Multifunctional Ionogels Incorporated with Lanthanide (Eu3+, Tb3+) Complexes Covalently Modified Multi-Walled Carbon Nanotubes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
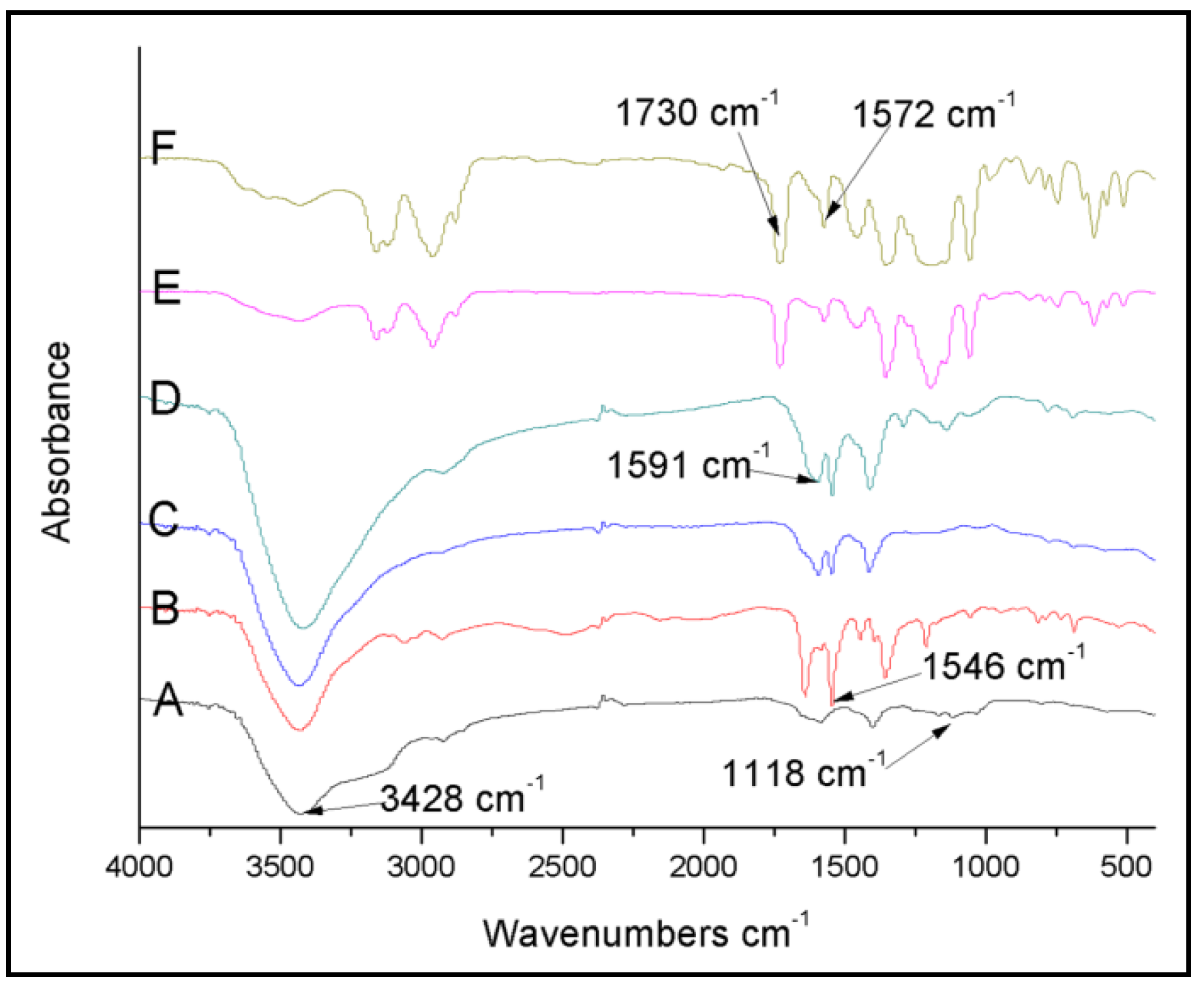
2.2. Characterizations
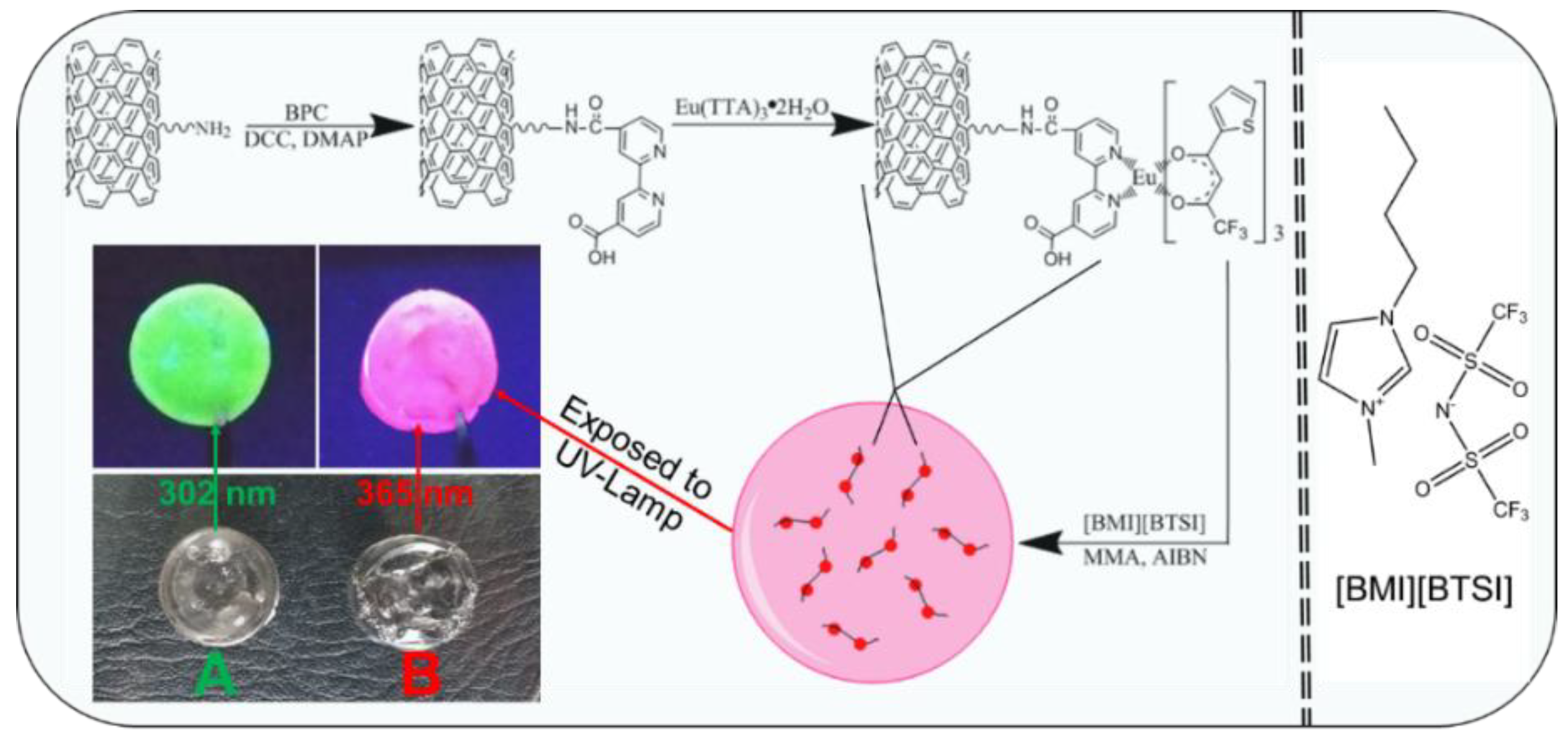
2.3. Preparation of Lanthanide Complexes Functionalized MWCNTs
2.4. Preparation of Lanthanide Complexes Functionalized MWCNTs Doped Luminescent Ionogels
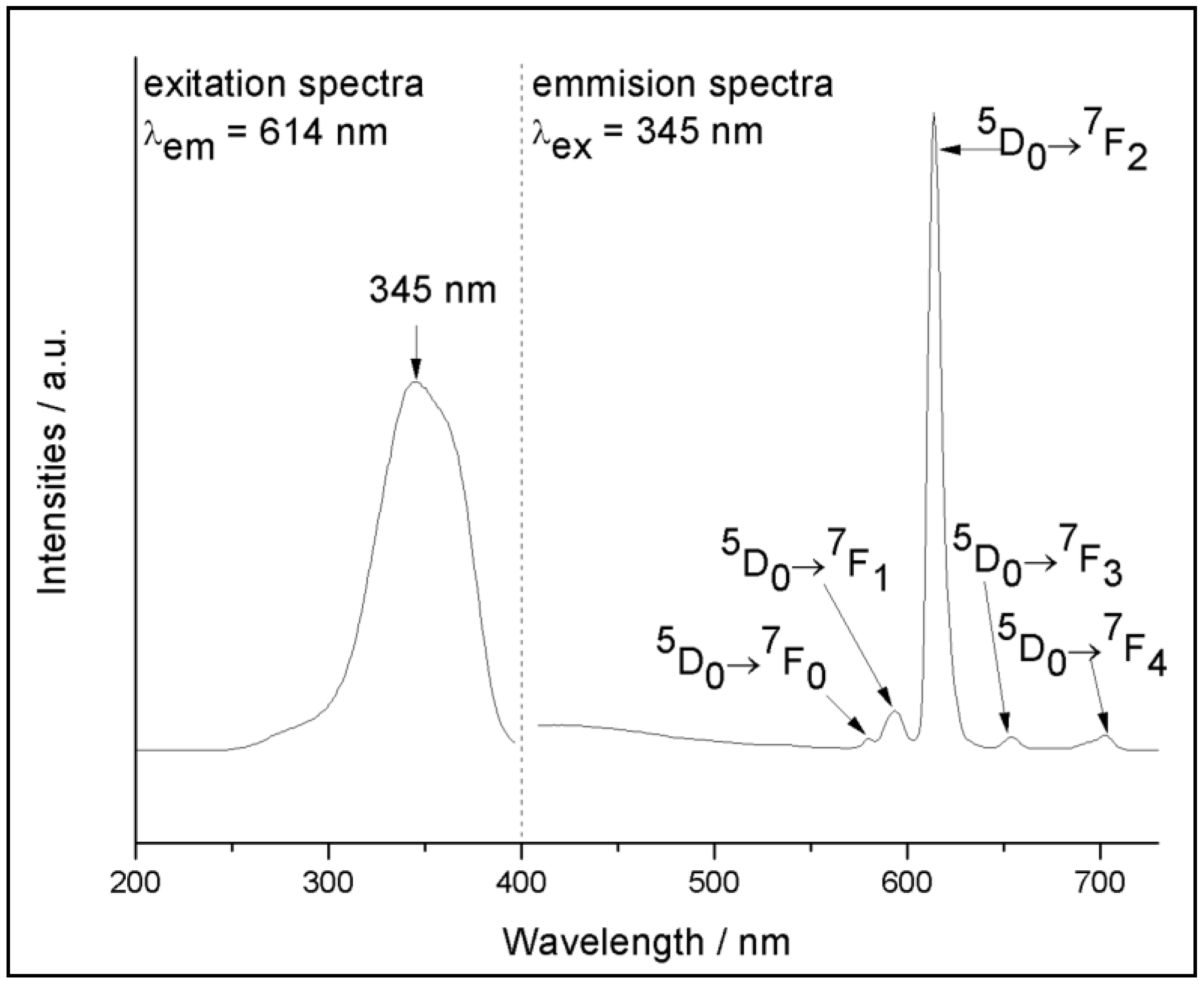
3. Results and Discussion
4. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Zhang, D.N.; Zhou, Y.; Cuan, J.; Gan, N. A lanthanide functionalized MOF hybrid for ratiometric luminescence detection of an anthrax biomarker. Cryst. Eng. Comm 2018, 20, 1264–1270. [Google Scholar] [CrossRef]
- Kemal, E.; Peters, R.; Bourke, S.; Fairclough, S.; Bergstrom-Mann, P.; Owen, D.M.; Sandiford, L.; Dailey, L.A.; Green, M. Magnetic conjugated polymer nanoparticles doped with a europium complex for biomedical imaging. Photochem. Photobiol. Sci. 2018, 17, 718–721. [Google Scholar] [CrossRef] [PubMed]
- Pablo, M.R.; Manuela, R.S. (Eds.) Lanthanide-doped Materials: From OLEDs to SIMs, 1st ed.; Elsevier: Amsterdam, the Netherlands, 2018; ISBN 978-0-12-813840-3. [Google Scholar]
- Zinna, F.; Pasini, M.; Galeotti, F.; Botta, C.; Bari, L.D.; Giovanella, U. Design of lanthanide-based OLEDs with remarkable circularly polarized electroluminescence. Adv. Funct. Mater. 2017, 27, 1603719. [Google Scholar] [CrossRef]
- Feng, J.; Zhang, H.J. Hybrid materials based on lanthanide organic complexes: A review. Chem. Soc. Rev. 2013, 42, 387–410. [Google Scholar] [CrossRef] [PubMed]
- Yan, B. Recent progress in photofunctional lanthanide hybrid materials. RSC Adv. 2012, 2, 9304–9324. [Google Scholar] [CrossRef]
- Binnemans, K. Lanthanide-based luminescent hybrid materials. Chem. Rev. 2009, 109, 4283–4374. [Google Scholar] [CrossRef] [PubMed]
- Yan, B. Photofunctional Rare Earth Hybrid Materials, 1st ed.; Springer: Singapore, 2017; ISBN 978-981-10-2957-8. [Google Scholar]
- Zheng, X.L.; Wang, M.Y.; Li, Q.P. Synthesis and luminescent properties of europium complexes covalently bonded to hybrid materials based on MCM-41 and poly(ionic liquids). Materials 2018, 11, 677. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Yang, Q.; Zhang, D.N.; Gan, N.; Li, Q.Q.; Cuan, J. Detection and removal of antibiotic tetracycline in water with a highly stable luminescent MOF. Sens. Actuators B 2018, 262, 137–143. [Google Scholar] [CrossRef]
- Ma, J.H.; Wang, T.R.; Liu, C.X.; Li, H.R. Tunable white-light emission of lanthanide (III) hybrid material based on hectorite. Chin. Chem. Lett. 2018, 29, 321–324. [Google Scholar] [CrossRef]
- Bideau, J.L.; Viau, L.; Vioux, A. Ionogels, ionic liquid based hybrid materials. Chem. Soc. Rev. 2011, 40, 907–925. [Google Scholar] [CrossRef] [PubMed]
- D’Anna, F.; Rizzo, C.; Vitale, P.; Lazzara, G.; Noto, R. Dicationic organic salts: gelators for ionic liquids. Soft Matter. 2014, 10, 9281–9292. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Danna, F.; Noto, R.; Zhang, M.; Weiss, R.G. Insights into the formation and structures of molecular gels by diimidazolium salt gelators in ionic liquids or “normal” solvents. Chem. Eur. J. 2016, 22, 11269–11282. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Arcudi, F.; Đorđević, L.; Dintcheva, N.T.; Noto, R.; D’Anna, F.; Prato, M. Nitrogen-doped carbon nanodots-ionogels: Preparation, characterization, and radical scavenging activity. ACS Nano 2018, 12, 1296–1305. [Google Scholar] [CrossRef] [PubMed]
- Li, X.W.; Li, S.J.; Zhang, Z.X.; Huang, J.; Yang, L.; Hirano, S. High-performance polymeric ionic liquid–silica hybrid ionogel electrolytes for lithium metal batteries. J. Mater. Chem. 2016, 4, 13822–13829. [Google Scholar] [CrossRef]
- Ma, J.; Yan, B. Multi-component hybrid soft ionogels for photoluminescence tuning and sensing organic solvent vapors. J. Colloid. Interface Sci. 2018, 513, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, H.Q.; Li, L.; Chen, R.J.; Guo, S.J. Ionogel electrolytes for high-performance lithium batteries: A review. Adv. Energy Mater. 2018, 8, 1702675. [Google Scholar] [CrossRef]
- Rizzo, C.; Marullo, S.; Campodonico, P.R.; Pibiri, I.; Dintcheva, N.T.; Noto, R.; Millan, D.; D’Anna, F. Self-sustaining supramolecular ionic liquid gels for dye adsorption. ACS Sustainable Chem. Eng. 2018, 6, 12453–12462. [Google Scholar] [CrossRef]
- Thoniyot, P.; Tan, M.J.; Karim, A.A.; Young, D.J.; Loh, X.J. Nanoparticle–hydrogel composites: concept, design, and applications of these promising, multi-functional materials. Adv. Sci. 2015, 2, 1400010. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Samanta, S.K. Soft-nanocomposites of nanoparticles and nanocarbons with supramolecular and polymer gels and their applications. Chem. Rev. 2016, 116, 11967–12028. [Google Scholar] [CrossRef] [PubMed]
- Li, H.R.; Wang, Y.G.; Wang, T.R.; Li, Z.Q. Ionic Liquids and Rare Earth Soft Luminescent Materials, Chen, J.; Springer: Berlin, Germany, 2016; ISBN 978-3-662-47510-2. [Google Scholar]
- Fan, Z.P.; Wang, Y.G.; Xue, Z.X.; Zhang, L.; Chen, Y.H.; Zhang, S.M. Preparation, characterization and luminescence of transparent thin film of ionogels. J. Sol-Gel Sci. Technol. 2014, 72, 328–333. [Google Scholar] [CrossRef]
- Zhou, F.; Wang, T.R.; Li, Z.Q.; Wang, Y.G. Transparent and luminescent ionogels composed of Eu3+-coordinated ionic liquids and poly(methyl methacrylate). Luminescence 2015, 30, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.G.; Feng, Y.; Zhao, H.S.; Gan, Q.Y.; Yu, X.Y. Luminescent organic–inorganic hybrid materials based on lanthanide containing ionic liquids and sylilated β-diketone. J. Sol-Gel Sci. Technol. 2011, 58, 711–715. [Google Scholar] [CrossRef]
- Wang, H.F.; Wang, Y.G.; Zhang, L.; Li, H.R. Transparent and luminescent ionogels based on lanthanide-containing ionic liquids and poly(methyl methacrylate) prepared through an environmentally friendly method. RSC Adv. 2013, 3, 8535–8540. [Google Scholar] [CrossRef]
- Mert, S.; Bankoglu, B.; Ozkan, A.; Atar, N.; Yola, M.L. Electrochemical sensing of ractopamine by carbon nitride nanotubes/ionic liquid nanohybrid in presence of other β-agonists. J. Mol. Liq. 2018, 254, 8–11. [Google Scholar] [CrossRef]
- Lunstroot, K.; Driesen, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Gorllerwalrand, C.; Binnemans, K.; Bellayer, S.; Viau, L.; Bideau, J.L. Lanthanide-doped luminescent ionogels. Dalton Trans. 2009, 298–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Čépe, K.; Zbořil, R. UV light-switchable transparent polymer films and invisible luminescent inks based on carbon dots and lanthanide complexes. J. Mater. Chem. C 2016, 4, 7253–7259. [Google Scholar] [CrossRef]
- Tigaa, R.A.; Monteiro, J.H.S.K.; Silva-Hernandez, S.; Bettencourt-Dias, A.D. LnIII-centered emission sensitized through fluorescent carbon dots. J. Lumin. 2017, 192, 1273–1277. [Google Scholar] [CrossRef]
- Gu, Q.Y.; Chen, J.N. Carbon-nanotube-based nano-emitters: A review. J. Lumin. 2018, 200, 181–188. [Google Scholar] [CrossRef]
- Li, Q.P.; Yan, B. Multi-walled carbon nanotube-based ternary rare earth (Eu3+, Tb3+) hybrid materials with organically modified silica–oxygen bridge. J. Colloid Interface Sci. 2012, 380, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Dai, B.; Deng, D.G.; Yu, H.; Lei, L.; Li, Y.Y.; Li, C.X.; Xu, S.Q. Eu3+-doped ionogel-functionalized carbon dot monoliths with bright white photoluminescence. RSC Adv. 2016, 6, 72149–72154. [Google Scholar] [CrossRef]
- Zhao, C.; Song, Y.J.; Qu, K.G.; Ren, J.S.; Qu, X.G. Luminescent rare-earth complex covalently modified single-walled carbon nanotubes: Design, synthesis, and DNA sequence-dependent red luminescence enhancement. Chem. Mater. 2010, 22, 5718–5724. [Google Scholar] [CrossRef]
- Melby, L.R.; Rose, N.J.; Abramson, E.; Caris, J.C. Synthesis and fluorescence of some trivalent lanthanide complexes. J. Am. Chem. Soc. 1964, 86, 5117–5125. [Google Scholar] [CrossRef]
- Brito, H.F.; Malta, O.L.; Menezes, J.F.S. Luminescent properties of diketonates of trivalent europium with dimethyl sulfoxide. J. Alloys Compd. 2000, 303–304, 336–339. [Google Scholar] [CrossRef]



© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Q. Multifunctional Ionogels Incorporated with Lanthanide (Eu3+, Tb3+) Complexes Covalently Modified Multi-Walled Carbon Nanotubes. Polymers 2018, 10, 1099. https://doi.org/10.3390/polym10101099
Li Q. Multifunctional Ionogels Incorporated with Lanthanide (Eu3+, Tb3+) Complexes Covalently Modified Multi-Walled Carbon Nanotubes. Polymers. 2018; 10(10):1099. https://doi.org/10.3390/polym10101099
Chicago/Turabian StyleLi, Qiuping. 2018. "Multifunctional Ionogels Incorporated with Lanthanide (Eu3+, Tb3+) Complexes Covalently Modified Multi-Walled Carbon Nanotubes" Polymers 10, no. 10: 1099. https://doi.org/10.3390/polym10101099




