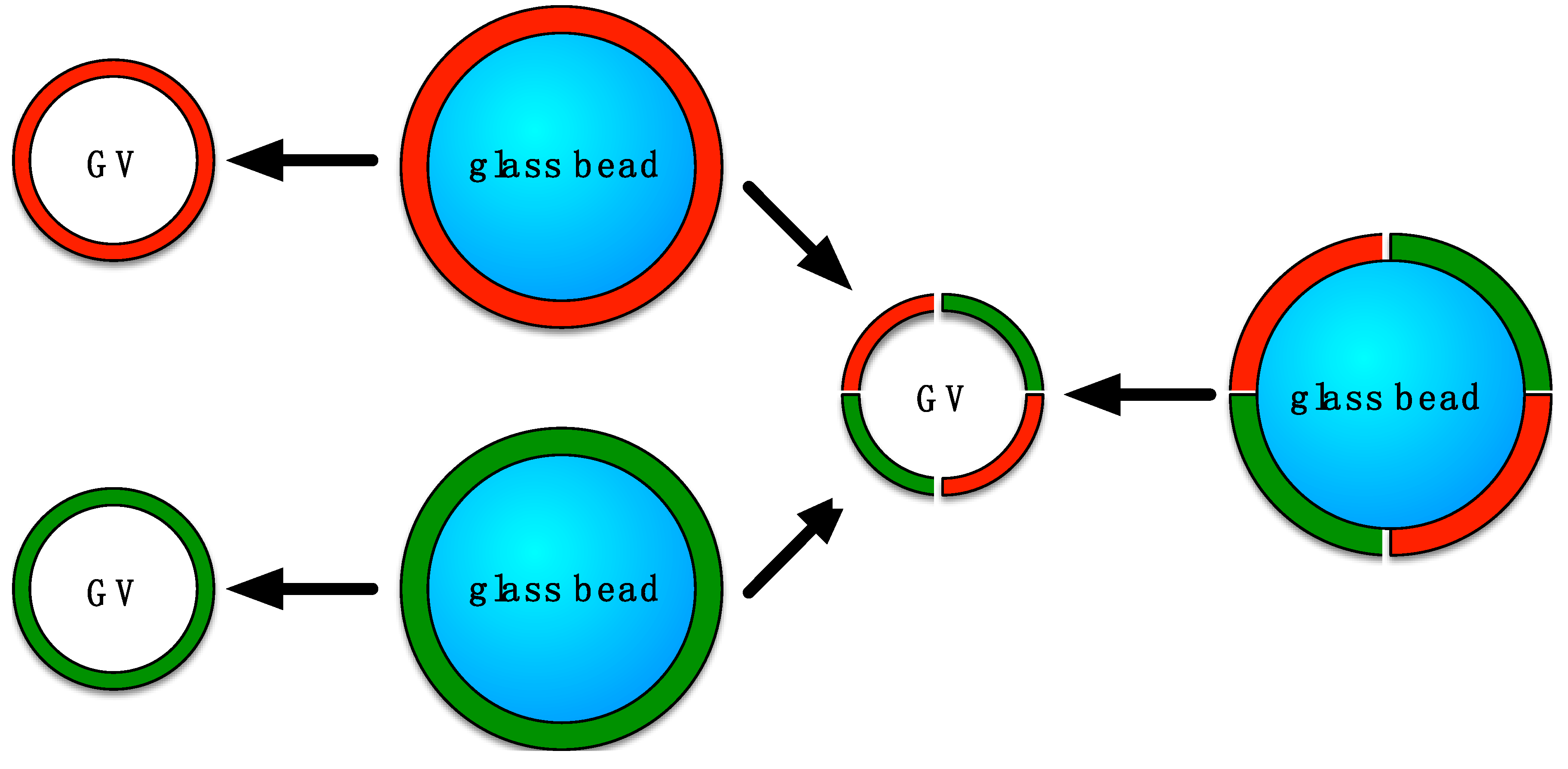
2.2. Preparation of Glass Beads
2.3. Liposome Formulation on Glass Beads
2.4. Preparation of Liposomes by Bead Gentle Hydration or by Electroformation
2.5. Microscopy Experiments
2.6. Generalized Polarization Experiment
3. Results and Discussion
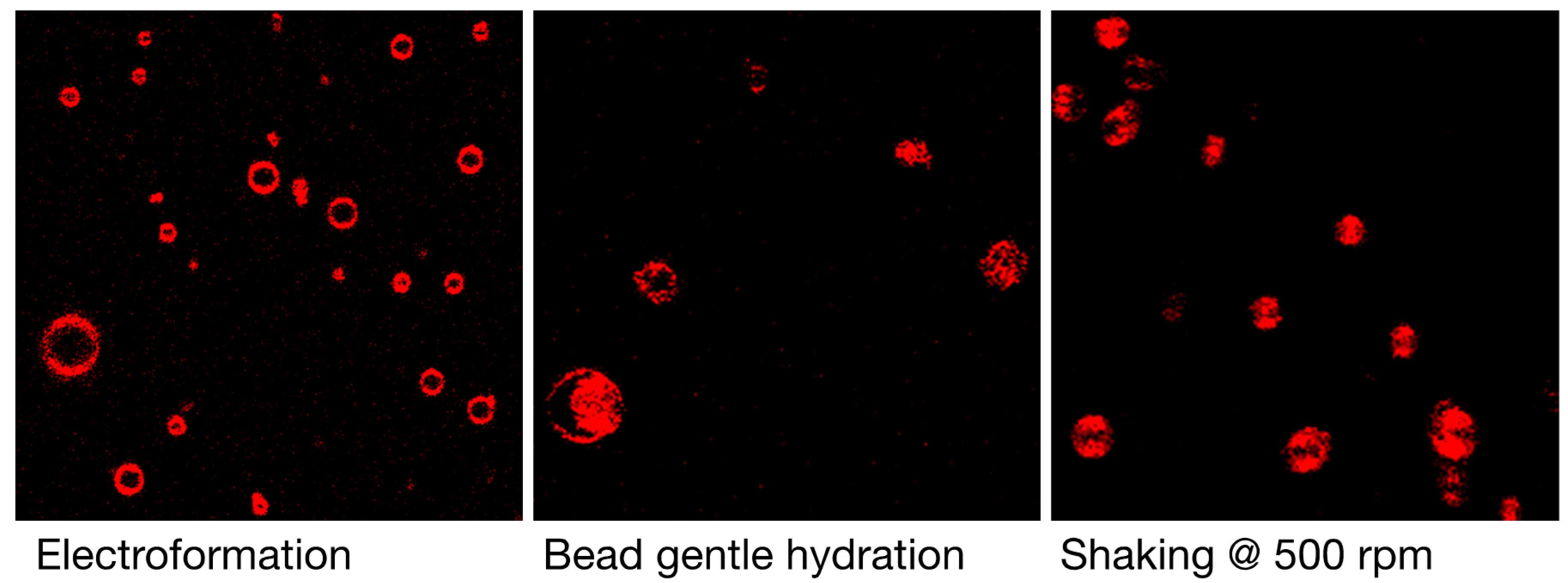
3.1. Qualitative and Quantitative Comparison of Different GV Formulation Techniques
3.2. GV Formulation in High Salt Conditions
3.3. Formulation of GVs from Phospholipid Mixtures in Pure Water
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Reeves, J.P.; Dowben, R.M. Formation and properties of thin-walled phospholipid vesicles. J. Cell. Physiol. 1969, 73, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Akashi, K.; Miyata, H.; Itoh, H.; Kinosita, K. Preparation of giant liposomes in physiological conditions and their characterization under an optical microscope. Biophys. J. 1996, 71, 3242–3250. [Google Scholar] [CrossRef]
- Angelova, M.I.; Dimitrov, D.S. Liposome electroformation. Faraday Discuss. 1986, 81, 303–311. [Google Scholar] [CrossRef]
- Estes, D.J.; Mayer, M. Giant liposomes in physiological buffer using electroformation in a flow chamber. Biochim. Biophys. Acta 2005, 1712, 152–160. [Google Scholar] [CrossRef] [PubMed]
- Pott, T.; Bouvrais, H.; Meleard, P. Giant unilamellar vesicle formation under physiologically relevant conditions. Chem. Phys. Lipids 2008, 154, 115–119. [Google Scholar] [CrossRef] [PubMed]
- Morales-Penningston, N.F.; Wu, J.; Farkas, E.R.; Goh, S.L.; Konyakhina, T.M.; Zheng, J.Y.; Webb, W.W.; Feigenson, G.W. Guv preparation and imaging: Minimizing artifacts. Biochim. Biophys. Acta 2010, 1798, 1324–1332. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.; Tanasescu, R.; Stefaniu, C.; Fedotenko, L.A.; Favarger, F.; Ishikawa, T.; Brezesinski, G.; Marques, C.M.; Zumbuehl, A. Bilayer properties of 1,3-diamidophospholipids. Langmuir 2015, 31, 1879–1884. [Google Scholar] [CrossRef] [PubMed]
- Horger, K.S.; Estes, D.J.; Capone, R.; Mayer, M. Films of agarose enable rapid formation of giant liposomes in solutions of physiologic ionic strength. J. Am. Chem. Soc. 2009, 131, 1810–1819. [Google Scholar] [CrossRef] [PubMed]
- Tsai, F.C.; Stuhrmann, B.; Koenderink, G.H. Encapsulation of active cytoskeletal protein networks in cell-sized liposomes. Langmuir 2011, 27, 10061–10071. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, A.; Tsai, F.C.; Koenderink, G.H.; Schmidt, T.F.; Itri, R.; Meier, W.; Schmatko, T.; Schroder, A.; Marques, C. Gel-assisted formation of giant unilamellar vesicles. Biophys. J. 2013, 105, 154–164. [Google Scholar] [CrossRef] [PubMed]
- Kresse, K.M.; Xu, M.; Pazzi, J.; Garcia-Ojeda, M.; Subramaniam, A.B. Novel application of cellulose paper as a platform for the macromolecular self-assembly of biomimetic giant liposomes. ACS Appl. Mater. Interfaces 2016, 8, 32102–32107. [Google Scholar] [CrossRef] [PubMed]
- Shum, H.C.; Lee, D.; Yoon, I.; Kodger, T.; Weitz, D.A. Double emulsion templated monodisperse phospholipid vesicles. Langmuir 2008, 24, 7651–7653. [Google Scholar] [CrossRef] [PubMed]
- Abkarian, M.; Loiseau, E.; Massiera, G. Continuous droplet interface crossing encapsulation (cdice) for high throughput monodisperse vesicle design. Soft Matter 2011, 7, 4610–4614. [Google Scholar] [CrossRef]
- Pautot, S.; Frisken, B.J.; Weitz, D.A. Production of unilamellar vesicles using an inverted emulsion. Langmuir 2003, 19, 2870–2879. [Google Scholar] [CrossRef]
- Pontani, L.L.; van der Gucht, J.; Salbreux, G.; Heuvingh, J.; Joanny, J.F.; Sykes, C. Reconstitution of an actin cortex inside a liposome. Biophys. J. 2009, 96, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, J.C.; Richmond, D.L.; Li, T.H.; Liu, A.P.; Parekh, S.H.; Fletcher, D.A. Unilamellar vesicle formation and encapsulation by microfluidic jetting. Proc. Natl. Acad. Sci. USA 2008, 105, 4697–4702. [Google Scholar] [CrossRef] [PubMed]
- Bastrop, M.; Meister, A.; Metz, H.; Drescher, S.; Dobner, B.; Mader, K.; Blume, A. The motional dynamics in bolaamphiphilic nanofibers and micellar aggregates: An esr spin probe study. J. Phys. Chem. B 2009, 113, 574–582. [Google Scholar] [CrossRef] [PubMed]
- Beck, P.; Liebi, M.; Kohlbrecher, J.; Ishikawa, T.; Ruegger, H.; Fischer, P.; Walde, P.; Windhab, E. Novel type of bicellar disks from a mixture of dmpc and dmpe-dtpa with complexed lanthanides. Langmuir 2010, 26, 5382–5387. [Google Scholar] [CrossRef] [PubMed]
- White, S.H. Solvent-free planar bilayer membranes—Novel method of formation. Biophys. J. 1978, 21, A123. [Google Scholar]
- Campillo, C.; Sens, P.; Koster, D.; Pontani, L.L.; Levy, D.; Bassereau, P.; Nassoy, P.; Sykes, C. Unexpected membrane dynamics unveiled by membrane nanotube extrusion. Biophys. J. 2013, 104, 1248–1256. [Google Scholar] [CrossRef] [PubMed]
- Karamdad, K.; Law, R.V.; Seddon, J.M.; Brooks, N.J.; Ces, O. Preparation and mechanical characterisation of giant unilamellar vesicles by a microfluidic method. Lab Chip 2015, 15, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Bayerl, T.M.; Bloom, M. Physical-properties of single phospholipid-bilayers adsorbed to micro glass-beads—A new vesicular model system studied by H-2-nuclear magnetic-resonance. Biophys. J. 1990, 58, 357–362. [Google Scholar] [CrossRef]
- Nourian, Z.; Roelofsen, W.; Danelon, C. Triggered gene expression in fed-vesicle microreactors with a multifunctional membrane. Angew. Chem. Int. Ed. 2012, 51, 3114–3118. [Google Scholar] [CrossRef] [PubMed]
- Parasassi, T.; Gratton, E.; Yu, W.M.; Wilson, P.; Levi, M. Two-photon fluorescence microscopy of laurdan generalized polarization domains in model and natural membranes. Biophys. J. 1997, 72, 2413–2429. [Google Scholar] [CrossRef]
- Bagatolli, L.A.; Gratton, E. Two photon fluorescence microscopy of coexisting lipid domains in giant unilamellar vesicles of binary phospholipid mixtures. Biophys. J. 2000, 78, 290–305. [Google Scholar] [CrossRef]
- Nagle, J.F.; Tristram-Nagle, S. Lipid bilayer structure. Curr. Opin. Struct. Biol. 2000, 10, 474–480. [Google Scholar] [CrossRef]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. Nih image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Rueden, C.T.; Hiner, M.C.; Eliceiri, K.W. The imagej ecosystem: An open platform for biomedical image analysis. Mol. Reprod. Dev. 2015, 82, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Sezgin, E.; Waithe, D.; Bernardino de la Serna, J.; Eggeling, C. Spectral imaging to measure heterogeneity in membrane lipid packing. ChemPhysChem 2015, 16, 1387–1394. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, S.A.; Tricerri, M.A.; Gunther, G.; Gratton, E. Laurdan generalized polarization: From cuvette to microscope. In Modern Research and Educational Topics in Microscopy; Méndez-Vilas, A., Diaz, J., Eds.; Formatex: Badajoz, Spain, 2007. [Google Scholar]
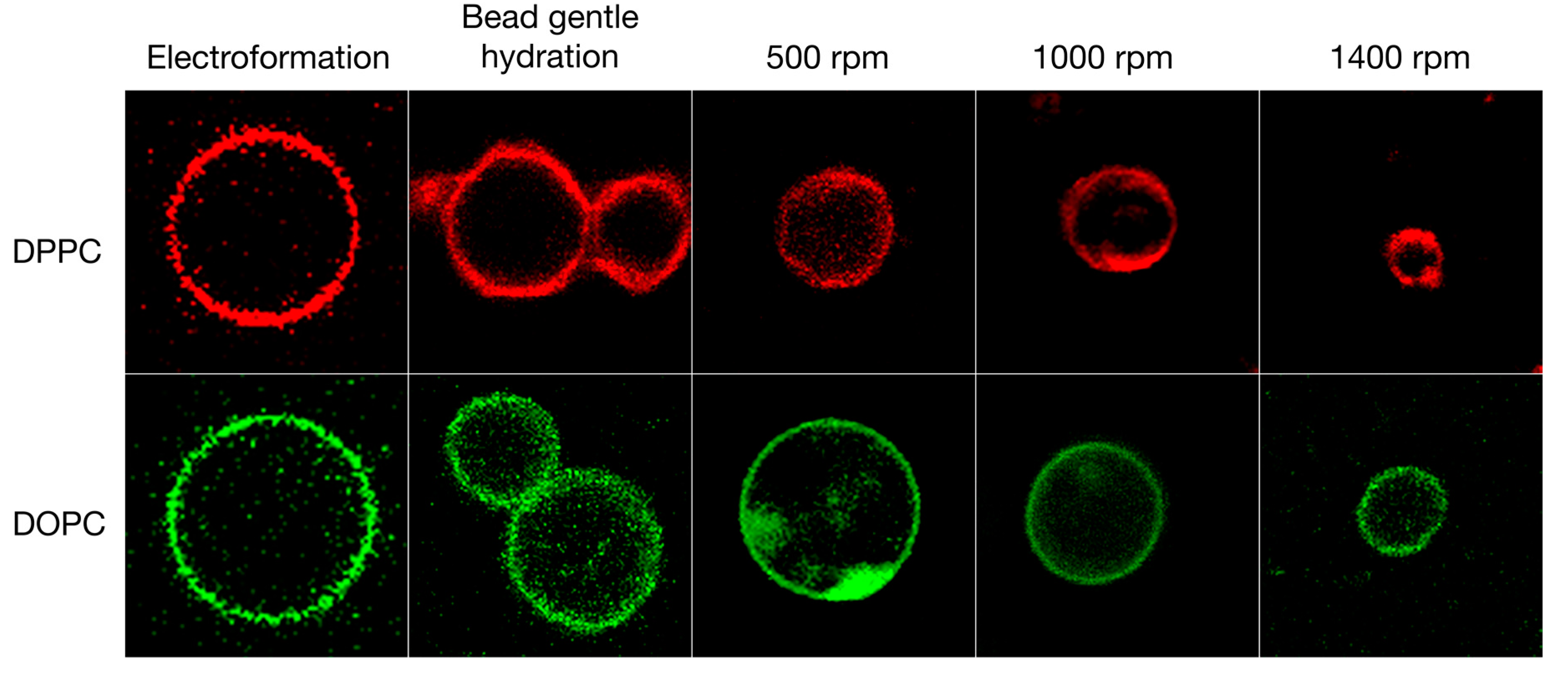
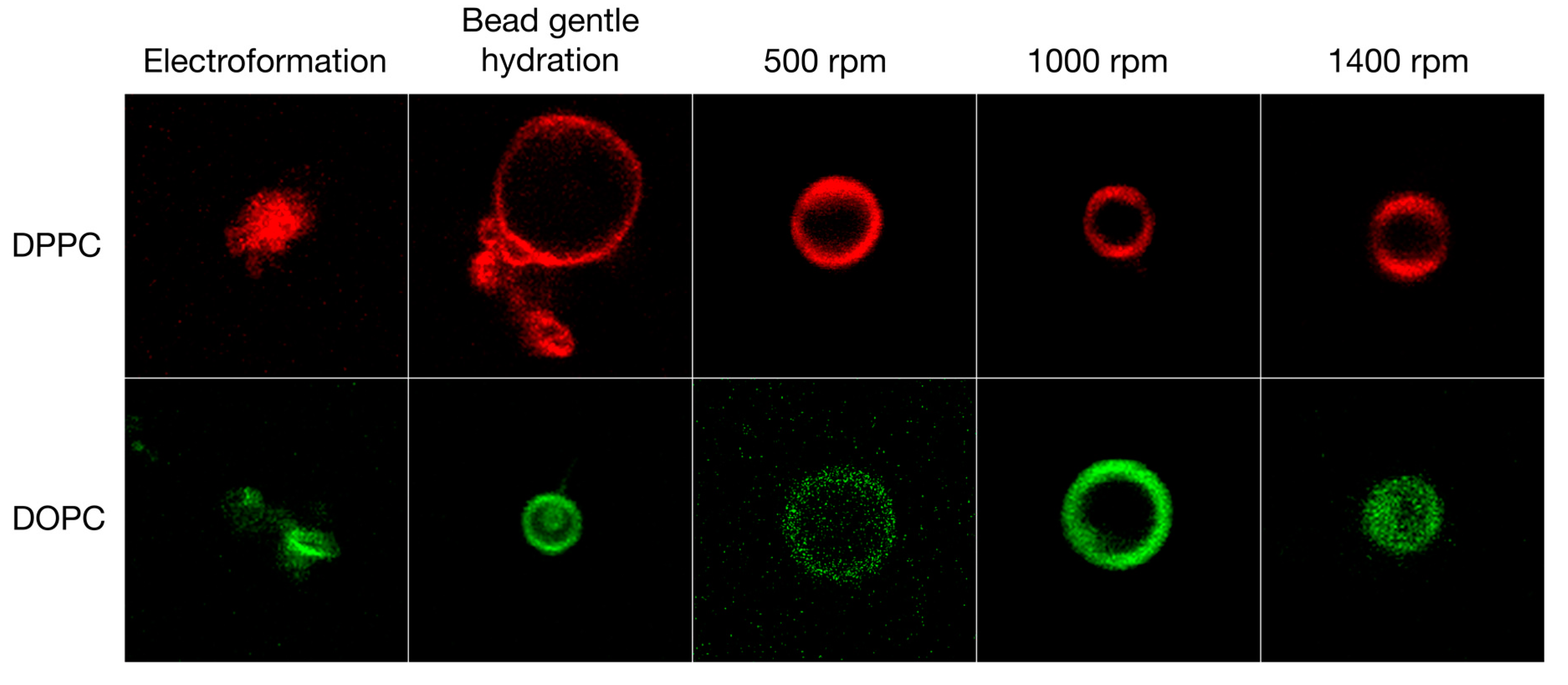
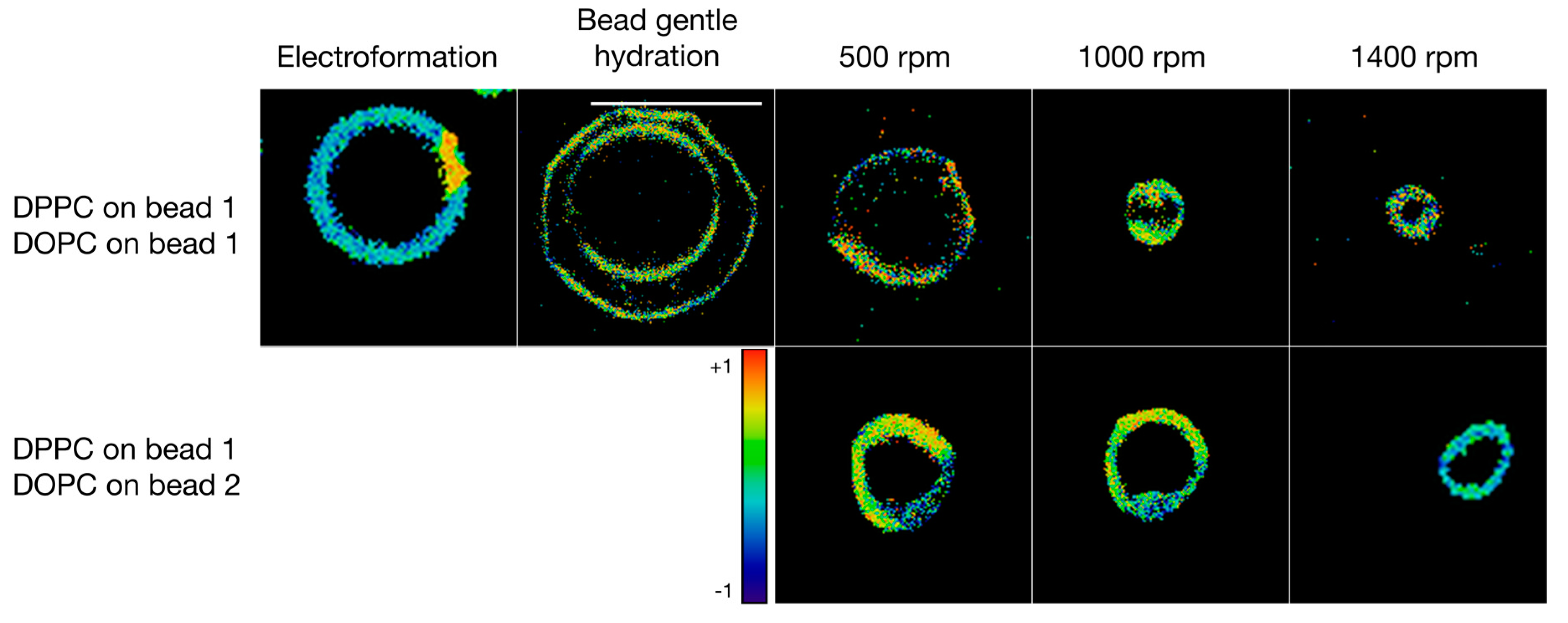


| Lipid | Solvent | Formulation | #GVs Counted | Size ± STD (μm) |
|---|---|---|---|---|
| DPPC | water | Electroformation | 24 | 9.9 ± 4.9 |
| Bead gentle hydration | 11 | 8.8 ± 4.1 | ||
| Shaking @ 500 rpm | 14 | 8.9 ± 1.3 | ||
| Shaking @ 1000 rpm | 26 | 7.8 ± 1.3 | ||
| Shaking @ 1400 rpm | 21 | 4.1 ± 1.1 | ||
| DPPC | PBS sucrose | Electroformation | - 1 | - 1 |
| Bead gentle hydration | 20 | 5.0 ± 2.6 | ||
| Shaking @ 500 rpm | 15 | 7.5 ± 1.2 | ||
| Shaking @ 1000 rpm | 20 | 4.9 ± 1.1 | ||
| Shaking @ 1400 rpm | 23 | 4.9 ± 0.9 | ||
| DOPC | water | Electroformation | 19 | 13.4 ± 5.6 |
| Bead gentle hydration | 26 | 7.9 ± 7.2 | ||
| Shaking @ 500 rpm | 17 | 11.2 ± 2.2 | ||
| Shaking @ 1000 rpm | 23 | 9.6 ± 1.6 | ||
| Shaking @ 1400 rpm | 25 | 5.9 ± 1.2 | ||
| DOPC | PBS sucrose | Electroformation | - 1 | - 1 |
| Bead gentle hydration | 13 | 8.8 ± 4.9 | ||
| Shaking @ 500 rpm | 21 | 7.8 ± 1.5 | ||
| Shaking @ 1000 rpm | 19 | 6.6 ± 1.2 | ||
| Shaking @ 1400 rpm | 16 | 5.0 ± 1.0 | ||
| DPPC on bead 1 DOPC on bead 1 | water | Electroformation | 31 | 17.3 ± 9.1 |
| Bead gentle hydration | 23 | 9.2 ± 6.1 | ||
| Shaking @ 500 rpm | 24 | 10.4 ± 1.3 | ||
| Shaking @ 1000 rpm | 14 | 5.9 ± 1.4 | ||
| Shaking @ 1400 rpm | 19 | 4.1 ± 1.2 | ||
| DPPC on bead 1 DOPC on bead 1 | PBS sucrose | Electroformation | - 1 | - 1 |
| Bead gentle hydration | 11 | 6.9 ± 2.3 | ||
| Shaking @ 500 rpm | 19 | 10.8 ± 1.3 | ||
| Shaking @ 1000 rpm | 21 | 6.1 ± 1.1 | ||
| Shaking @ 1400 rpm | 19 | 4.6 ± 0.6 | ||
| DPPC on bead 1 DOPC on bead 2 | water | Electroformation | - 2 | - 2 |
| Bead gentle hydration | 29 | 5.3 ± 2.8 | ||
| Shaking @ 500 rpm | 16 | 8.3 ± 1.4 | ||
| Shaking @ 1000 rpm | 17 | 7.8 ± 1.3 | ||
| Shaking @ 1400 rpm | 21 | 4.6 ± 1.0 | ||
| DPPC on bead 1 DOPC on bead 2 | PBS sucrose | Electroformation | - 2 | - 2 |
| Bead gentle hydration | 21 | 4.7 ± 2.7 | ||
| Shaking @ 500 rpm | 18 | 8.2 ± 1.3 | ||
| Shaking @ 1000 rpm | 16 | 5.6 ± 1.1 | ||
| Shaking @ 1400 rpm | 24 | 4.2 ± 0.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanasescu, R.; Mettal, U.; Colom, A.; Roux, A.; Zumbuehl, A. Facile and Rapid Formation of Giant Vesicles from Glass Beads. Polymers 2018, 10, 54. https://doi.org/10.3390/polym10010054
Tanasescu R, Mettal U, Colom A, Roux A, Zumbuehl A. Facile and Rapid Formation of Giant Vesicles from Glass Beads. Polymers. 2018; 10(1):54. https://doi.org/10.3390/polym10010054
Chicago/Turabian StyleTanasescu, Radu, Ute Mettal, Adai Colom, Aurélien Roux, and Andreas Zumbuehl. 2018. "Facile and Rapid Formation of Giant Vesicles from Glass Beads" Polymers 10, no. 1: 54. https://doi.org/10.3390/polym10010054
APA StyleTanasescu, R., Mettal, U., Colom, A., Roux, A., & Zumbuehl, A. (2018). Facile and Rapid Formation of Giant Vesicles from Glass Beads. Polymers, 10(1), 54. https://doi.org/10.3390/polym10010054