A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CPE
2.3. Preparation of SPI and SPI/CPE Films
2.4. Characterization
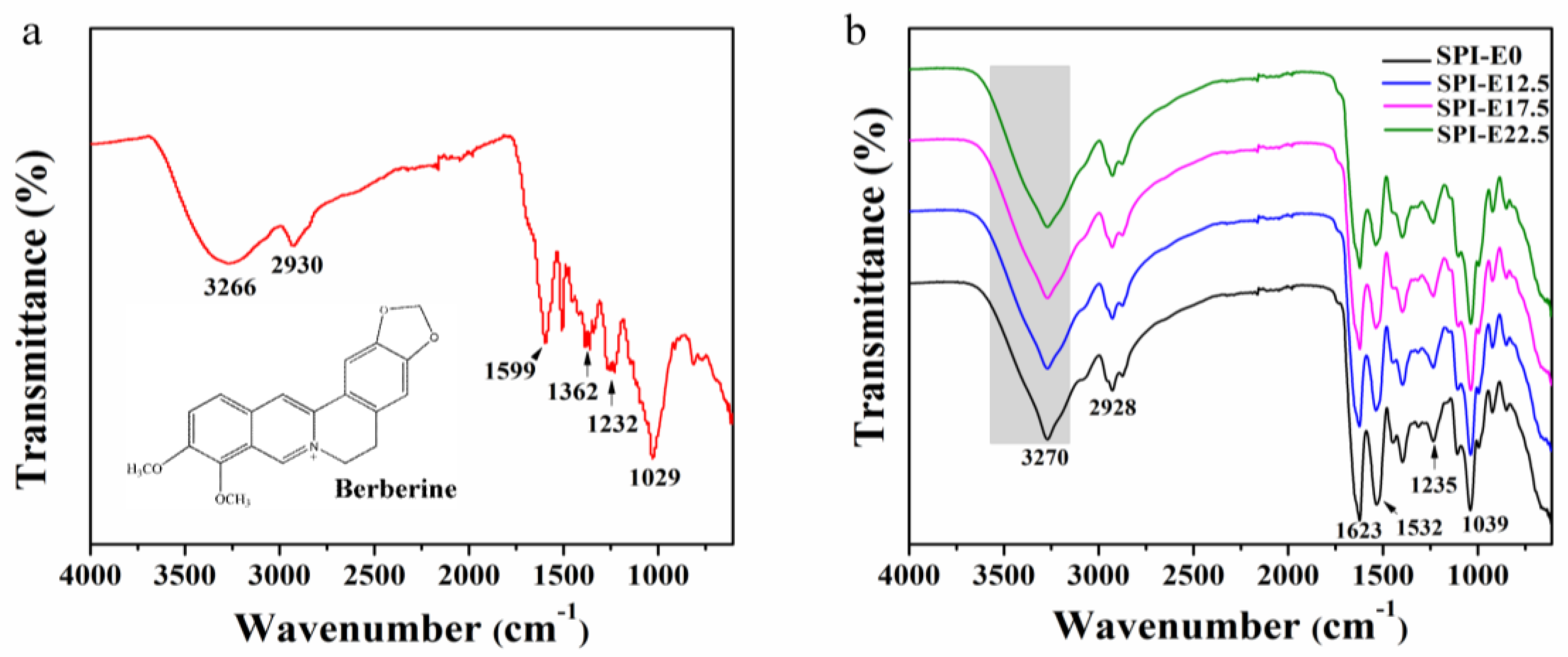
2.4.1. FTIR Spectroscopy
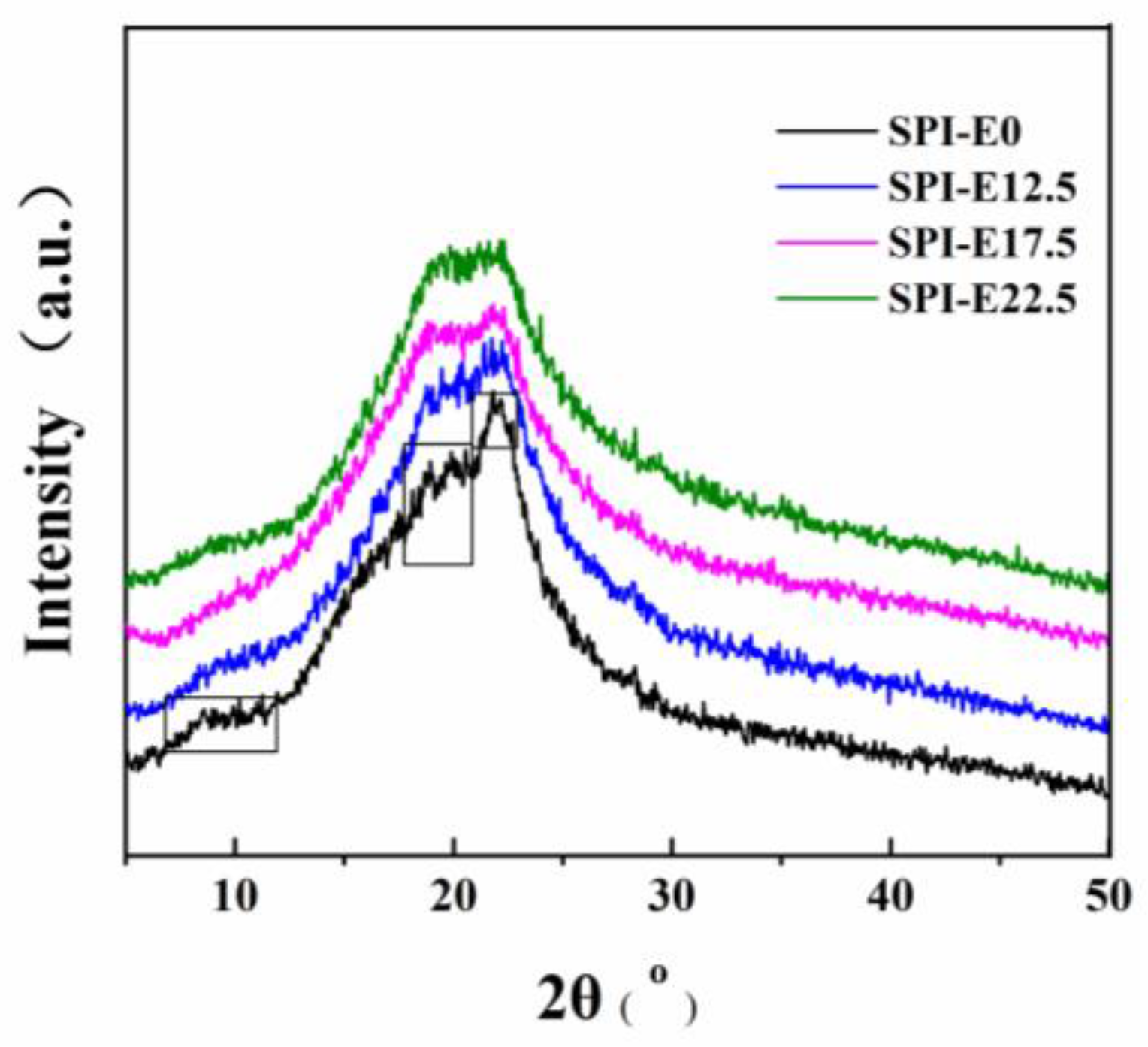
2.4.2. XRD Analysis
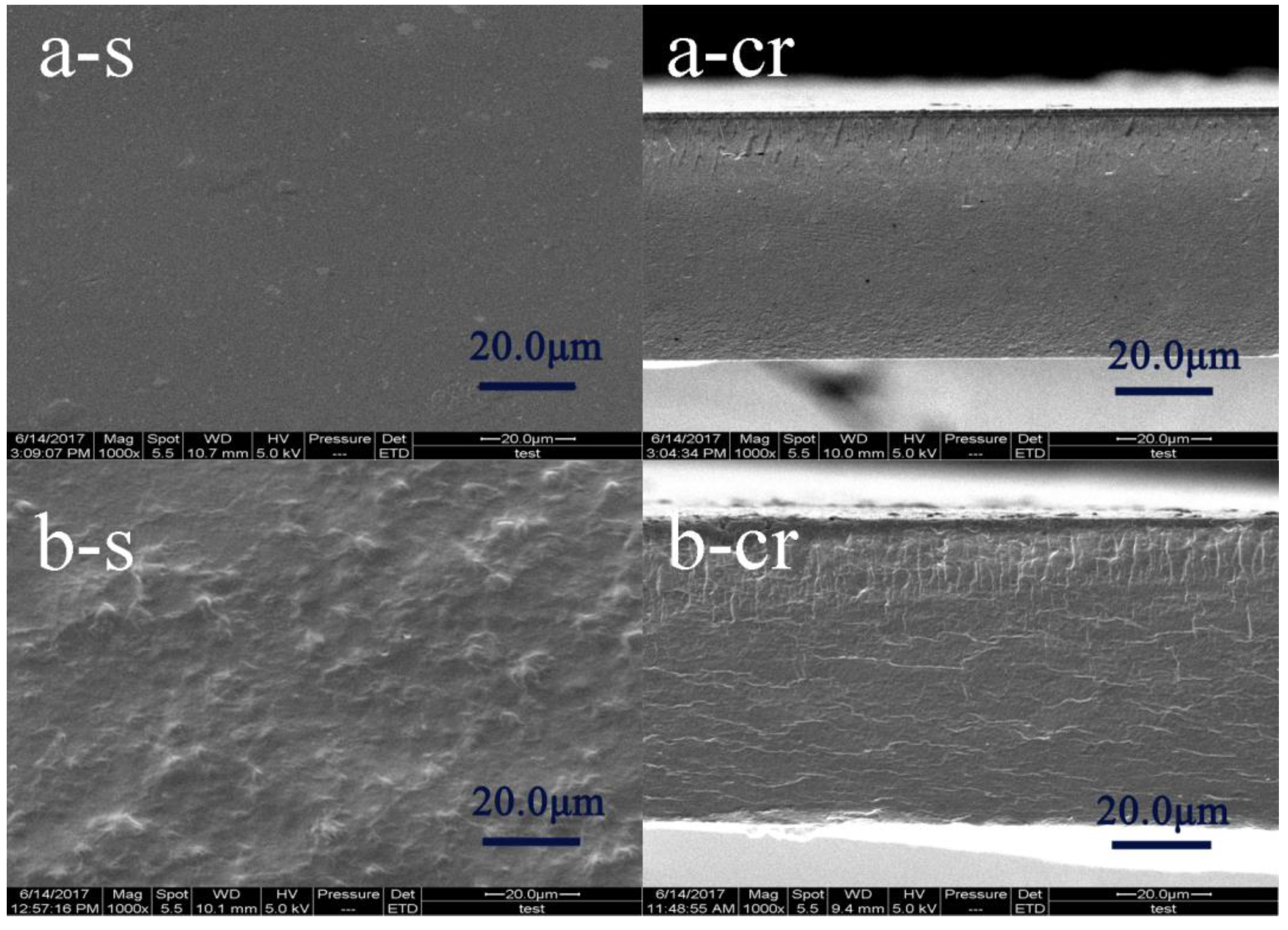
2.4.3. SEM Observation
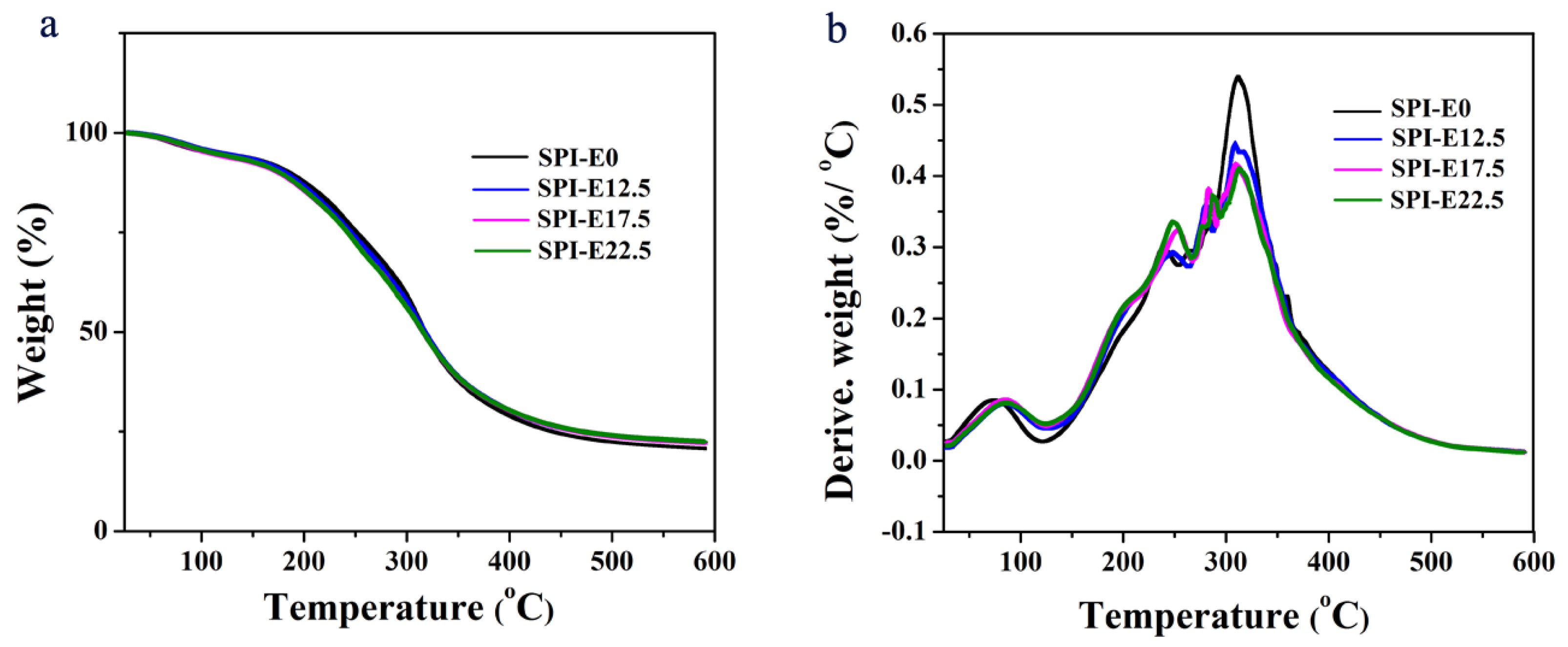
2.4.4. TGA Analysis
2.4.5. Rheological Measurement
2.4.6. Mechanical Testing
2.4.7. WVP Measurement
2.4.8. OP Measurement
2.4.9. Color and Light Transmission Analysis
2.4.10. Determination of Antibacterial Activity
2.4.11. Evaluation of Antioxidant Activity
2.4.12. Statistical Analysis
3. Results and Discussion
3.1. FTIR Spectroscopy of Films
3.2. XRD Patterns of Films
3.3. Morphologies of the Films
3.4. Thermal Analysis
3.5. Rheological Properties of FFS
3.6. Mechanical Properties of the Films
3.7. WVP
3.8. OP
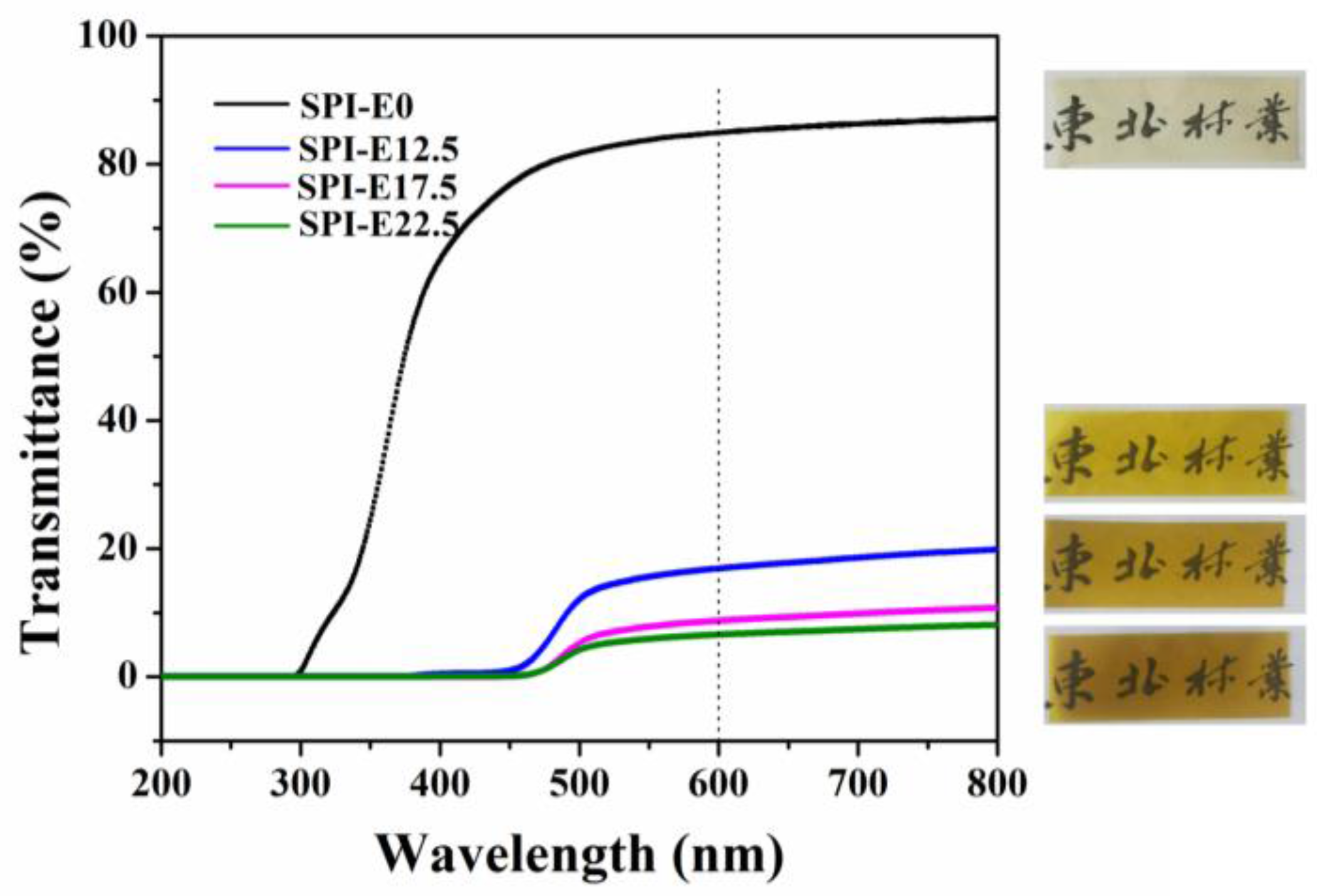
3.9. Color and Light Transmission
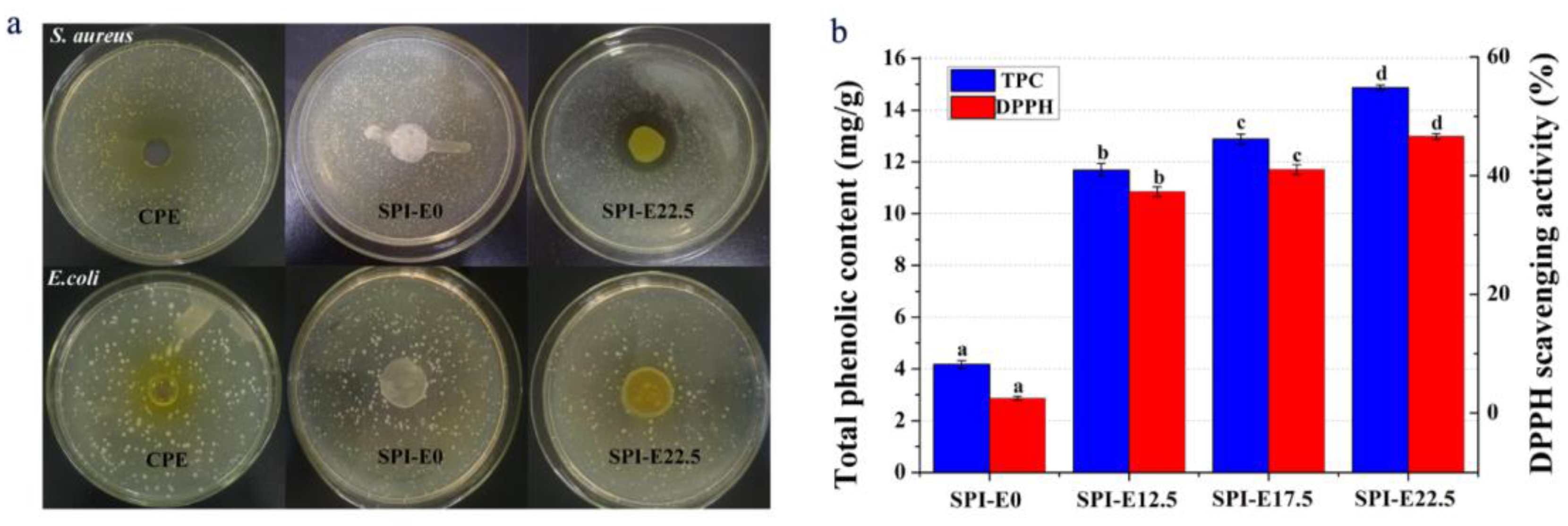
3.10. Antibacterial Activity
3.11. Antioxidant Activity
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Gómez-Estaca, J.; Gavara, R.; Catalá, R.; Hernández-Muñoz, P. The potential of proteins for producing food packaging materials: A review. Packag. Technol. Sci. 2016, 29, 203–224. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.-M.; Ha, C.-S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38, 1629–1652. [Google Scholar] [CrossRef]
- Nur Hanani, Z.A.; Roos, Y.H.; Kerry, J.P. Use and application of gelatin as potential biodegradable packaging materials for food products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Cao, N.; Fu, Y.; He, J. Preparation and physical properties of soy protein isolate and gelatin composite films. Food Hydrocoll. 2007, 21, 1153–1162. [Google Scholar] [CrossRef]
- Cho, S.Y.; Lee, S.Y.; Rhee, C. Edible oxygen barrier bilayer film pouches from corn zein and soy protein isolate for olive oil packaging. LWT Food Sci. Technol. 2010, 43, 1234–1239. [Google Scholar] [CrossRef]
- Zhao, S.; Yao, J.; Fei, X.; Shao, Z.; Chen, X. An antimicrobial film by embedding in situ synthesized silver nanoparticles in soy protein isolate. Mater. Lett. 2013, 95, 142–144. [Google Scholar] [CrossRef]
- Lucera, A.; Costa, C.; Conte, A.; Del Nobile, M.A. Food applications of natural antimicrobial compounds. Front. Microbiol. 2012, 3, 287. [Google Scholar] [CrossRef] [PubMed]
- Vuddanda, P.R.; Chakraborty, S.; Singh, S. Berberine: A potential phytochemical with multispectrum therapeutic activities. Expert Opin. Investig. Drugs 2010, 19, 1297–1307. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.Y.; Kim, M.H.; Han, J.M.; Hong, J.; Lee, T.H.; Kim, S.H.; Yang, W.M. The anti-inflammatory potential of cortex phellodendron in vivo and in vitro: Down-regulation of no and inos through suppression of nf-kappab and mapk activation. Int. Immunopharmacol. 2014, 19, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Sur, B.; Shim, I.; Lee, H.; Hahm, D.H. Phellodendron amurense and its major alkaloid compound, berberine ameliorates scopolamine-induced neuronal impairment and memory dysfunction in rats. Korean J. Physiol. Pharmacol. 2012, 16, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Ong, E.S.; Len, S.M. Pressurized hot water extraction of berberine, baicalein and glycyrrhizin in medicinal plants. Anal. Chim. Acta 2003, 482, 81–89. [Google Scholar] [CrossRef]
- Krishnaiah, D.; Bono, A.; Sarbatly, R.; Anisuzzaman, S.M. Antioxidant activity and total phenolic content of an isolated Morinda citrifolia L. Methanolic extract from poly-ethersulphone (PES) membrane separator. J. King Saud Univ. Eng. Sci. 2015, 27, 63–67. [Google Scholar] [CrossRef]
- Ricci, A.; Olejar, K.J.; Parpinello, G.P.; Kilmartin, P.A.; Versari, A. Application of fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl. Spectrosc. Rev. 2015, 50, 407–442. [Google Scholar] [CrossRef]
- Salari, R.; Bazzaz, B.S.; Rajabi, O.; Khashyarmanesh, Z. New aspects of saccharomyces cerevisiae as a novel carrier for berberine. DARU J. Pharm. Sci. 2013, 21, 73. [Google Scholar] [CrossRef] [PubMed]
- Denavi, G.A.; Pérez-Mateos, M.; Añón, M.C.; Montero, P.; Mauri, A.N.; Gómez-Guillén, M.C. Structural and functional properties of soy protein isolate and cod gelatin blend films. Food Hydrocoll. 2009, 23, 2094–2101. [Google Scholar] [CrossRef]
- Ahmad, M.; Nirmal, N.P.; Danish, M.; Chuprom, J.; Jafarzedeh, S. Characterisation of composite films fabricated from collagen/chitosan and collagen/soy protein isolate for food packaging applications. RSC Adv. 2016, 6, 82191–82204. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Guerrero, P.; De la Caba, K. Characterization of agar/soy protein biocomposite films: Effect of agar on the extruded pellets and compression moulded films. Carbohydr. Polym. 2016, 151, 408–416. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Song, F.; Wang, X.L.; Wang, Y.Z. Development of soy protein isolate/waterborne polyurethane blend films with improved properties. Colloids Surf. B 2012, 100, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Hoque, M.S.; Benjakul, S.; Prodpran, T. Effect of heat treatment of film-forming solution on the properties of film from cuttlefish (Sepia pharaonis) skin gelatin. J. Food Eng. 2010, 96, 66–73. [Google Scholar] [CrossRef]
- Liu, X.; Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Simultaneously toughening and strengthening soy protein isolate-based composites via carboxymethylated chitosan and halloysite nanotube hybridization. Materials 2017, 10, 653. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, P.; Nur Hanani, Z.A.; Kerry, J.P.; de la Caba, K. Characterization of soy protein-based films prepared with acids and oils by compression. J. Food Eng. 2011, 107, 41–49. [Google Scholar] [CrossRef]
- Su, J.-F.; Huang, Z.; Zhao, Y.-H.; Yuan, X.-Y.; Wang, X.-Y.; Li, M. Moisture sorption and water vapor permeability of soy protein isolate/poly(vinyl alcohol)/glycerol blend films. Ind. Crops Prod. 2010, 31, 266–276. [Google Scholar] [CrossRef]
- Wang, H.; Hu, D.; Ma, Q.; Wang, L. Physical and antioxidant properties of flexible soy protein isolate films by incorporating chestnut (Castanea mollissima) bur extracts. LWT Food Sci. Technol. 2016, 71, 33–39. [Google Scholar] [CrossRef]
- Xu, F.; Dong, Y.; Zhang, W.; Zhang, S.; Li, L.; Li, J. Preparation of cross-linked soy protein isolate-based environmentally-friendly films enhanced by ptge and pam. Ind. Crops Prod. 2015, 67, 373–380. [Google Scholar] [CrossRef]
- El Miri, N.; Abdelouahdi, K.; Zahouily, M.; Fihri, A.; Barakat, A.; Solhy, A.; El Achaby, M. Bio-nanocomposite films based on cellulose nanocrystals filled polyvinyl alcohol/chitosan polymer blend. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Liu, P.; Xu, H.; Zhao, Y.; Yang, Y. Rheological properties of soy protein isolate solution for fibers and films. Food Hydrocoll. 2017, 64, 149–156. [Google Scholar] [CrossRef]
- Moreno, O.; Pastor, C.; Muller, J.; Atarés, L.; González, C.; Chiralt, A. Physical and bioactive properties of corn starch—Buttermilk edible films. J. Food Eng. 2014, 141, 27–36. [Google Scholar] [CrossRef]
- Ma, Q.; Du, L.; Yang, Y.; Wang, L. Rheology of film-forming solutions and physical properties of tara gum film reinforced with polyvinyl alcohol (PVA). Food Hydrocoll. 2017, 63, 677–684. [Google Scholar] [CrossRef]
- Diftis, N.G.; Biliaderis, C.G.; Kiosseoglou, V.D. Rheological properties and stability of model salad dressing emulsions prepared with a dry-heated soybean protein isolate–dextran mixture. Food Hydrocoll. 2005, 19, 1025–1031. [Google Scholar] [CrossRef]
- Osano, J.P.; Hosseini-Parvar, S.H.; Matia-Merino, L.; Golding, M. Emulsifying properties of a novel polysaccharide extracted from basil seed (Ocimum bacilicum L.): Effect of polysaccharide and protein content. Food Hydrocoll. 2014, 37, 40–48. [Google Scholar] [CrossRef]
- Cho, S.Y.; Rhee, C. Sorption characteristics of soy protein films and their relation to mechanical properties. LWT Food Sci. Technol. 2002, 35, 151–157. [Google Scholar] [CrossRef]
- Kim, K.M.; Weller, C.L.; Hanna, M.A.; Gennadios, A. Heat curing of soy protein films at selected temperatures and pressures. LWT Food Sci. Technol. 2002, 35, 140–145. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the barrier and mechanical properties of corn starch-based edible films: Effect of citric acid and carboxymethyl cellulose. Ind. Crops Prod. 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Hu, D.; Wang, H.; Wang, L. Physical properties and antibacterial activity of quaternized chitosan/carboxymethyl cellulose blend films. LWT Food Sci. Technol. 2016, 65, 398–405. [Google Scholar] [CrossRef]
- Alves, M.M.; Gonçalves, M.P.; Rocha, C.M.R. Effect of ferulic acid on the performance of soy protein isolate-based edible coatings applied to fresh-cut apples. LWT Food Sci. Technol. 2017, 80, 409–415. [Google Scholar] [CrossRef]
- Ustunol, Z.; Mert, B. Water solubility, mechanical, barrier, and thermal properties of cross-linked whey protein isolate-based films. J. Food Sci. 2006, 69. [Google Scholar] [CrossRef]
- Wojtyczka, R.D.; Dziedzic, A.; Kepa, M.; Kubina, R.; Kabala-Dzik, A.; Mularz, T.; Idzik, D. Berberine enhances the antibacterial activity of selected antibiotics against coagulase-negative staphylococcus strains in vitro. Molecules 2014, 19, 6583–6596. [Google Scholar] [CrossRef] [PubMed]
- Sivarooban, T.; Hettiarachchy, N.S.; Johnson, M.G. Physical and antimicrobial properties of grape seed extract, nisin, and edta incorporated soy protein edible films. Food Res. Int. 2008, 41, 781–785. [Google Scholar] [CrossRef]
- Friesen, K.; Chang, C.; Nickerson, M. Incorporation of phenolic compounds, rutin and epicatechin, into soy protein isolate films: Mechanical, barrier and cross-linking properties. Food Chem. 2015, 172, 18–23. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.-X.; Zhao, M. Physical and chemical modification of SPI as a potential means to enhance small peptide contents and antioxidant activity found in hydrolysates. Innov. Food Sci. Emerg. Technol. 2010, 11, 677–683. [Google Scholar] [CrossRef]
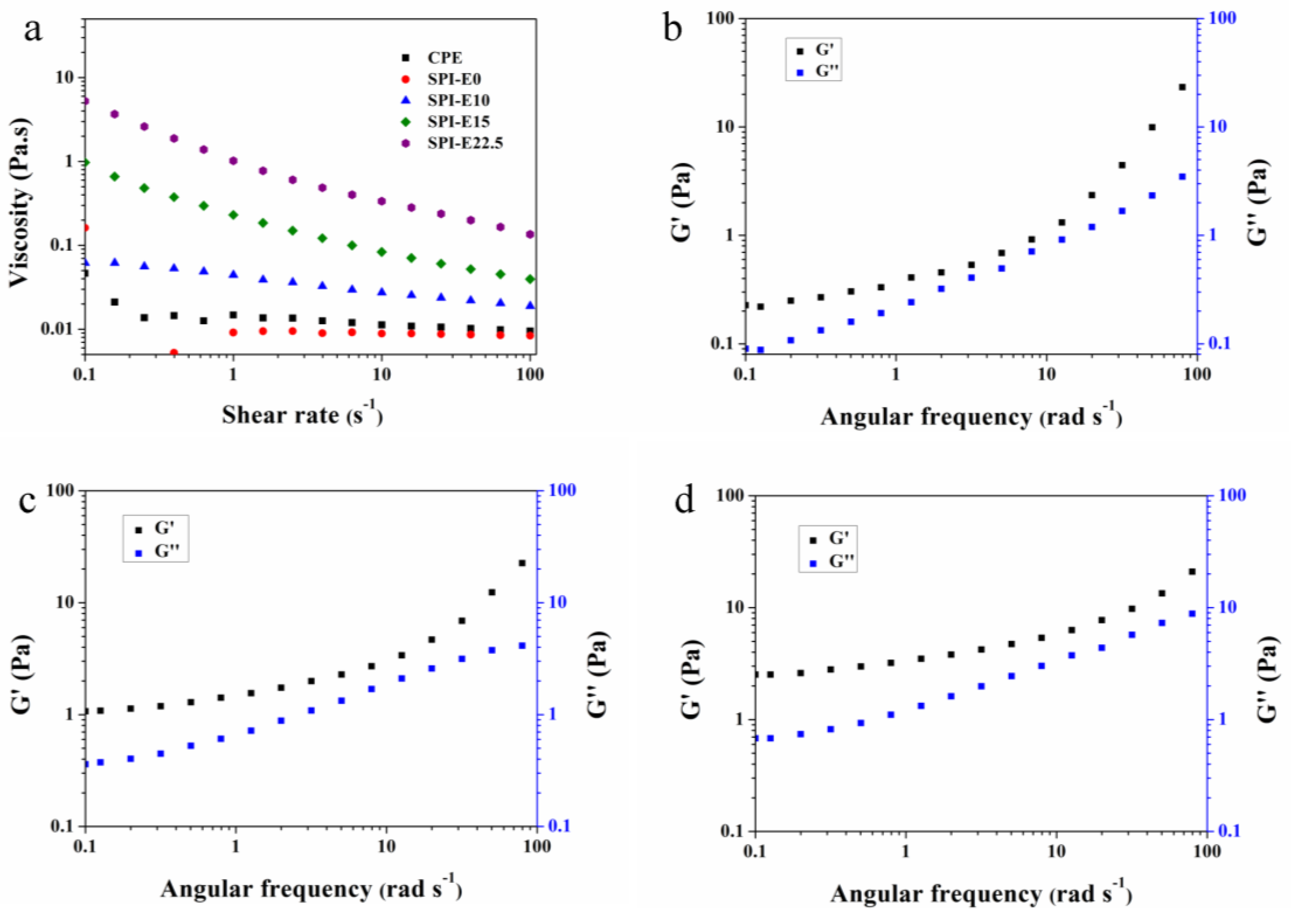
| Sample Code | Ostwald-de Waele Model | ||
|---|---|---|---|
| κ | n | R2 | |
| CPE | 0.014 | 0.923 | 0.999 |
| SPI-E0 | 0.010 | 0.970 | 0.998 |
| SPI-E10 | 0.040 | 0.835 | 0.999 |
| SPI-E15 | 0.185 | 0.662 | 0.998 |
| SPI-E22.5 | 0.920 | 0.584 | 0.997 |
| Sample Code | TS (MPa) | E (%) | WVP (g mm−2·s−1·Pa−1 × 10−10) | OP (cm3mm·m−2·day−1·atm−1 × 1011) |
|---|---|---|---|---|
| SPI-E0 | 5.57 ± 0.04 a | 130.13 ± 3.18 g | 2.56 ± 0.14 c | 0.18 ± 0.01 c |
| SPI-E10 | 5.68 ± 0.28 a | 88.73 ± 4.97 f | 2.44 ± 0.12 b,c | 0.16 ± 0.04 c |
| SPI-E12.5 | 5.70 ± 0.17 a | 73.07 ± 2.65 e | 2.36 ± 0.07 a,b | 0.13 ± 0.02 b,c |
| SPI-E15 | 6.00 ± 0.40 a | 56.73 ± 2.22 d | 2.34 ± 0.13 a | 0.12 ± 0.02 b,c |
| SPI-E17.5 | 5.01 ± 0.37 b | 49.00 ± 2.85 c | 2.29 ± 0.03 a,b | 0.10 ± 0.02 a,b |
| SPI-E20 | 4.73 ± 0.02 b | 37.43 ± 0.05 b | 2.24 ± 0.04 a | 0.08 ± 0.01 a |
| SPI-E22.5 | 4.50 ± 0.16 b | 31.00 ± 2.61 a | 2.19 ± 0.01 a | 0.08 ± 0.01 a |
| Sample Code | L* | A* | B* |
|---|---|---|---|
| SPI-E0 | 96.38 ± 0.11 a | −1.80 ± 0.06 b | 10.67 ± 0.57 a |
| SPI-E12.5 | 83.42 ± 0.15 b | −3.85 ± 0.16 a | 88.76 ± 0.94 b |
| SPI-E17.5 | 75.05 ± 0.51 d | 4.97 ± 0.48 c | 93.16 ± 0.37 d |
| SPI-E22.5 | 72.84 ± 0.58 f | 7.52 ± 0.54 e | 93.94 ± 0.04 d |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, S.; Wang, L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers 2018, 10, 71. https://doi.org/10.3390/polym10010071
Liang S, Wang L. A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract. Polymers. 2018; 10(1):71. https://doi.org/10.3390/polym10010071
Chicago/Turabian StyleLiang, Shumin, and Lijuan Wang. 2018. "A Natural Antibacterial-Antioxidant Film from Soy Protein Isolate Incorporated with Cortex Phellodendron Extract" Polymers 10, no. 1: 71. https://doi.org/10.3390/polym10010071




