High-Efficient and Recyclable Magnetic Separable Catalyst for Catalytic Hydrogenolysis of β-O-4 Linkage in Lignin
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Fe3O4
2.3. Synthesis of Pd-Fe3O4
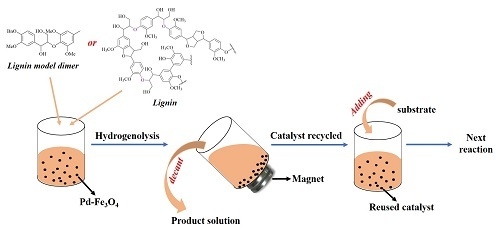
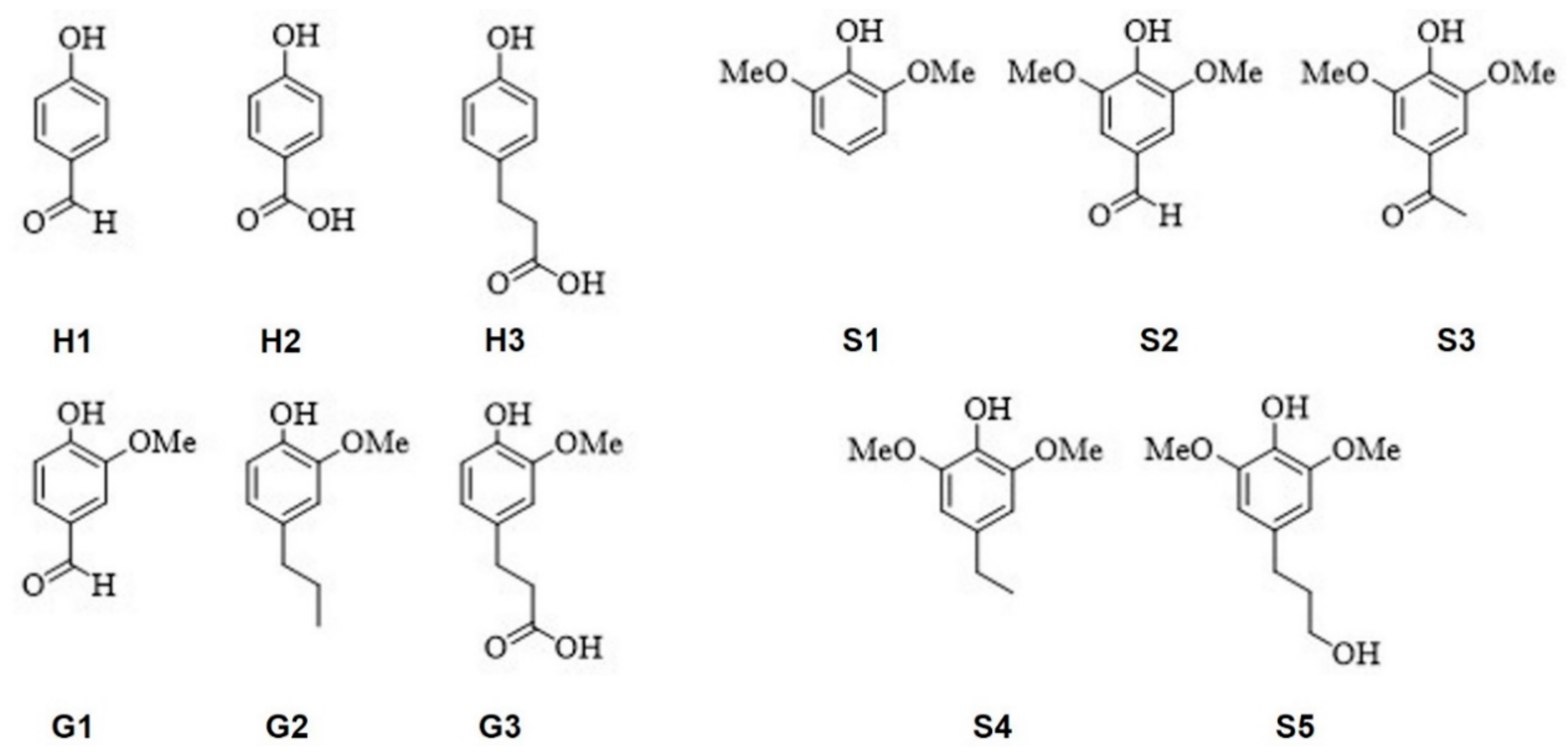
2.4. General Procedure for Lignin Model-Dimer Depolymerization Reaction
2.5. General Procedure for Bagasse Lignin Extraction
2.6. General Procedure for Bagasse Lignin Depolymerization Reaction
2.7. Catalyst Characterization
2.8. Analytical Methods
2.9. Recycling of the Catalysts
3. Results and Discussions
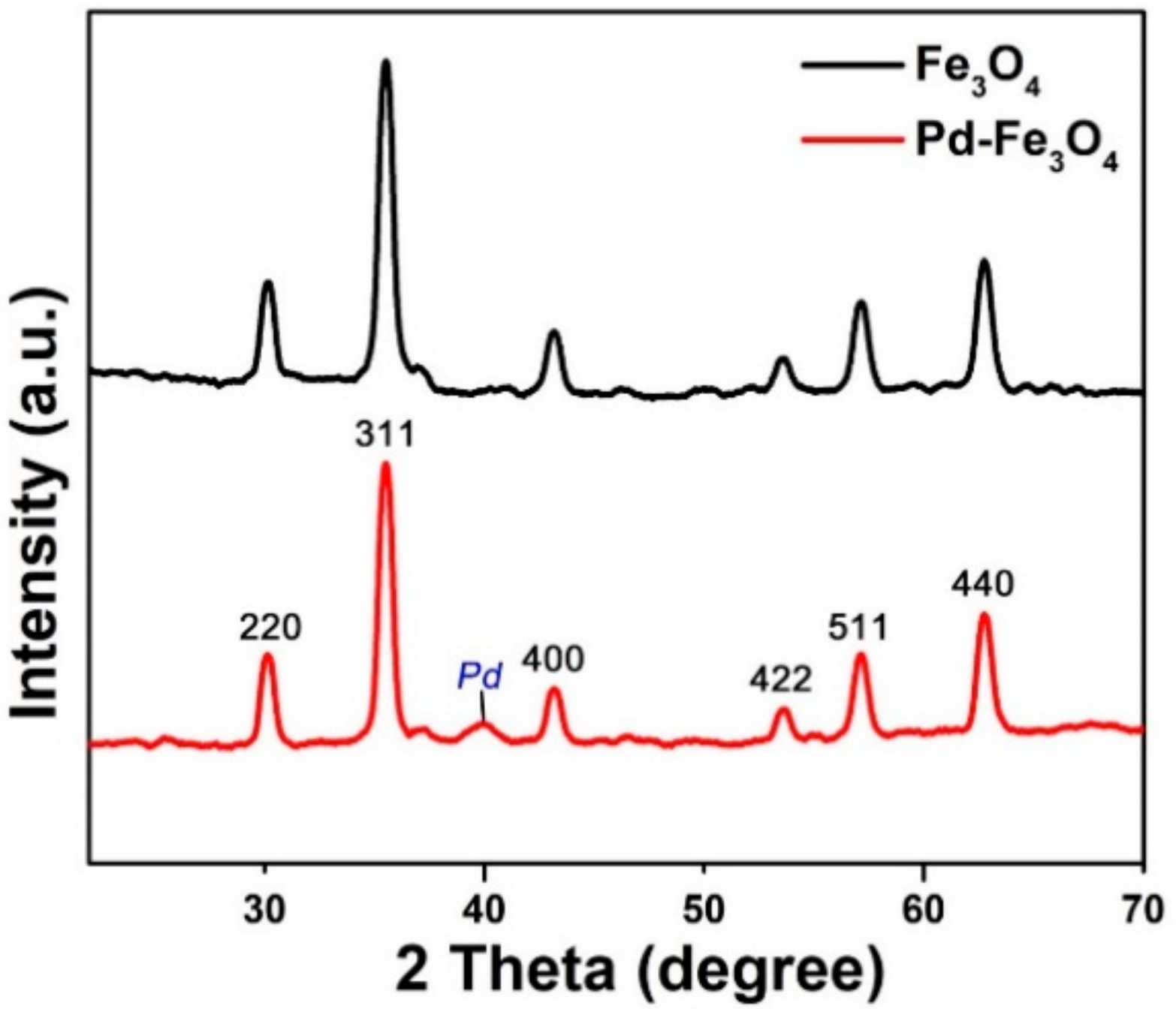
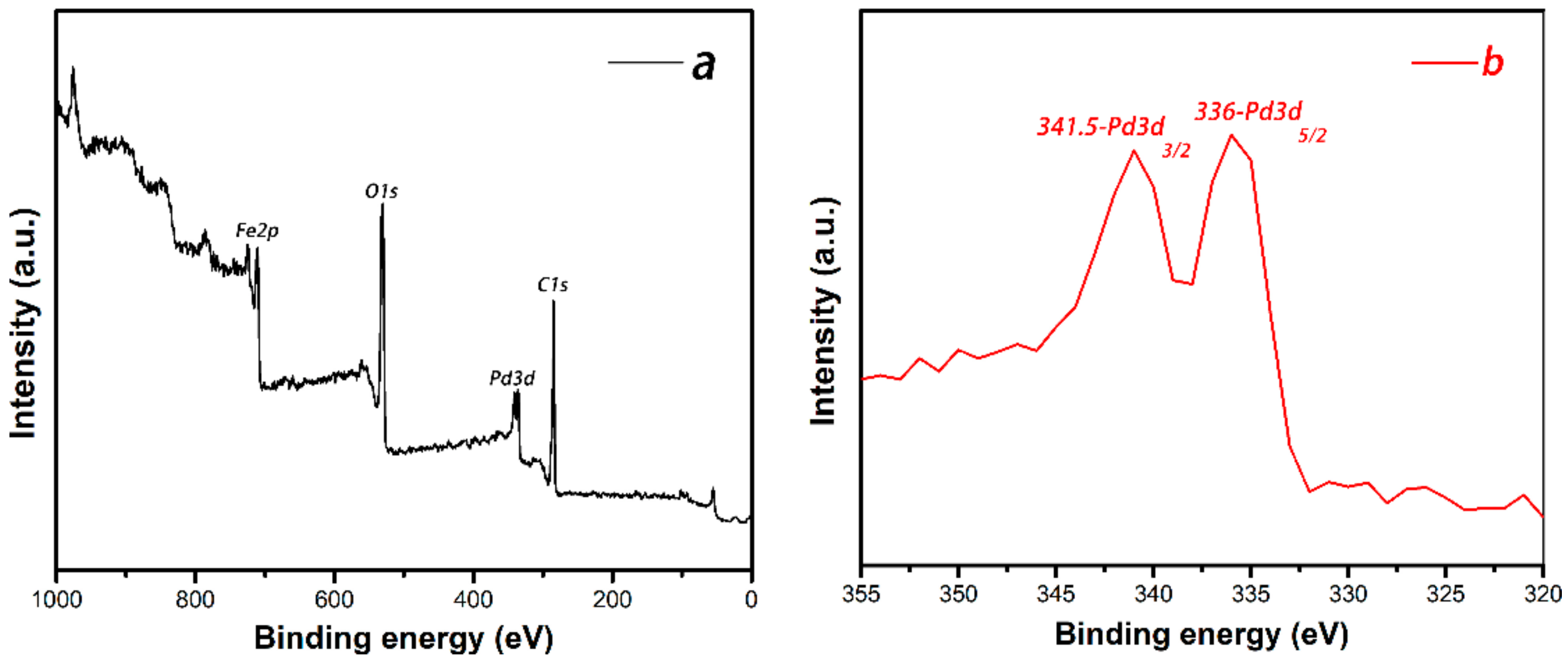
3.1. Preparation and Characterization of the Pd-Fe3O4 Catalyst
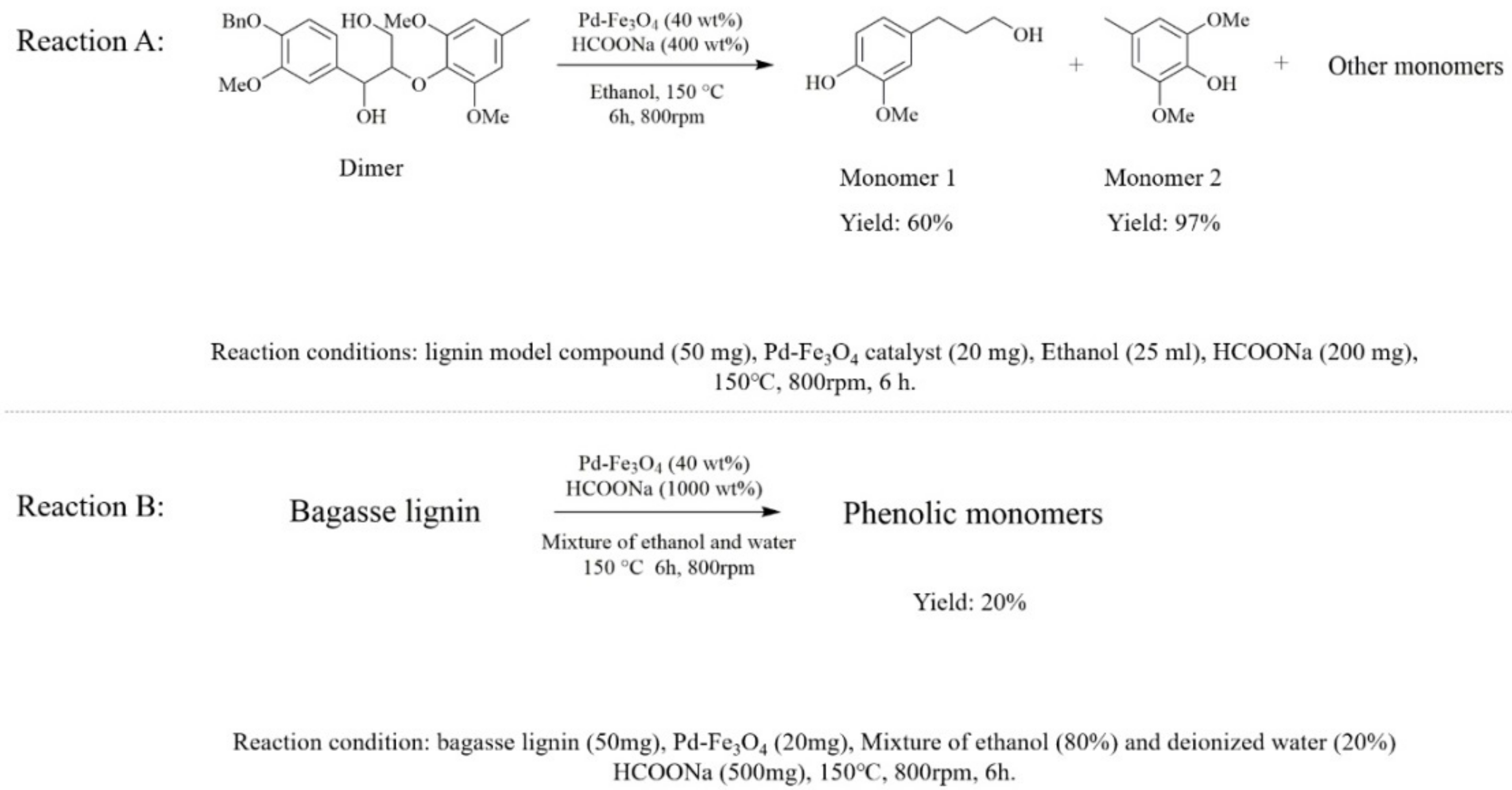
3.2. Catalytic Hydrogenolysis of β-O-4 Model Compound and Bagasse Lignin by Pd-Fe3O4
3.3. Recyclability of Pd-Fe3O4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Azadi, P.; Inderwildi, O.R.; Farnood, R.; King, D.A. Liquid fuels, hydrogen and chemicals from lignin: A critical review. Renew. Sustain. Energy Rev. 2013, 21, 506–523. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van den Bosch, S.; Koelewijn, S.F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: An interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- Upton, B.M.; Kasko, A.M. Strategies for the conversion of lignin to high-value polymeric materials: Review and perspective. Chem. Rev. 2016, 116, 2275–2306. [Google Scholar] [CrossRef] [PubMed]
- Subbotina, E.; Galkin, M.V.; Samec, J.S.M. Pd/C-catalyzed hydrogenolysis of dibenzodioxocin lignin model compounds using silanes and water as hydrogen source. ACS Sustain. Chem. Eng. 2017, 5, 3726–3731. [Google Scholar] [CrossRef]
- Gosselink, R.J.A.; de Jong, E.; Guran, B.; Abächerli, A. Co-ordination network for lignin—Standardisation, production and applications adapted to market requirements (eurolignin). Ind. Crops Prod. 2004, 20, 121–129. [Google Scholar] [CrossRef]
- Lora, J.H.; Glasser, W.G. Recent industrial applications of lignin: A sustainable alternative to nonrenewable materials. J. Polym. Environ. 2002, 10, 39–48. [Google Scholar] [CrossRef]
- Bozell, J.J.; Holladay, J.E.; Johnson, D.; White, J.F. Top value added chemicals from biomass. Nato Adv. Sci. Inst. 2007, 2, 263–275. [Google Scholar]
- Verziu, M.; Tirsoaga, A.; Cojocaru, B.; Bucur, C.; Tudora, B.; Richel, A.; Aguedo, M.; Samikannu, A.; Mikkola, J.P. Hydrogenolysis of lignin over ru-based catalysts: The role of the ruthenium in a lignin fragmentation process. Mol. Catal. 2018, 450, 65–76. [Google Scholar] [CrossRef]
- Hu, L.; Pan, H.; Zhou, Y.; Zhang, M. Methods to improve lignin’s reactivity as a phenol substitute and as replacement for other phenolic compounds: A brief review. Bioresources 2011, 6, 3515–3525. [Google Scholar]
- Li, C.; Zhao, X.; Wang, A.; Huber, G.W.; Zhang, T. Catalytic transformation of lignin for the production of chemicals and fuels. Chem. Rev. 2015, 115, 11559–11624. [Google Scholar] [CrossRef] [PubMed]
- Klein, I.; Marcum, C.; Kenttämaa, H.; Abu-Omar, M.M. Mechanistic investigation of the Zn/Pd/C catalyzed cleavage and hydrodeoxygenation of lignin. Green Chem. 2016, 18, 2399–2405. [Google Scholar] [CrossRef]
- Zakzeski, J.; Bruijnincx, P.C.A.; Jongerius, A.L.; Weckhuysen, B.M. The catalytic valorization of lignin for the production of renewable chemicals. Chem. Rev. 2010, 110, 3552–3599. [Google Scholar] [CrossRef] [PubMed]
- Evtuguin, D.V.; Neto, C.P.; Silva, A.M.S.; Domingues, P.M.; Amado, F.M.L.; Robert, D.; Faix, O. Comprehensive study on the chemical structure of dioxane lignin from plantation eucalyptus globulus wood. J. Agric. Food Chem. 2001, 49, 4252–4261. [Google Scholar] [CrossRef] [PubMed]
- Chu, S.; Subrahmanyam, A.V.; Huber, G.W. The pyrolysis chemistry of a β-O-4 type oligomeric lignin model compound. Green Chem. 2013, 15, 125–136. [Google Scholar] [CrossRef]
- Song, Q.; Wang, F.; Cai, J.; Wang, Y.; Zhang, J.; Yu, W.; Xu, J. Lignin depolymerization (LDP) in alcohol over nickel-based catalysts via a fragmentation–hydrogenolysis process. Energy Environ. Sci. 2013, 6, 994–1007. [Google Scholar] [CrossRef] [Green Version]
- Sergeev, A.G.; Hartwig, J.F. Selective, nickel-catalyzed hydrogenolysis of aryl ethers. Science 2011, 332, 439–443. [Google Scholar] [CrossRef] [PubMed]
- Shimanskaya, E.; Stepacheva, A.A.; Sulman, E.; Rebrov, E.; Matveeva, V. Lignin-containing feedstock hydrogenolysis for biofuel component production. Bull. Chem. React. Eng. Catal. 2018, 13, 74–81. [Google Scholar] [CrossRef]
- Tyrone Ghampson, I.; Sepúlveda, C.; Garcia, R.; García Fierro, J.L.; Escalona, N.; DeSisto, W.J. Comparison of alumina- and sba-15-supported molybdenum nitride catalysts for hydrodeoxygenation of guaiacol. Appl. Catal. Gen. 2012, 435–436, 51–60. [Google Scholar] [CrossRef]
- Xiao, L.-P.; Wang, S.; Li, H.; Li, Z.; Shi, Z.-J.; Xiao, L.; Sun, R.-C.; Fang, Y.; Song, G. Catalytic hydrogenolysis of lignins into phenolic compounds over carbon nanotube supported molybdenum oxide. ACS Catal. 2017, 7, 7535–7542. [Google Scholar] [CrossRef]
- Barta, K.; Warner, G.R.; Beach, E.S.; Anastas, P.T. Depolymerization of organosolv lignin to aromatic compounds over cu-doped porous metal oxides. Green Chem. 2014, 16, 191–196. [Google Scholar] [CrossRef]
- Warner, G.; Hansen, T.S.; Riisager, A.; Beach, E.S.; Barta, K.; Anastas, P.T. Depolymerization of organosolv lignin using doped porous metal oxides in supercritical methanol. Bioresour. Technol. 2014, 161, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Galkin, M.V.; Sawadjoon, S.; Rohde, V.; Dawange, M.; Samec, J.S.M. Mild heterogeneous palladium-catalyzed cleavage of β-O-4′-ether linkages of lignin model compounds and native lignin in air. ChemCatChem 2014, 6, 179–184. [Google Scholar] [CrossRef]
- Liu, X.; Lu, G.; Guo, Y.; Guo, Y.; Wang, Y.; Wang, X. Catalytic transfer hydrogenolysis of 2-phenyl-2-propanol over palladium supported on activated carbon. J. Mol. Catal. A Chem. 2006, 252, 176–180. [Google Scholar] [CrossRef]
- Paone, E.; Espro, C.; Pietropaolo, R.; Mauriello, F. Selective arene production from transfer hydrogenolysis of benzyl phenyl ether promoted by a co-precipitated Pd/Fe3O4 catalyst. Catal. Sci. Technol. 2016, 6, 7937–7941. [Google Scholar] [CrossRef]
- Long, Y.; Liang, K.; Niu, J.; Tong, X.; Yuan, B.; Ma, J. Agglomeration of Pd0 nanoparticles causing different catalytic activities of suzuki carbonylative cross-coupling reactions catalyzed by PdII and Pd0 immobilized on dopamine-functionalized magnetite nanoparticles. New J. Chem. 2015, 39, 2988–2996. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, Z.; Liu, B.; Li, J. Silica coated magnetic Fe3O4 nanoparticles supported phosphotungstic acid: A novel environmentally friendly catalyst for the synthesis of 5-ethoxymethylfurfural from 5-hydroxymethylfurfural and fructose. Catal. Sci. Technol. 2013, 3, 2104–2122. [Google Scholar] [CrossRef]
- Xia, S.; Du, W.; Zheng, L.; Chen, P.; Hou, Z. A thermally stable and easily recycled core–shell Fe2O3@cumgal catalyst for hydrogenolysis of glycerol. Catal. Sci. Technol. 2014, 4, 912–916. [Google Scholar] [CrossRef]
- Zhu, M.; Diao, G. Magnetically recyclable Pd nanoparticles immobilized on magnetic Fe3O4@C nanocomposites: Preparation, characterization, and their catalytic activity toward suzuki and heck coupling reactions. J. Phys. Chem. C 2011, 115, 24743–24749. [Google Scholar] [CrossRef]
- Gracheva, I.E.; Olchowik, G.; Gareev, K.G.; Moshnikov, V.A.; Kuznetsov, V.V.; Olchowik, J.M. Investigations of nanocomposite magnetic materials based on the oxides of iron, nickel, cobalt and silicon dioxide. J. Phys. Chem. Solids 2013, 74, 656–663. [Google Scholar] [CrossRef]
- Sun, S.; Zeng, H. Size-controlled synthesis of magnetite nanoparticles. J. Am. Chem. Soc. 2002, 124, 8204–8205. [Google Scholar] [CrossRef] [PubMed]
- Jaiswal, M.K.; Gupta, U.; Vishnoi, P. A covalently conjugated MoS2/Fe3O4 magnetic nanocomposite as an efficient & reusable catalyst for H2 production. Dalton Trans. 2018, 47, 287–291. [Google Scholar] [PubMed]
- Wu, Z.S.; Yang, S.; Sun, Y.; Parvez, K.; Feng, X.; Mullen, K. 3D nitrogen-doped graphene aerogel-supported Fe3O4 nanoparticles as efficient electrocatalysts for the oxygen reduction reaction. J. Am. Chem. Soc. 2012, 134, 9082–9085. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.; Wang, D.-W.; Li, F.; Zhang, L.; Li, N.; Wu, Z.-S.; Wen, L.; Lu, G.Q.M.; Cheng, H.-M. Graphene-wrapped Fe3O4 anode material with improved reversible capacity and cyclic stability for lithium ion batteries. Chem. Mater. 2010, 22, 5306–5313. [Google Scholar] [CrossRef]
- Mao, G.-Y.; Yang, W.-J.; Bu, F.-X.; Jiang, D.-M.; Zhao, Z.-J.; Zhang, Q.-H.; Fang, Q.-C.; Jiang, J.-S. One-step hydrothermal synthesis of Fe3O4@C nanoparticles with great performance in biomedicine. J. Mater. Chem. B 2014, 2, 4481–4488. [Google Scholar] [CrossRef]
- Opris, C.; Cojocaru, B.; Gheorghe, N.; Tudorache, M.; Coman, S.M.; Parvulescu, V.I.; Duraki, B.; Krumeich, F.; van Bokhoven, J.A. Lignin fragmentation over magnetically recyclable composite Co@Nb2O5@Fe3O4 catalysts. J. Catal. 2016, 339, 209–227. [Google Scholar] [CrossRef]
- Wuang, S.C.; Neoh, K.G.; Kang, E.-T.; Pack, D.W.; Leckband, D.E. Synthesis and functionalization of polypyrrole-Fe3O4 nanoparticles for applications in biomedicine. J. Mater. Chem. 2007, 17, 3354–3362. [Google Scholar] [CrossRef]
- Bhat, P.B.; Inam, F.; Bhat, B.R. Nickel hydroxide/cobalt-ferrite magnetic nanocatalyst for alcohol oxidation. ACS Comb. Sci. 2014, 16, 397–402. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.K.; Jang, S.; Kim, J.Y.; Sharma, S.; Basavaraju, K.C.; Kim, M.-G.; Kim, K.-R.; Lee, J.S.; Lee, H.H.; Kim, D.-P. One-pot defunctionalization of lignin-derived compounds by dual-functional Pd50Ag50/Fe3O4/N-rGO catalyst. ACS Catal. 2015, 5, 6964–6972. [Google Scholar] [CrossRef]
- Espro, C.; Gumina, B.; Paone, E.; Mauriello, F. Upgrading lignocellulosic biomasses: Hydrogenolysis of platform derived molecules promoted by heterogeneous Pd-Fe catalysts. Catalysts 2017, 7, 78. [Google Scholar] [CrossRef]





| Cycle Times | Substrate | Catalyst | Solvent | Temperature | Time | Yield % |
|---|---|---|---|---|---|---|
| 1 | Dimer | Pd-Fe3O4 | Ethanol | 150 °C | 6 h | 97 |
| 2 | Dimer | Pd-Fe3O4 | Ethanol | 150 °C | 6 h | 97 |
| 3 | Dimer | Pd-Fe3O4 | Ethanol | 150 °C | 6 h | 95 |
| 4 | Dimer | Pd-Fe3O4 | Ethanol | 150 °C | 6 h | 94 |
| 5 | Dimer | Pd-Fe3O4 | Ethanol | 150 °C | 6 h | 95 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Zhao, C.; Lu, F. High-Efficient and Recyclable Magnetic Separable Catalyst for Catalytic Hydrogenolysis of β-O-4 Linkage in Lignin. Polymers 2018, 10, 1077. https://doi.org/10.3390/polym10101077
Huang J, Zhao C, Lu F. High-Efficient and Recyclable Magnetic Separable Catalyst for Catalytic Hydrogenolysis of β-O-4 Linkage in Lignin. Polymers. 2018; 10(10):1077. https://doi.org/10.3390/polym10101077
Chicago/Turabian StyleHuang, Jingtao, Chengke Zhao, and Fachuang Lu. 2018. "High-Efficient and Recyclable Magnetic Separable Catalyst for Catalytic Hydrogenolysis of β-O-4 Linkage in Lignin" Polymers 10, no. 10: 1077. https://doi.org/10.3390/polym10101077