Spectroscopic Techniques for the Characterization of Polymer Nanocomposites: A Review
Abstract
:1. Introduction
2. Fluorescence Spectroscopy
3. Solid-State NMR Spectroscopy
4. Infrared and Raman Spectroscopy
4.1. Infrared Spectroscopy
4.2. Raman Spectroscopy
5. Conclusions
Conflicts of Interest
References
- Jancar, J.; Douglas, J.F.; Starr, F.W.; Kumar, S.K.; Cassagnau, P.; Lesser, A.J.; Sternstein, S.S.; Buehler, M.J. Current issues in research on structure-property relationships in polymer nanocomposites. Polymer 2010, 51, 3321–3343. [Google Scholar] [CrossRef]
- Bokobza, L. Mechanical and electrical properties of elastomer nanocomposites based on different carbon nanomaterials. C J. Carbon Res. 2017, 3, 10. [Google Scholar] [CrossRef]
- Bokobza, L. The reinforcement of elastomeric networks by fillers. Macromol. Mater. Eng. 2004, 289, 607–621. [Google Scholar] [CrossRef]
- Wang, S.B.; Mark, J.E. In-situ precipitation of reinforcing titania fillers. Polym. Bull. 1987, 17, 271–277. [Google Scholar] [CrossRef]
- Mark, J.E. Novel reinforcement techniques for elastomers. J. Appl. Polym. Sci. 1992, 50, 273–282. [Google Scholar] [CrossRef]
- McCarthy, D.W.; Mark, J.E.; Schaefer, D.W. Synthesis, structure, and properties of hybrid organic-Iinorganic composites based on polysiloxanes. I. Poly(dimethylsiloxane) elastomers containing silica. J. Polym. Sci. Part B 1998, 36, 1167–1189. [Google Scholar] [CrossRef]
- Yuan, Q.W.; Mark, J.E. Reinforcement of poly(dimethylsiloxane) networks by blended and in-situ generated silica fillers having various sizes, size distributions, and modified surfaces. Macromol. Chem. Phys. 1999, 200, 206–220. [Google Scholar] [CrossRef]
- Hajji, P.; David, L.; Gerard, J.F.; Pascault, J.P.; Vigier, G. Synthesis, structure and morphology of polymer-silica hybrid nanocomposites based on hydroxyetyl methacrylate. J. Polym. Sci. Part B 1999, 37, 3172–3187. [Google Scholar] [CrossRef]
- Matĕjka, L.; Dukh, O.; Kolařík, J. Reinforcement of crosslinked rubbery epoxies by in-situ formed silica. Polymer 2000, 41, 1449–1459. [Google Scholar] [CrossRef]
- Matĕjka, L.; Dukh, O. Organic-inorganic hybrid networks. Macromol. Symp. 2001, 171, 181–188. [Google Scholar] [CrossRef]
- Dewimille, L.; Bresson, B.; Bokobza, L. Synthesis, structure and morphology of poly(dimethylsiloxane) networks filled with in situ generated silica particles. Polymer 2005, 46, 4135–4143. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of poly(dimethylsiloxane) by sol-gel in situ generated silica and titania particles. eXPRESS Polym. Lett. 2010, 4, 355–363. [Google Scholar] [CrossRef]
- Wen, J.; Mark, J.E. Precipitation of silica-titania mixed-oxide fillers into poly(dimethylsiloxane) networks. Rubber Chem. Technol. 1994, 67, 806–819. [Google Scholar] [CrossRef]
- Breiner, J.M.; Mark, J.E. Preparation, structure, growth mechanisms and properties of siloxane composites containing silica, titania or mixed silica-titania phases. Polymer 1998, 39, 5483–5493. [Google Scholar] [CrossRef]
- Giannelis, E.P. Polymer Layered Silicate composites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Krishnamoorti, R.; Vaia, R.A.; Giannelis, E.P. Structure and dynamics of polymer-layered silicate nanocomposites. Chem. Mater. 1996, 8, 1728–1734. [Google Scholar] [CrossRef]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Formation and properties of nylon 6 nanocomposites. Polím. Ciênc. Technol. 2003, 13, 212–217. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty years of polymer-clay nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Alena, K.; Dagmar, M.; Gerard, J.F.; Miroslav, S. Polymer/clay nanocomposites and their gas barrier properties. Polym. Compos. 2013, 34, 1418–1424. [Google Scholar]
- Bokobza, L.; Rahmani, M.; Belin, C.; El Bounia, N.-E. Blends of carbon blacks and multiwall carbon nanotubes as reinforcing fillers for hydrocarbon rubbers. J. Polym. Sci. Part B 2008, 46, 1939–1951. [Google Scholar] [CrossRef]
- Galimberti, M.; Coombs, M.; Cipolleti, V.; Riccio, P.; Riccò, T.; Pandini, S.; Conzatti, L. Enhancement of mechanical reinforcement due to hybrid filler networking promoted by an organoclay in hydrocarbon-based nanocomposites. Appl. Clay Sci. 2012, 65–66, 57–66. [Google Scholar] [CrossRef]
- Ma, P.-C.; Siddiqui, N.A.; Marom, G.; Kim, J.-K. Dispersion and functionalization of carbon nanotubes for polymer-based nanocomposites: A review. Compos. Part A 2010, 41, 1345–1367. [Google Scholar] [CrossRef]
- Punetha, V.D.; Rana, S.; Yoo, H.J.; Chaurasia, A.; McLeskey, J.T., Jr.; Ramasamy, M.S.; Sahoo, N.G.; Cho, J.W. Functionalization of carbon nanomaterials for advanced polymer nanocomposites: A comparison study between CNT and graphene. Prog. Polym. Sci. 2017, 67, 1–47. [Google Scholar] [CrossRef]
- Zhang, X.; Hou, L.; Samori, P. Coupling carbon nanomaterials with photochromic molecules for the optically responsive materials. Nat. Commun. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Joung, D.; Zhai, L.; Das, S.; Khondaker, S.I.; Seal, S. Graphene based materials: Past, present and future. Prog. Mater. Sci. 2011, 56, 1178–1271. [Google Scholar] [CrossRef]
- Xu, L.; Cheng, L. Graphite oxide under high pressure: A Raman spectroscopic study. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, S.-M.; Oh, Y.-S.; Yang, Y.-H.; Lim, Y.S.; Yoon, D.Y.; Lee, C.; Kim, J.-Y.; Ruoff, R.S. Unoxidized graphene/alimina nanocomposite: Fracture- and wear-resistance effects of graphene on alumina matrix. Sci. Rep. 2014, 4. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectra of carbon-based materials (from graphite to carbon black) and of some silicone composites. C J. Carbon Res. 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Viculis, L.M.; Mack, J.J.; Mayer, O.M.; Hahn, H.T.; Kaner, R.B. Intercalation and exfoliation routes to graphite nanoplatelets. J. Mater. Chem. 2005, 15, 974–978. [Google Scholar] [CrossRef]
- Geng, Y.; Wang, S.J.; Kim, J.-K. Preparation of graphite nanoplatelets and graphene sheets. J. Colloid Interface 2009, 336, 592–598. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Zhao, W. Rubber/Graphite Nanocomposites. In Rubber Nanocomposites: Preparation, Properties, and Applications; Thomas, S., Stephen, R., Eds.; John Wiley & Sons (Asia): Singapore, 2010. [Google Scholar]
- Kuilla, T.; Bhadra, S.; Yao, D.; Kim, N.H. Recent advances in graphene based polymer composites. Prog. Polym. Sci. 2010, 35, 1350–1375. [Google Scholar] [CrossRef]
- Li, B.; Zhong, W.-H. Review on polymer/graphite nanoplatelet nanocomposites. J. Mater. Sci. 2011, 46, 5595–5614. [Google Scholar] [CrossRef]
- Sengupta, R.; Bhattacharya, M.; Bandyopadhyay, S.; Bhowmick, A.K. A review on the mechanical and electrical properties of graphite and modified reinforced polymer composites. Prog. Polym. Sci. 2011, 36, 638–670. [Google Scholar] [CrossRef]
- Singh, K.; Ohlan, A.; Dhawan, S.K. Polymer-Graphene nanocomposites: Preparation, Characterization, Properties, and Applications. In Nanocomposites—New Trends and Developments; Ebrahimi, F., Ed.; Intech: Rijeka, Croatia, 2012. [Google Scholar]
- Cai, M.; Thorpe, D.; Adamson, D.H.; Schniepp, H.C. Methods of graphite exfoliation. J. Mater. Chem. 2012, 22, 24992–25002. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Dimiev, A.M.; Ceriotti, G.; Metzger, A.; Kim, N.D.; Tour, J.M. Chemical Mass Production of graphene nanoplatelets in ~100% yield. ACS Nano 2016, 10, 274–279. [Google Scholar] [CrossRef] [PubMed]
- Thomas, S.; Rouxel, D.; Ponnamma, D. (Eds.) Spectroscopy of Polymer Nanocomposites, 1st ed.; Elsevier: Oxford, UK, 2016. [Google Scholar]
- Bokobza, L. Investigation of local dynamics of polymer chains in the bulk by the excimer fluorescence technique. Prog. Polym. Sci. 1990, 15, 337–360. [Google Scholar] [CrossRef]
- George, G.A. Characterization of solid polymers by luminescence techniques. Pure Appl. Chem. 1985, 57, 945–954. [Google Scholar] [CrossRef]
- Zammarano, M.; Maupin, P.H.; Sung, L.-P.; Gilman, J.W.; McCarthy, E.D.; Kim, Y.S.; Fox, D.M. Revealing the interface in polymer nanocomposites. ACS Nano 2011, 5, 3391–3399. [Google Scholar] [CrossRef] [PubMed]
- Maupin, P.H.; Gilman, J.W.; Harris, R.H.; Bellayer, S.; Bur, A.J.; Roth, C.; Murariu, M.; Morgan, A.B.; Harris, J.D. Optical probes for monitoring intercalation and exfoliation in melt-processed polymer nanocomposites. Macromol. Rapid Commun. 2004, 25, 788–792. [Google Scholar] [CrossRef]
- Rittigstein, P.; Torkelson, J.M. Polymer-nanoparticle interfacial interactions in polymer nanocomposites: Confinement effects on glass transition temperature and suppression of physical aging. J. Polym. Sci. Part B 2006, 44, 2935–2943. [Google Scholar] [CrossRef]
- Clough, J.M.; Creton, C.; Craig, S.L.; Sibesma, R.P. Covalent bond scission in the Mullins effect of a filled elastomer: Real-time visualization with mechanoluminescence. Adv. Funct. Mater. 2016, 26, 9063–9074. [Google Scholar] [CrossRef]
- Böhme, U.; Scheler, U. Interfaces in polymer nanocomposites—An NMR study. AIP Conf. Proc. 2016. [Google Scholar] [CrossRef]
- Mirau, P.A. Solid-state characterization of polymer interfaces. In Chapter in Modern Magnetic Resonance; Webb, A., Ed.; Springer: Dordrecht, The Netherlands, 2006. [Google Scholar]
- Mirau, P.A.; Heffner, S.A.; Schilling, M. Fast magic-angle spinning proton NMR studies of polymers at surfaces and interfaces. Solid State Nucl. Magn. Res. 2000, 16, 47–53. [Google Scholar] [CrossRef]
- Legrand, A.P.; Hommel, H.; Tuel, A.; Vidal, A.; Balard, H.; Papirer, E.; Levitz, P.; Czernichowski, M.; Erre, R.; Van Damme, H.; et al. Hydroxyls of silica powders. Adv. Colloids Interface Sci. 1990, 33, 91–330. [Google Scholar] [CrossRef]
- Avolio, R.; Gentle, G.; Avella, M.; Capitani, D.; Errico, M.E. Synthesis and characterization of poly(methylmethacrylate)/silica nanocomposites: Study of the interphase by solid-state NMR and structure/properties relationships. J. Polym. Sci. Part A 2010, 48, 5618–5629. [Google Scholar] [CrossRef]
- Olejniczak, S.; Kaźmierski, S.; Pallathadka, P.K.; Potrzebowski, M.J. A review on advances of high-resolution solid state NMR spectroscopy in structural studies of polymer/clay nanocomposites. Polimery 2007, 52, 713–721. [Google Scholar]
- Rodrigues, T.; Tavares, M.I.B.; Soares, I.; Moreira, A.; Ferreira, A. The use of solid state NMR to characterize high density polyethylene/organoclay nanocomposites. Chem. Chem. Technol. 2009, 3, 187–190. [Google Scholar]
- Li, W.; Hou, L.; Chen, Z. An NMR Investigation of Phase Structure and Chain Dynamics in the Polyethylene/Montmorillonite Nanocomposites. J. Nanomater. 2013. [Google Scholar] [CrossRef]
- Lorthioir, C.; Lauprêtre, F.; Soulestin, J.; Lefebvre, J.-M. Segmental dynamics of poly(ethylene oxide) chains in a model polymer/clay intercalated phase: Solid-state NMR investigation. Macromolecules 2009, 42, 218–230. [Google Scholar] [CrossRef]
- Cahill, L.S.; Yao, Z.; Adronov, A.; Penner, J.; Moonoosawmy, K.R.; Kruse, P.; Goward, G.R. Polymer-functionalized carbon nanotubes investigated by solid-state nuclear magnetic resonance and scanning tunneling microscopy. J. Phys. Chem. B 2004, 108, 11412–11418. [Google Scholar] [CrossRef]
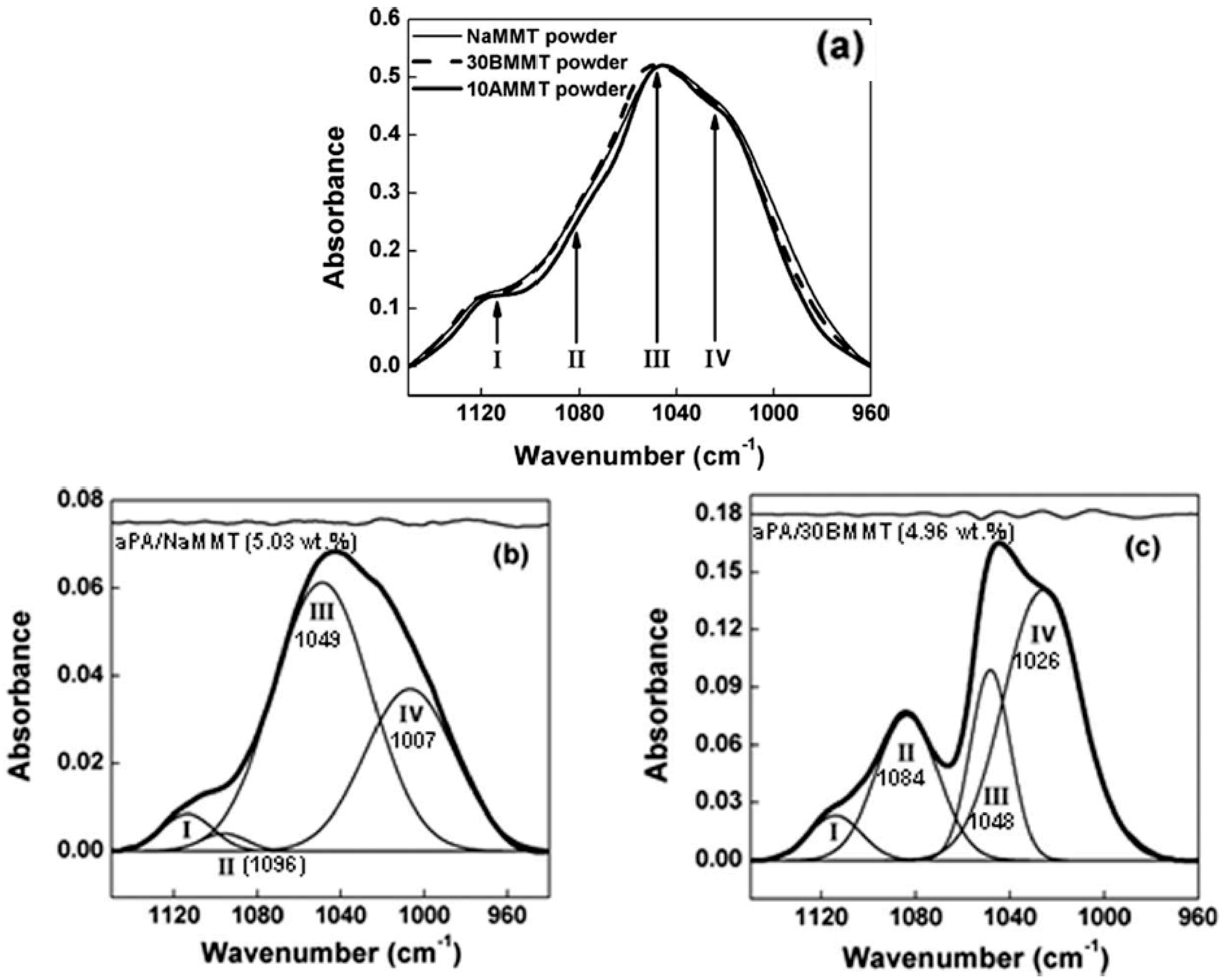
- Cole, K.C. Use of infrared spectroscopy to characterize clay intercalation and exfoliation in polymer nanocomposites. Macromolecules 2008, 41, 834–843. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bhuvana, S.; Loo, L.S. Characterization of layered silicate dispersion in polymer nanocomposites using Fourier transform infrared spectroscopy. J. Appl. Polym. Sci. 2012, 125, E175–E180. [Google Scholar] [CrossRef]
- Bokobza, L.; Diop, A.L. Reinforcement of silicone rubbers by sol-gel in situ generated particles. In Rubber Nanocomposites: Preparation, Properties, and Applications; Thomas, S., Stephen, R., Eds.; John Wiley & Sons (Asia): Singapore, 2010. [Google Scholar]
- Titus, E.; Ali, N.; Cabral, G.; Gracio, J.; Babu, P.R.; Jackson, M.J. Chemically functionalized carbon nanotubes and their characterization using thermogravimetric analysis, Fourier transform infrared, and Raman spectroscopy. J. Mater. Eng. Perform. 2006, 15, 182–186. [Google Scholar] [CrossRef]
- Hussain, S.; Jha, P.; Chouksey, A.; Raman, R.; Islam, S.S.; Islam, T.; Choudhary, P.K. Spectroscopic investigation of modified single wall carbon nanotube (SWCNT). J. Modern Phys. 2011, 2, 538–543. [Google Scholar] [CrossRef]
- Le, V.T.; Ngo, C.L.; Le, Q.T.; Ngo, T.T.; Nguyen, D.N.; Vu, M.T. Surface modification and functionalization of carbon nanotube with some organic compounds. Adv. Nat. Sci. 2013, 4. [Google Scholar] [CrossRef]
- Lee, S.H.; Choi, S.H.; Kim, S.Y.; Choi, J.I.; Lee, J.R.; Youn, R. Degradation and dynamic properties of poly(amide-co-imide)/carbon nanotube composite films. Polym. Polym. Compos. 2010, 18, 381–390. [Google Scholar]
- Deniz, A.E.; Vural, H.A.; Ortaç, B.; Uyar, T. Gold nanoparticle/polymer nanofibrous composites by laser ablation and electrospinning. Mater. Lett. 2011, 65, 2941–2943. [Google Scholar] [CrossRef]
- Al-Attabi, N.Y.; Kaur, G.; Adhikan, R.; Cass, P.; Bown, M.; Evans, M.; Gunatillake, P.; Malherbe, F.; Yu, A. Preparation and characterization of highly conductive polyurethane composites containing graphene and gold nanoparticles. J. Mater. Sci. 2017, 52, 11774–11784. [Google Scholar] [CrossRef]
- Kausar, A. Polyaniline composites with nanodiamond, carbon nanotube and silver nanoparticle: preparation and properties. Am. J. Polym. Sci. Eng. 2015, 3, 149–160. [Google Scholar]
- Bokobza, L. Filled elastomers: A new approach based on measurements of chain orientation. Polymer 2001, 42, 5415–5423. [Google Scholar] [CrossRef]
- Bokobza, L. Infrared analysis of elastomeric composites under uniaxial extension. Macromol. Symp. 2005, 220, 45–59. [Google Scholar] [CrossRef]
- Bokobza, L.; Buffeteau, T.; Desbat, B. Mid- and near-infrared investigation of molecular orientation in elastomeric networks. Appl. Spectrosc. 2000, 54, 360–365. [Google Scholar] [CrossRef]
- Besbes, S.; Cermelli, I.; Bokobza, L.; Monnerie, L.; Bahar, I.; Erman, B.; Herz, J. Segmental orientation in model networks of poly(dimethylsiloxane): Fourier transform infrared dichroism measurements and theoretical interpretation. Macromolecules 1992, 25, 1949–1954. [Google Scholar] [CrossRef]
- Cole, K.C.; Perrin-Sarazin, F.; Dorval-Douville, G. Infrared spectroscopic characteriztion of polymer and clay platelet orientation in blown films based on polypropylene-clay nanocomposite. Macromol. Symp. 2005, 230, 1–10. [Google Scholar] [CrossRef]
- Bokobza, L.; Garnaud, G.; Beaunier, P.; Bruneel, J.-L. Vibrational and electrical investigations of a uniaxially stretched polystyrene/carbon nanotube composite. Vib. Spectrosc. 2013, 67, 6–13. [Google Scholar] [CrossRef]
- Dazzi, A.; Prater, C.B. AFM-IR: Technology and applications in nanoscale infrared spectroscopy and chemical imaging. Chem. Rev. 2017, 117, 5146–5173. [Google Scholar] [CrossRef] [PubMed]
- Marcott, C.; Lo, M.; Dillon, E.; Kjoller, K.; Prater, C. Interface analysis of composites using AFM-based nanoscale IR and mechanical spectroscopy. Microsc. Today 2015, 23, 38–45. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Bokobza, L.; Couzi, M.; Bruneel, J.-L. Raman Spectroscopy of polymer-carbon nanomaterial composites. Rubber Chem. Technol. 2017, 90, 37–59. [Google Scholar] [CrossRef]
- Bounos, G.; Andrikopoulos, K.S.; Karachalios, T.K.; Voyiatzis, G.A. Evaluation of multi-walled carbon nanotube concentrations by Raman spectroscopy. Carbon 2014, 76, 301–309. [Google Scholar] [CrossRef]
- Everall, N.J.; Lumsdon, J.; Christopher, D.J. The effect of laser-induced heating upon the vibrational Raman spectra of graphites and carbon fibres. Carbon 1991, 29, 133–137. [Google Scholar] [CrossRef]
- Kao, C.C.; Young, R.J. A Raman spectroscopic investigation of heating effects and the deformation behaviour of epoxy/SWNT composites. Compos. Sci. Technol. 2004, 64, 2291–2295. [Google Scholar] [CrossRef]
- Yan, X.; Kitahama, Y.; Sato, H.; Suzuki, T.; Han, X.; Itoh, T.; Bokobza, L.; Ozaki, Y. Laser heating effect on Raman spectra of styrene-butadiene rubber/multiwalled carbon nanotube nanocomposites. Chem. Phys. Lett. 2012, 523, 87–91. [Google Scholar] [CrossRef]
- Bokobza, L.; Pflock, T.; Lindemann, A.; Kwiryn, D.; Dos Santos Claro, P. Thermal conductivity and mechanical properties of composites based on multiwall carbon nanotubes and styrene-butadiene rubber. Kautsch. Gummi Kunstst. 2014, 67, 45–50. [Google Scholar]
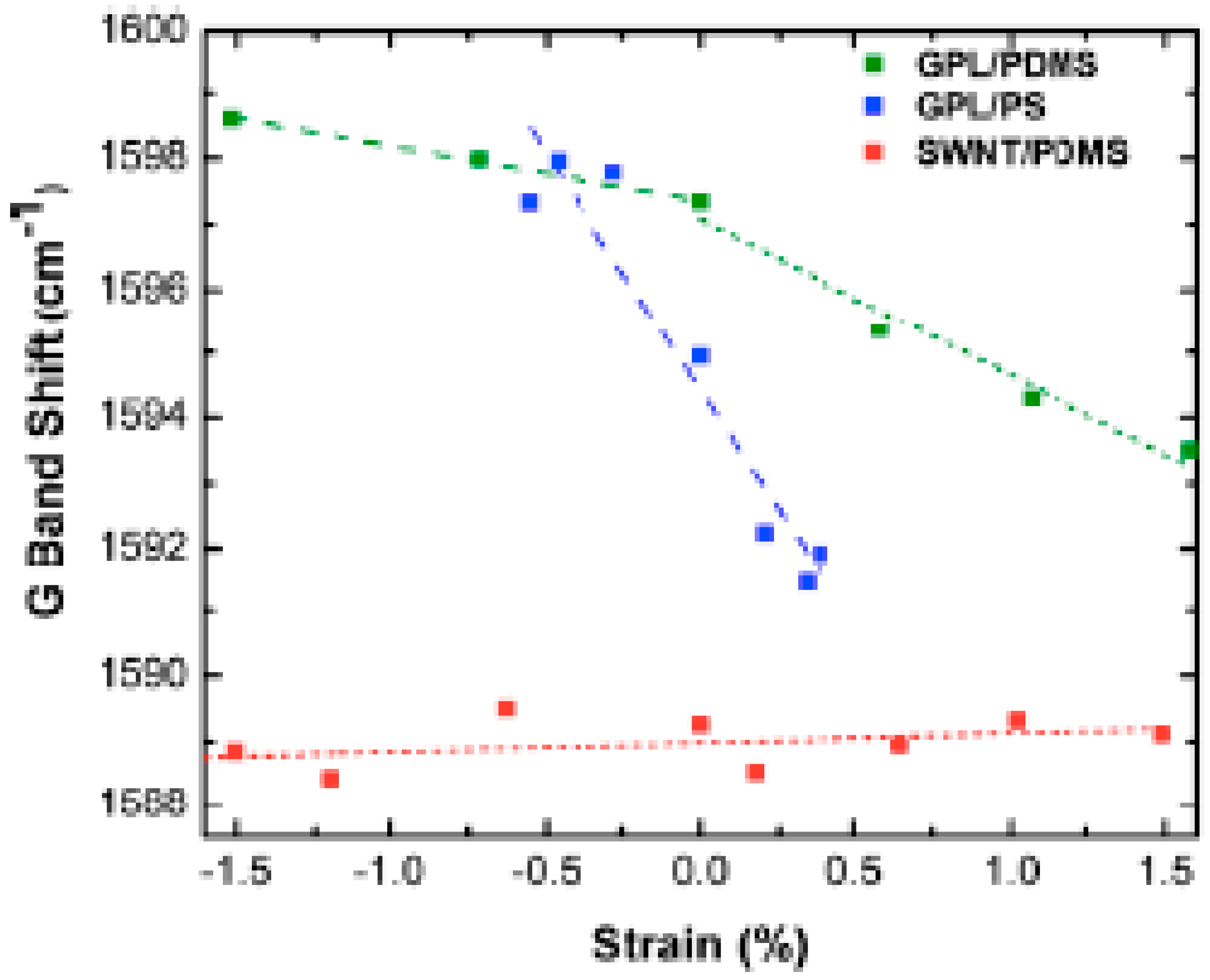
- Galiotis, C.; Batchelder, D.N. Strain dependences of the first- and second-order Raman spectra of carbon fibres. J. Mater. Sci. Lett. 1988, 7, 545–547. [Google Scholar] [CrossRef]
- Srivastava, I.; Mehta, R.J.; Yu, Z.-Z.; Schadler, L.; Koratkar, N. Raman study of interfacial load transfer in graphene nanocomposites. Appl. Phys. Lett. 2011, 98, 063102. [Google Scholar] [CrossRef]
- Frogley, M.D.; Ravich, D.; Wagner, H.D. Mechanical properties of carbon nanoparticle-reinforced elastomers. Compos. Sci. Technol. 2003, 63, 1647–1654. [Google Scholar] [CrossRef]
- Bokobza, L.; Zhang, J. Raman spectroscopic characterization of multiwall carbon nanotubes and of composites. eXPRESS Polym. Lett. 2012, 6, 601–608. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman spectroscopic investigation of carbon-based materials and their composites. Comparison between carbon nanotubes and carbon black. Chem. Phys. Lett. 2013, 590, 153–159. [Google Scholar] [CrossRef]
- Beigbeder, A.; Linares, M.; Devalckenaere, M.; Degée, P.; Claes, M.; Beljonne, D.; Lazzaroni, R.; Dubois, P. CH- interactions as the driving force for silicone-based nanocomposites with exceptional properties. Adv. Mater. 2008, 20, 1003–1007. [Google Scholar] [CrossRef]
- Bokobza, L. Some issues in rubber nanocomposites: New opportunities for silicone materials. Viewpoint. Silicon 2009, 1, 141–145. [Google Scholar] [CrossRef]
- Bokobza, L.; Rahmani, M. Carbon nanotubes: Exceptional reinforcing fillers for silicon rubbers. Kautsch. Gummi Kunstst. 2009, 62, 112–117. [Google Scholar]
- Kumar, N.; Mignuzzi, S.; Su, W.; Roy, D. Tip-enhanced Raman spectroscopy: principles and applications. EPJ Tech. Instrum. 2015. [Google Scholar] [CrossRef]
- Kurouski, D. Advances of tip-enhanced Raman spectroscopy (TERS) in electrochemistry, biochemistry, and surface science. Vib. Spectrosc. 2017, 91, 3–15. [Google Scholar] [CrossRef]
- Saito, Y.; Yanagi, K. Using a nano light source to investigate small-scale composite materials. SPIE 2008. [Google Scholar] [CrossRef]
- Yano, T.; Inouye, Y.; Kawata, S. Nanoscale uniaxial pressure effect of a carbon nanotube bundle on tip-enhanced near-field Raman spectra. Nano Lett. 2006, 6, 1269–1273. [Google Scholar] [CrossRef] [PubMed]
- Yano, T.; Ichimura, T.; Kuwahara, S.; H’Dhili, F.; Uetsuki, K.; Okuno, Y.; Verma, P.; Kawata, S. Tip-enhanced nano-Raman analytical imaging of locally induced strain distribution in carbon nanotubes. Nat. Commun. 2013. [Google Scholar] [CrossRef] [PubMed]
- Vantasin, S.; Yan, X.-L.; Suzuki, T.; Ozaki, Y. Tip-enhanced Raman scattering of nanomaterials. e-J. Surface Sci. Technol. 2015, 13, 329–338. [Google Scholar] [CrossRef]
- Suzuki, T.; Yan, X.; Kitahama, Y.; Sato, H.; Itoh, T.; Miura, T.; Ozaki, Y. Tip-enhanced Raman spectroscopy study of local interactions at the interface of styrene-butadiene rubber/multiwalled carbon nanotube nanocomposites. J. Phys. Chem. C 2013, 117, 1436–1440. [Google Scholar] [CrossRef]
- Giesfeldt, K.S.; Connatser, R.M.; De Jesús, M.A.; Dutta, P.; Sepaniak, M.J. Gold-polymer nanocomposites: Studies of their optical properties and their potential as SERS substrates. J. Raman Spectrosc. 2005, 36, 1134–1142. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Dahanayaka, D.H.; Bumm, L.A.; Li, Z.; Watanabe, F.; Sharma, R.; Xu, Y.; Biris, A.S.; Norton, M.G.; et al. Tailored polymer-metal fractal nanocomposites: An approach to highly active surface enhanced Raman scattering substrates. Nanotechnology 2009, 20. [Google Scholar] [CrossRef] [PubMed]
- Fateixa, S.; Girão, A.V.; Nogueira, H.I.S.; Trindade, T. Polymer based silver nanocomposites as versatile solid film and aqueous emulsion SERS substrates. J. Mater. Chem. 2011, 21, 15629–15636. [Google Scholar] [CrossRef] [Green Version]
- Rao, V.K.; Radhakrishnan, T.P. Tuning the SERS response with Ag-Au nanoparticle-embedded polymer thin film substrates. ACS Appl. Mater. Interfaces 2015, 7, 12767–12773. [Google Scholar] [CrossRef] [PubMed]
- Schlücker, S. Surface-enhanced Raman spectroscopy: Concepts and chemical applications. Angew. Chem. Int. Ed. 2014, 53, 4756–4795. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.; Zhi, X.; Zhang, A. Application of graphene in surface-enhanced Raman spectroscopy. Nano Biomed. Eng. 2017, 9, 49–56. [Google Scholar] [CrossRef]
- Carboni, D.; Lasio, B.; Alzari, V.; Mariani, A.; Loche, D.; Casula, M.F.; Malfatti, L.; Innocenzi, P. Graphene-mediated surface enhanced Raman scattering in silica mesoporous nanocomposite films. Phys. Chem. Chem. Phys. 2014, 16, 25809–25818. [Google Scholar] [CrossRef] [PubMed]








© 2017 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bokobza, L. Spectroscopic Techniques for the Characterization of Polymer Nanocomposites: A Review. Polymers 2018, 10, 7. https://doi.org/10.3390/polym10010007
Bokobza L. Spectroscopic Techniques for the Characterization of Polymer Nanocomposites: A Review. Polymers. 2018; 10(1):7. https://doi.org/10.3390/polym10010007
Chicago/Turabian StyleBokobza, Liliane. 2018. "Spectroscopic Techniques for the Characterization of Polymer Nanocomposites: A Review" Polymers 10, no. 1: 7. https://doi.org/10.3390/polym10010007



