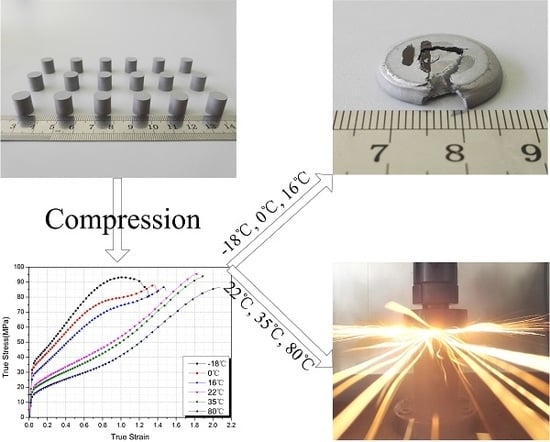
Influence of Temperature on the Mechanical Properties and Reactive Behavior of Al-PTFE under Quasi-Static Compression
Abstract
:1. Introduction
2. Materials and Experiments
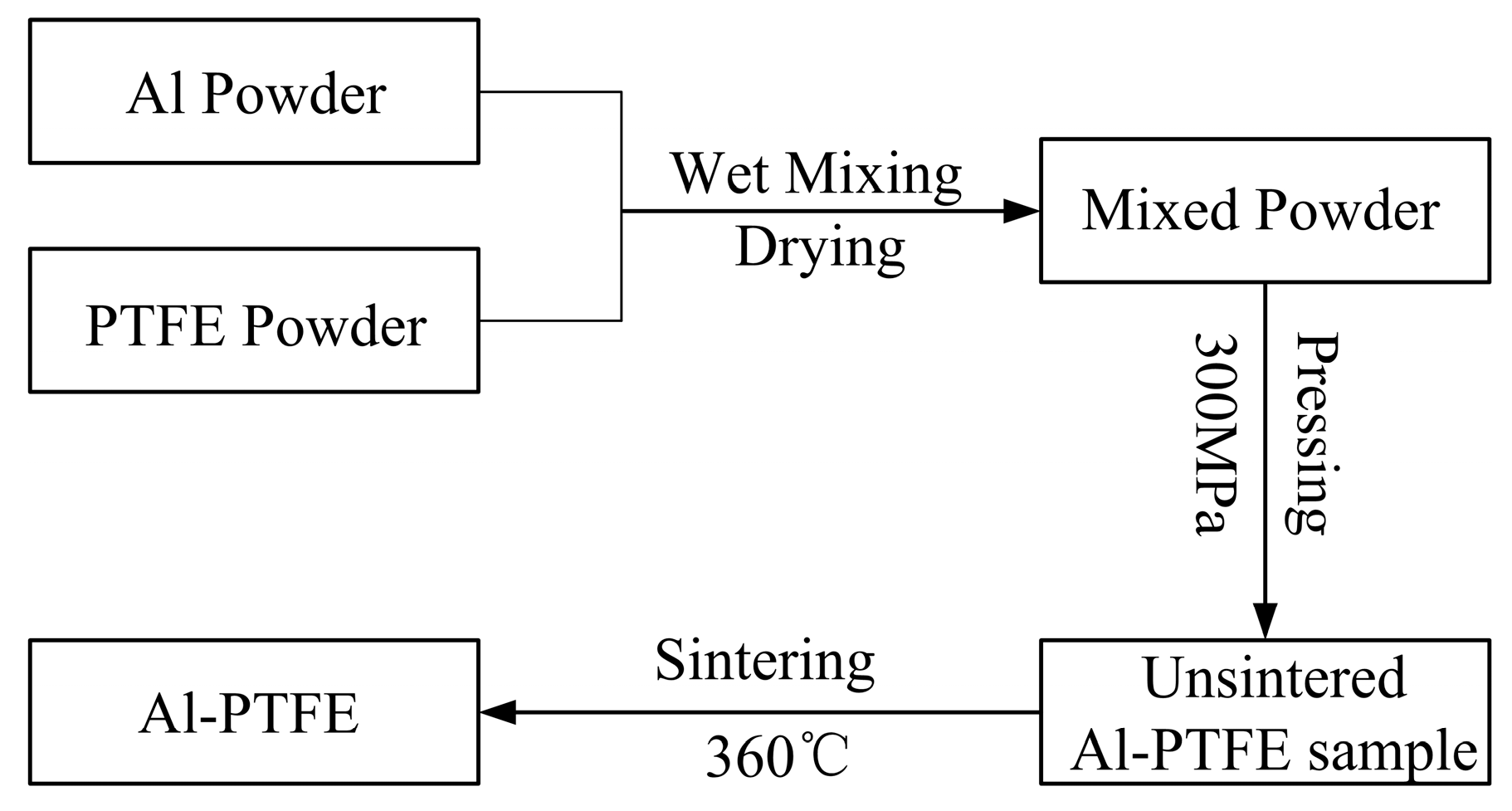
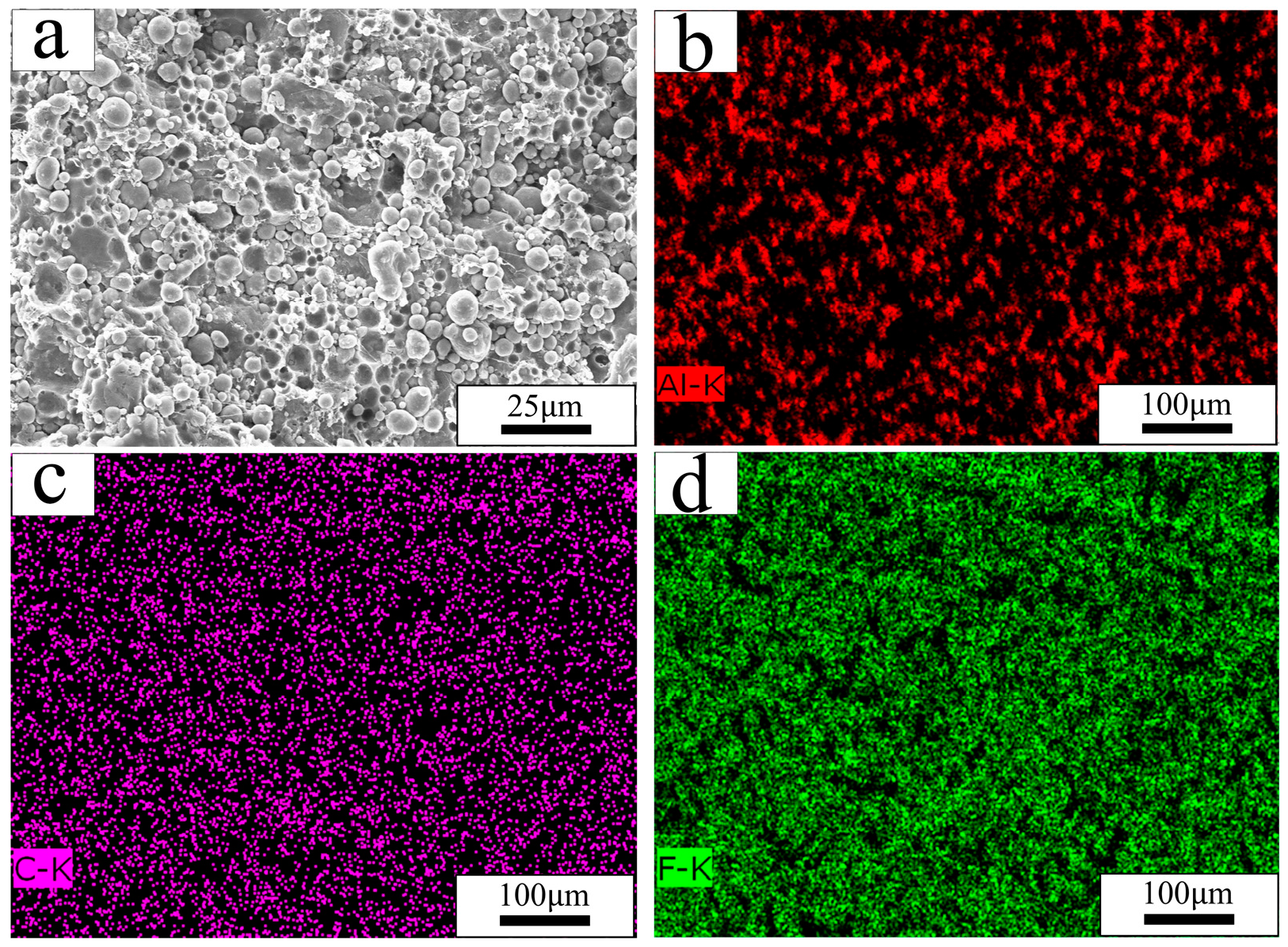
2.1. Sample Preparation
2.2. Quasi-Static Compression Tests
3. Results and Discussion
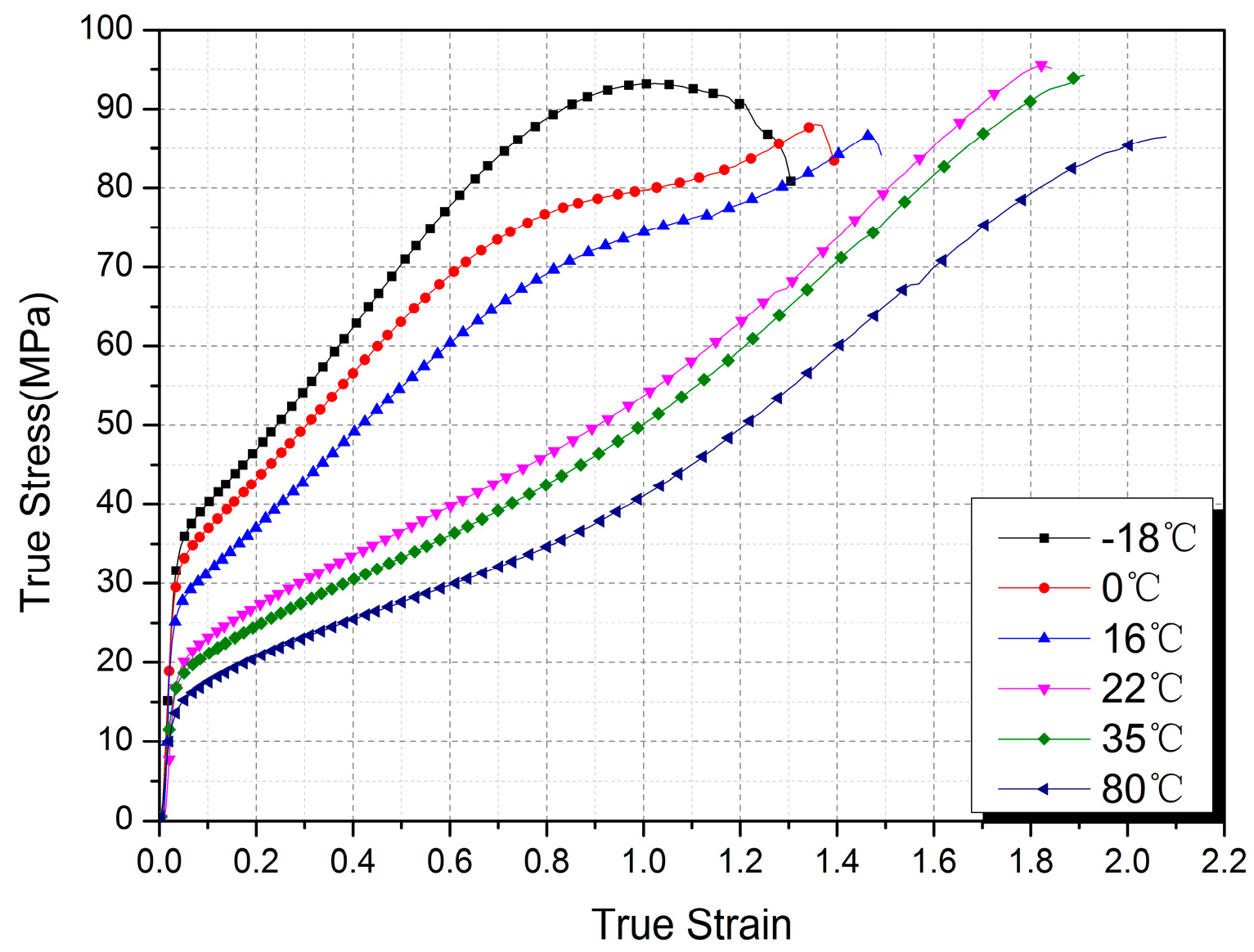
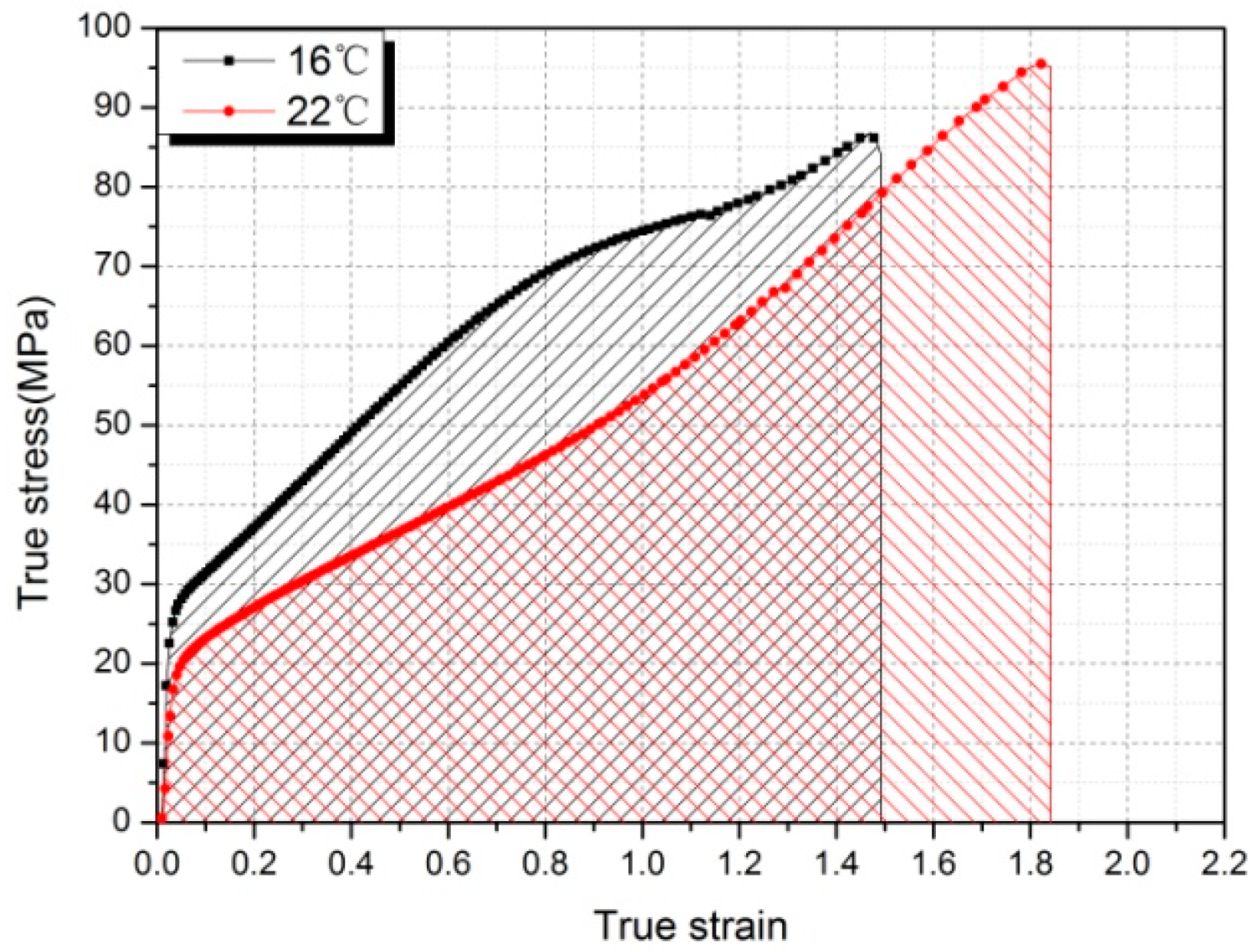
3.1. Mechanical Response
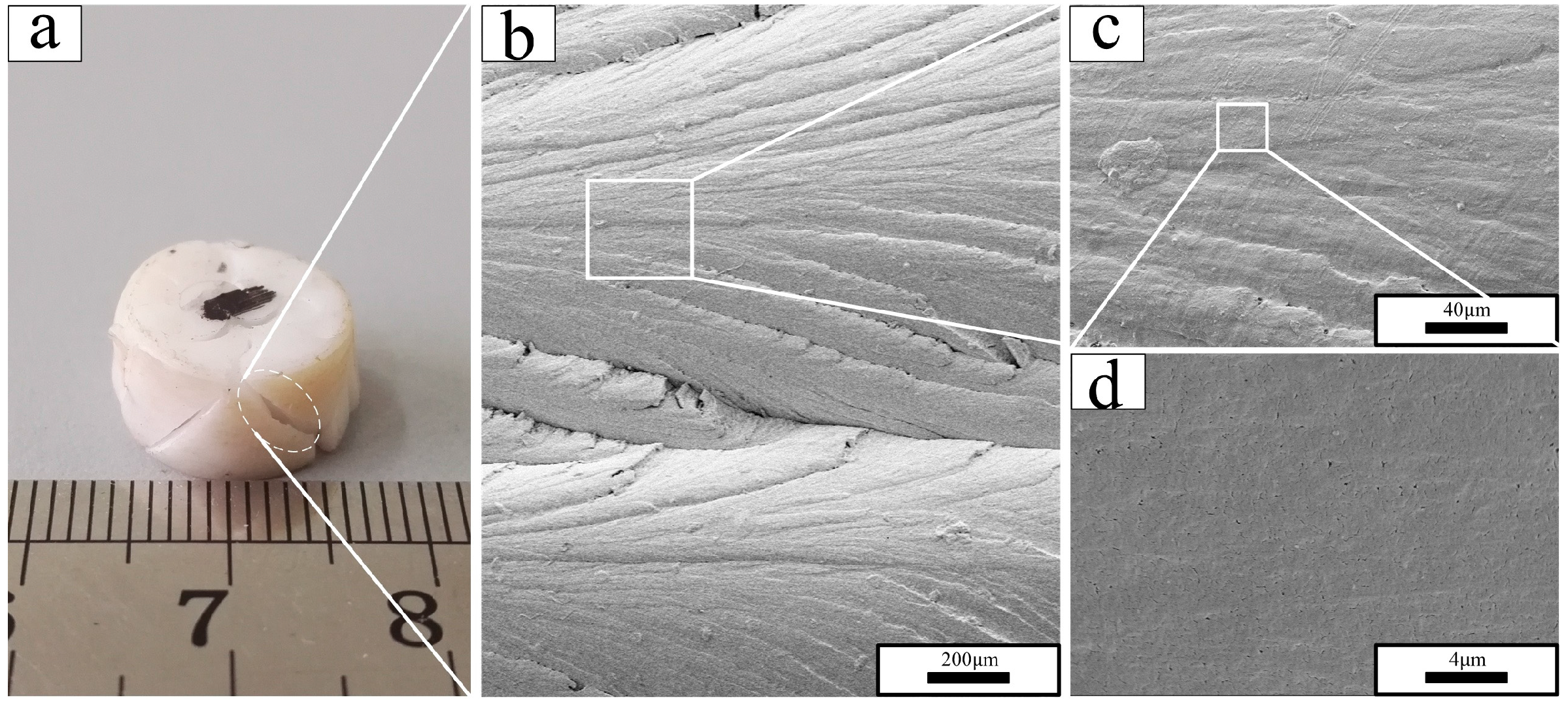
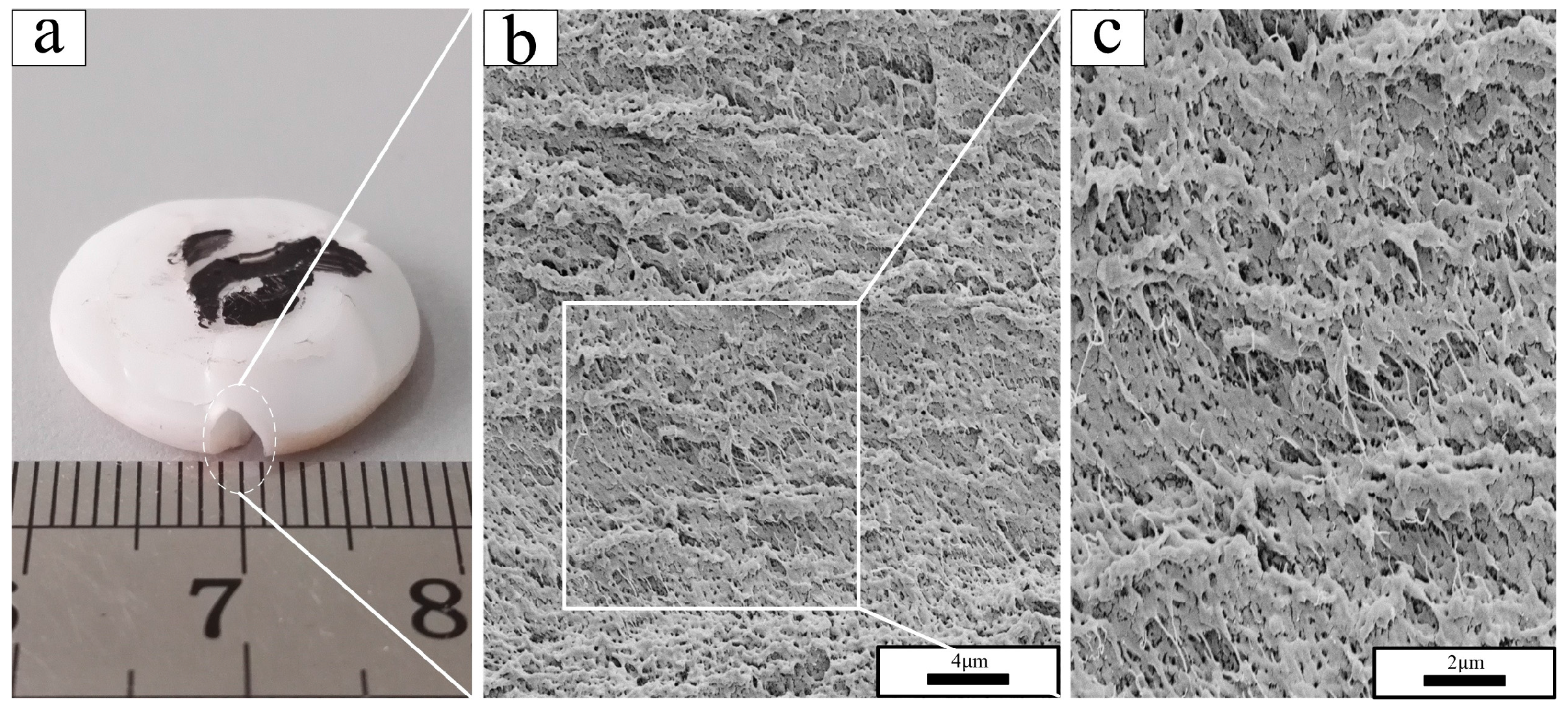
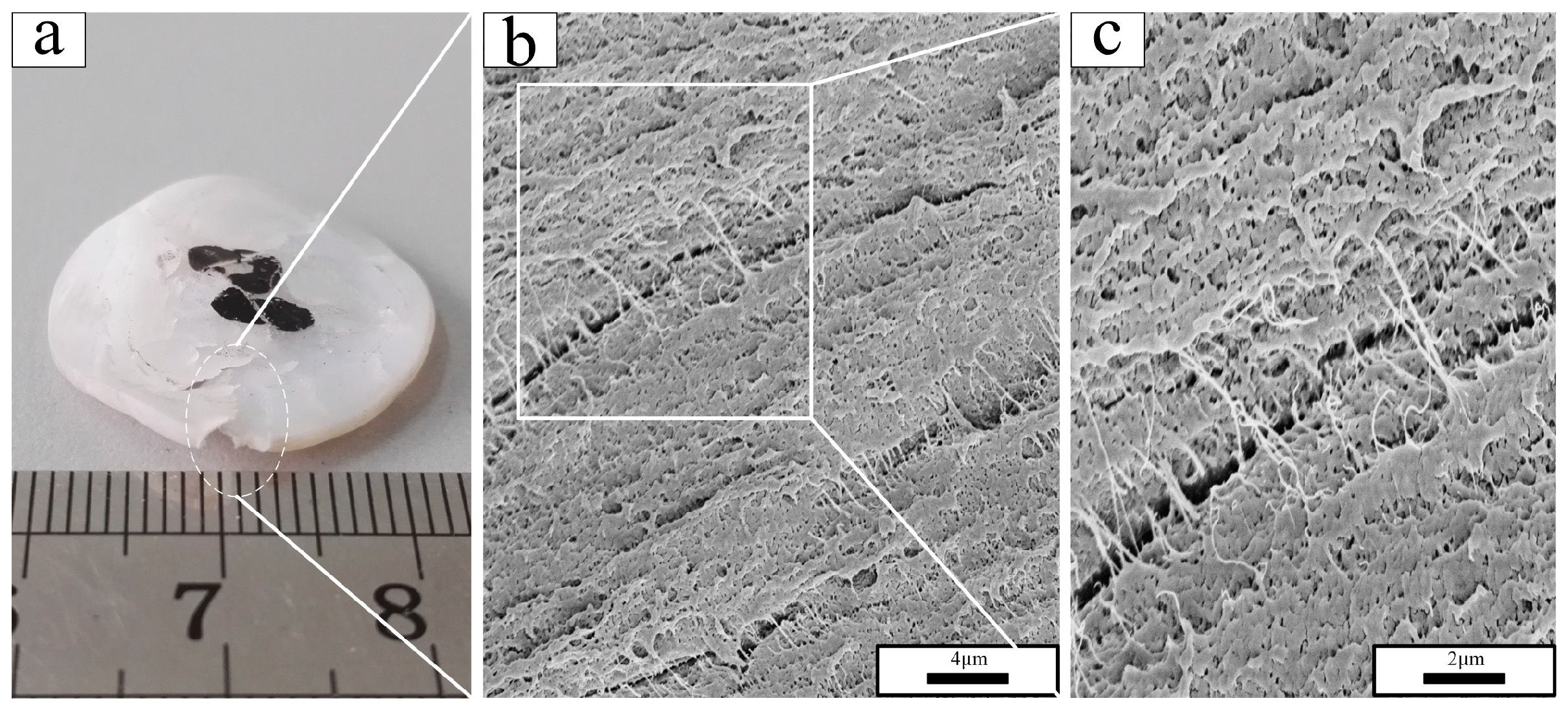
3.2. Fractography
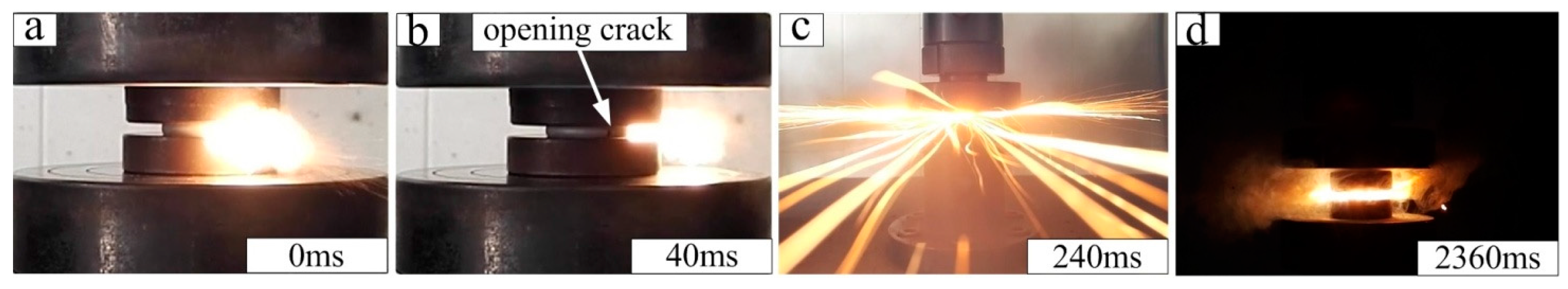
3.3. Reaction Phenomenon under Quasi-Static Compression
4. Conclusions
- (1)
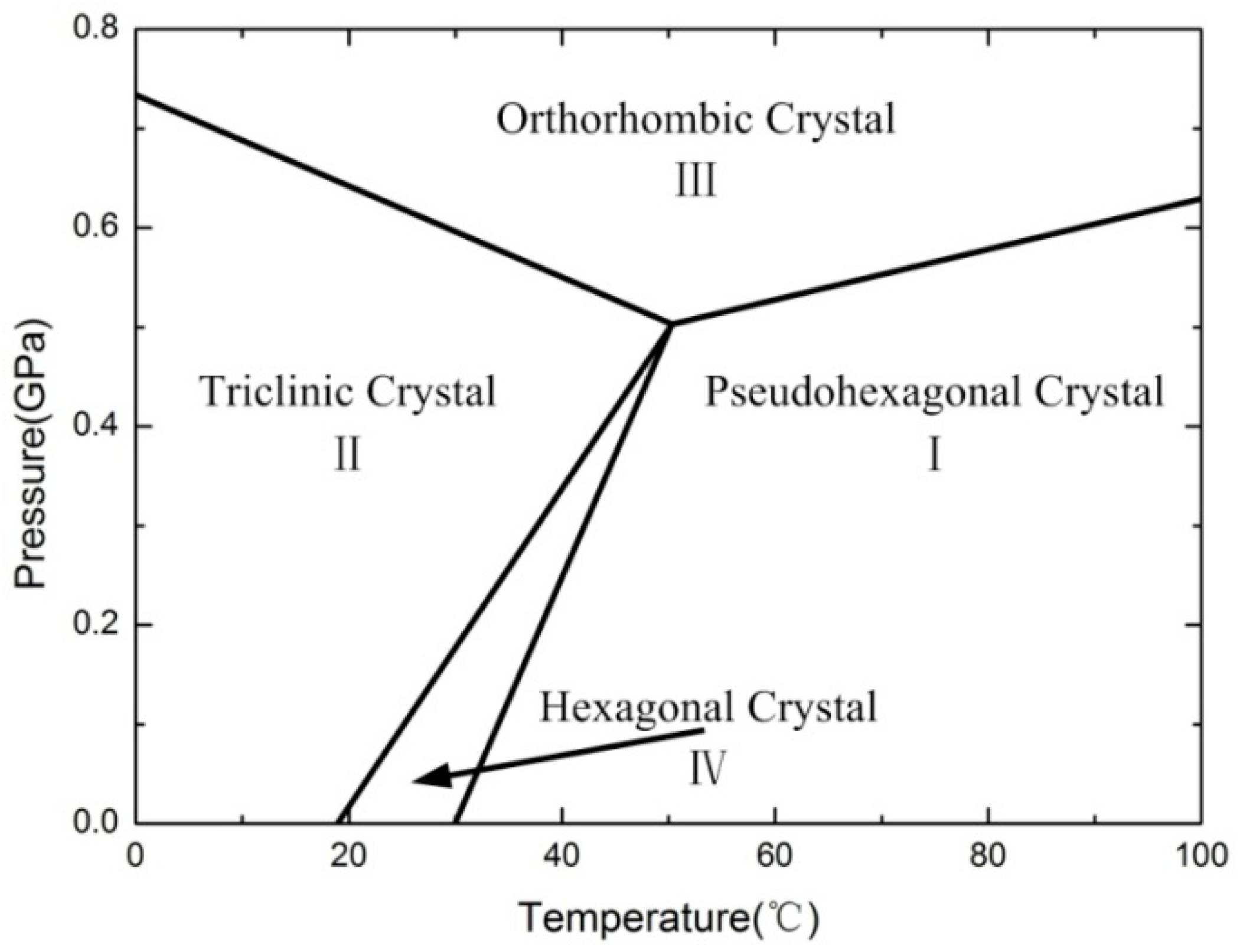
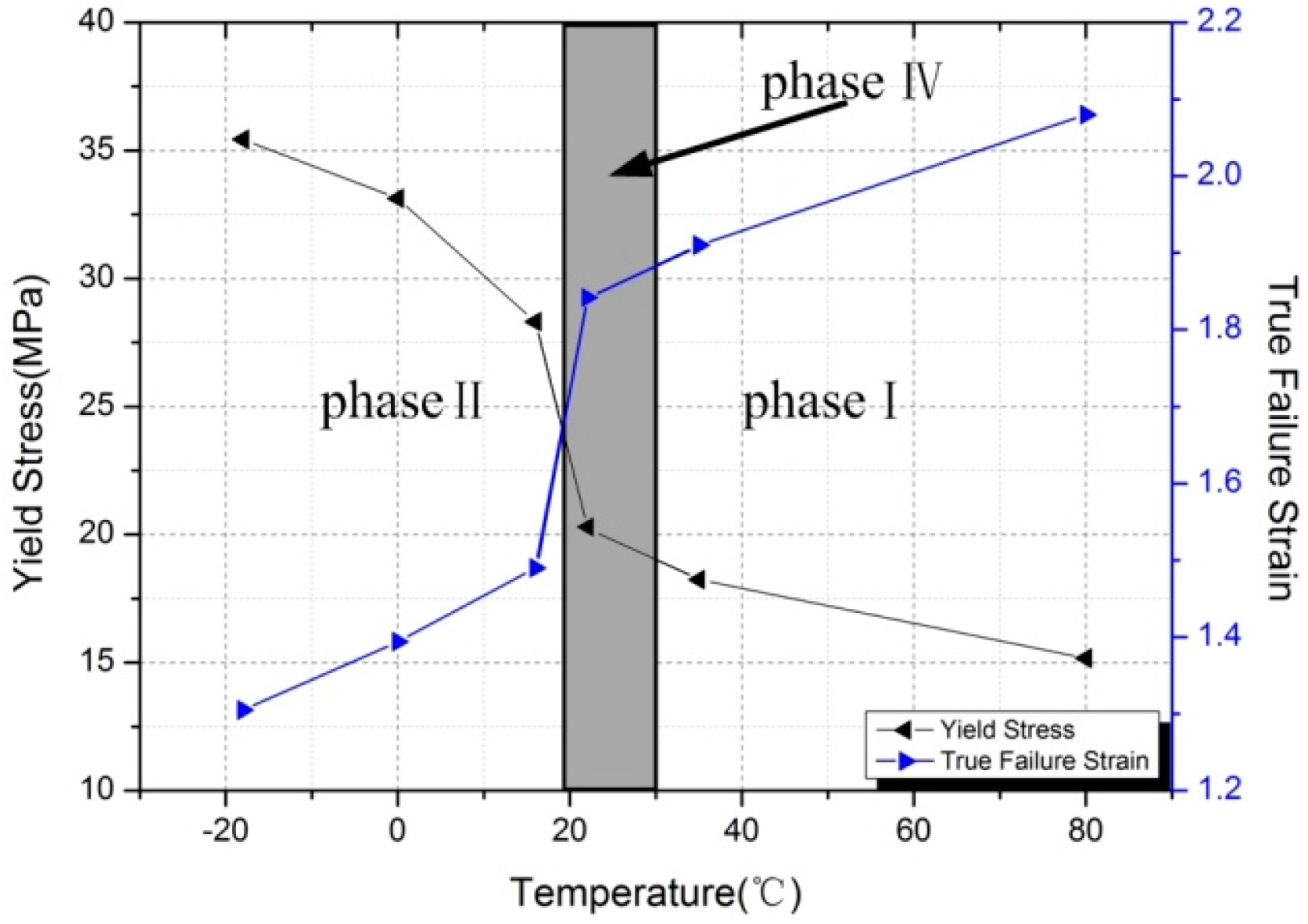
- The mechanical behavior of Al-PTFE is strongly temperature-dependent, and a transition from brittleness to ductility with increased temperature is observed. The yield stress, true failure strain, and flow stress exhibit an unusual change resulting from the temperature-induced crystalline phase transition of the PTFE matrix between phases II and IV.
- (2)
- In the scanning electron micrographs, the PTFE matrix showed two different fracture mechanisms: brittle fracture with a smooth fracture surface in phase II (below 19 °C) and ductile fracture with the formation of PTFE fibrils on the rough fracture surface in phases IV and I (above 19 °C).
- (3)
- This work demonstrates that although Al-PTFE has been considered inert under quasi-static compression, the reaction phenomenon in phases IV and I necessitates the consideration of safety during service at temperatures above 19 °C.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Joshi, V.S. Process for Making Polytetrafluoroethylene-Aluminum Composite and Product Made. U.S. Patent US6,547,993 B1, 15 April 2003. [Google Scholar]
- Dolgoborodov, A.Y.; Makhov, M.N.; Kolbanev, I.V.; Streletskiĭ, A.N.; Fortov, V.E. Detonation in an aluminum-Teflon mixture. JETP Lett. 2005, 81, 311–314. [Google Scholar] [CrossRef]
- Raftenberg, M.N.; Mock, W., Jr.; Kirby, G.C. Modeling the Impact Deformation of Rods of a Pressed PTFE/Al Composite Mixture. Int. J. Impact Eng. 2008, 35, 1735–1744. [Google Scholar] [CrossRef]
- Ge, C.; Dong, Y.X.; Maimaitituersun, W. Mircroscale simulation on mechanical properties of Al/PTFE composite based on real microstructures. Materials 2016, 9, 590. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Nesterenko, V.F.; Vecchio, K.S.; Jiang, F.; Herbold, E.B.; Benson, D.J.; Addiss, J.W.; Walley, S.M.; Proud, W.G. The influence of metallic particle size on the mechanical properties of polytetrafluoroethylene-Al-W powder composties. Appl. Phys. Lett. 2008, 92, 031903. [Google Scholar] [CrossRef]
- Xu, S.L.; Yang, S.Q.; Zhang, W. The mechanical behaviors of polytetrafluoroethylene/Al/W energetic composites. J. Phys. Condens. Mater. 2009, 21, 285401. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Zhang, J.; Qiao, L.; Shi, A.S.; Zhang, Y.G.; He, Y.; Guan, Z.W. Experimental study of the compression properties of Al/W/PTFE granular composites under elevated strain rates. Mater. Sci. Eng. A 2013, 581, 48–55. [Google Scholar] [CrossRef]
- Wang, L.; Liu, J.X.; Li, S.K.; Zhang, X.B. Investigation on reaction energy, mechanical bebavior and impact insensitivity of W-PTFE-Al composites with different W percentage. Mater. Des. 2016, 92, 397–404. [Google Scholar] [CrossRef]
- Ge, C.; Maimaitituersun, W.; Dong, Y.X.; Tian, C. A study on the mechanical properties and impact-induced initiation characteristics of brittle PTFE/Al/W reactive materials. Materials 2017, 10, 452. [Google Scholar] [CrossRef]
- Feng, B.; Fang, X.; Li, Y.C.; Wang, H.X.; Mao, Y.M.; Wu, S.Z. An initiation phenomenon of Al-PTFE under quasi-static compression. Chem. Phys. Lett. 2015, 637, 38–41. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.C.; Wu, S.Z.; Wang, H.X.; Tao, Z.M.; Fang, X. A crack-induced initiation mechanism of Al-PTFE under quasi-static compression and the investigation of influencing factors. Mater. Des. 2016, 108, 411–417. [Google Scholar] [CrossRef]
- Koo, G.P.; Andrews, R.D. Mechanical behavior of Polytetrafluoroethylene around the room-temperature first-order transition. Polym. Eng. Sci. 1969, 9, 268–276. [Google Scholar] [CrossRef]
- Bunn, C.W.; Howells, E.R. Structures of molecules and crystals of fluoro-carbons. Nature 1954, 174, 549–551. [Google Scholar] [CrossRef]
- Mccrum, N.G. An internal friction study of polytetrafluoroethylene. J. Polym. Sci. 1959, 34, 355–369. [Google Scholar] [CrossRef]
- Sperati, C.A.; Starkweather, H.W. Fluorine-containing polymers. II. Polytetrafluoroethylene. Adv. Polym. Sci. 1961, 2/4, 465–495. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Gray, G.T. The influence of temperature and strain rate on the tensile and compressive constitutive response of four fluoropolymers. J. Phys. IV 2006, 134, 935–940. [Google Scholar] [CrossRef]
- Wu, C.K.; Nicol, M. Reman spectra of high pressure phase and phase transition of polytetrafluoroethylene (Teflon). Chem. Phys. Lett. 1973, 21, 153–157. [Google Scholar] [CrossRef]
- Clark, E.S. The molecular conformations of polytetrafluoroethylene: Forms II and IV. Polymer 1999, 40, 4659–4665. [Google Scholar] [CrossRef]
- Bunn, C.W.; Cobbold, A.J.; Palmer, R.P. The fine structure of polytetrafluoroethylene. J. Polym. Sci. 1958, 28, 365–376. [Google Scholar] [CrossRef]
- Weeks, J.J.; Sanchez, I.C.; Eby, R.K.; Poser, C.I. Order-disorder transitions in polytetrafluoroethylene. Polymer 1980, 21, 325–331. [Google Scholar] [CrossRef]
- Cox, J.M.; Wright, B.A.; Wright, W.W. Thermal degradation of fluorine-containing polymers. Part I. Degradation in vacuum. J. Appl. Polym. Sci. 1964, 8, 2935–2950. [Google Scholar] [CrossRef]
- Nielson, D.B.; Tanner, R.L.; Lund, G.K. High Strength Reactive Materials. U.S. Patent US20030096897 A1, 22 May 2003. [Google Scholar]
- Speerschneider, C.J.; Li, C.H. A Correlation of Mechanical Properties and Microstructure of Polytetrafluoroethylene at Various Temperatures. J. Appl. Phys. 1963, 34, 3004–3007. [Google Scholar] [CrossRef]
- Brown, E.N.; Rae, P.J.; Orler, E.B.; Gray, G.T., III; Dattelbaum, D.M. The effect of crystallinity on the fracture of polytetrafluoroethylene (PTFE). Mater. Sci. Eng. C 2006, 26, 1338–1343. [Google Scholar] [CrossRef]
- Rae, P.J.; Dattelbaum, D.M. The properties of poly(tetrafluoroethylene) (PTFE) in compression. Polymer 2004, 45, 7615–7625. [Google Scholar] [CrossRef]
- Rae, P.J.; Brown, E.N. The properties of poly(tetrafluoroethylene) (PTFE) in tension. Polymer 2005, 46, 8128–8140. [Google Scholar] [CrossRef]
- Brown, E.N.; Dattelbaum, D.M. The role of crystalline phase on fracture and microstructure evolution of polytetrafluoroethylene (PTFE). Polymer 2005, 46, 3056–3068. [Google Scholar] [CrossRef]
- O’Leary, K.; Geil, P.H. Polytetrafluoroethylene fibril structure. J. Appl. Phys. 1967, 38, 4169–4181. [Google Scholar] [CrossRef]
- Kitamura, T.; Okabe, S.; Tanigaki, M.; Kurumada, K.; Ohshima, M.; Zanazawa, S. Morphology change in polytetrafluoroethylene (PTFE), porous membrance caused by heat treatment. Polym. Eng. Sci. 2000, 40, 809–817. [Google Scholar] [CrossRef]
- Ariawan, A.B.; Ebnesajjad, S.; Hatzikiriakos, S.G. Properties of polytetrafluoroethylene (PTFE) paste extrudates. Polym. Eng. Sci. 2002, 42, 1247–1259. [Google Scholar] [CrossRef]
- Jie, M.; Tang, C.Y.; Li, Y.P.; Li, C.C. Damage evolution and energy dissipation of polymers with crazes. Theor. Appl. Fract. Mech. 1998, 28, 165–174. [Google Scholar] [CrossRef]
- Aglan, H.; Gan, Y.; El-Hadeki, M.; Faughnan, P.; Bryan, C. Evaluation of fatigue fracture resistance of unfilled and filled polytetrafluoroethylene materials. J. Mater. Sci. 1999, 34, 83–97. [Google Scholar] [CrossRef]
- Sun, B.N.; Hsiao, C.C. Nonlinear quasi-fracture behavior of polymers. J. Appl. Phys. 1985, 57, 170–174. [Google Scholar] [CrossRef]
- Marissen, R. Craze growth mechanics. Polymer 2000, 41, 1119–1129. [Google Scholar] [CrossRef]
- Feng, B.; Li, Y.C.; Hao, H.; Wang, H.X.; Hao, Y.F.; Fang, X. A mechanism of hot-spots formation at the crack tip of Al-PTFE under quasi-static compression. Propellants Explos. Pyrotech. 2017, 42, 1–8. [Google Scholar] [CrossRef]
- Fuller, K.N.G.; Fox, P.G.; Field, J.E. The temperature rise at the tip of fast-moving cracks in glassy polymers. Proc. R. Soc. A 1975, 341, 537–557. [Google Scholar] [CrossRef]
- Swallowe, G.M.; Field, J.E.; Horn, L.A. Measurements of transient high temperatures during the deformation of polymers. J. Mater. Sci. 1986, 21, 4089–4096. [Google Scholar] [CrossRef]
- Zhang, J.W.; Jiang, H.; Jiang, C.K.; Cheng, Q.; Kang, G.Z. In-situ observation of temperature rise during scratch testing of poly (methylmethacrylate) and polycarbonate. Tribol. Int. 2016, 95, 1–4. [Google Scholar] [CrossRef]



© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-X.; Fang, X.; Feng, B.; Gao, Z.-R.; Wu, S.-Z.; Li, Y.-C. Influence of Temperature on the Mechanical Properties and Reactive Behavior of Al-PTFE under Quasi-Static Compression. Polymers 2018, 10, 56. https://doi.org/10.3390/polym10010056
Wang H-X, Fang X, Feng B, Gao Z-R, Wu S-Z, Li Y-C. Influence of Temperature on the Mechanical Properties and Reactive Behavior of Al-PTFE under Quasi-Static Compression. Polymers. 2018; 10(1):56. https://doi.org/10.3390/polym10010056
Chicago/Turabian StyleWang, Huai-Xi, Xiang Fang, Bin Feng, Zhen-Ru Gao, Shuang-Zhang Wu, and Yu-Chun Li. 2018. "Influence of Temperature on the Mechanical Properties and Reactive Behavior of Al-PTFE under Quasi-Static Compression" Polymers 10, no. 1: 56. https://doi.org/10.3390/polym10010056