Hyaluronic Acid-Based Nanomaterials for Cancer Therapy
Abstract
:1. Introduction
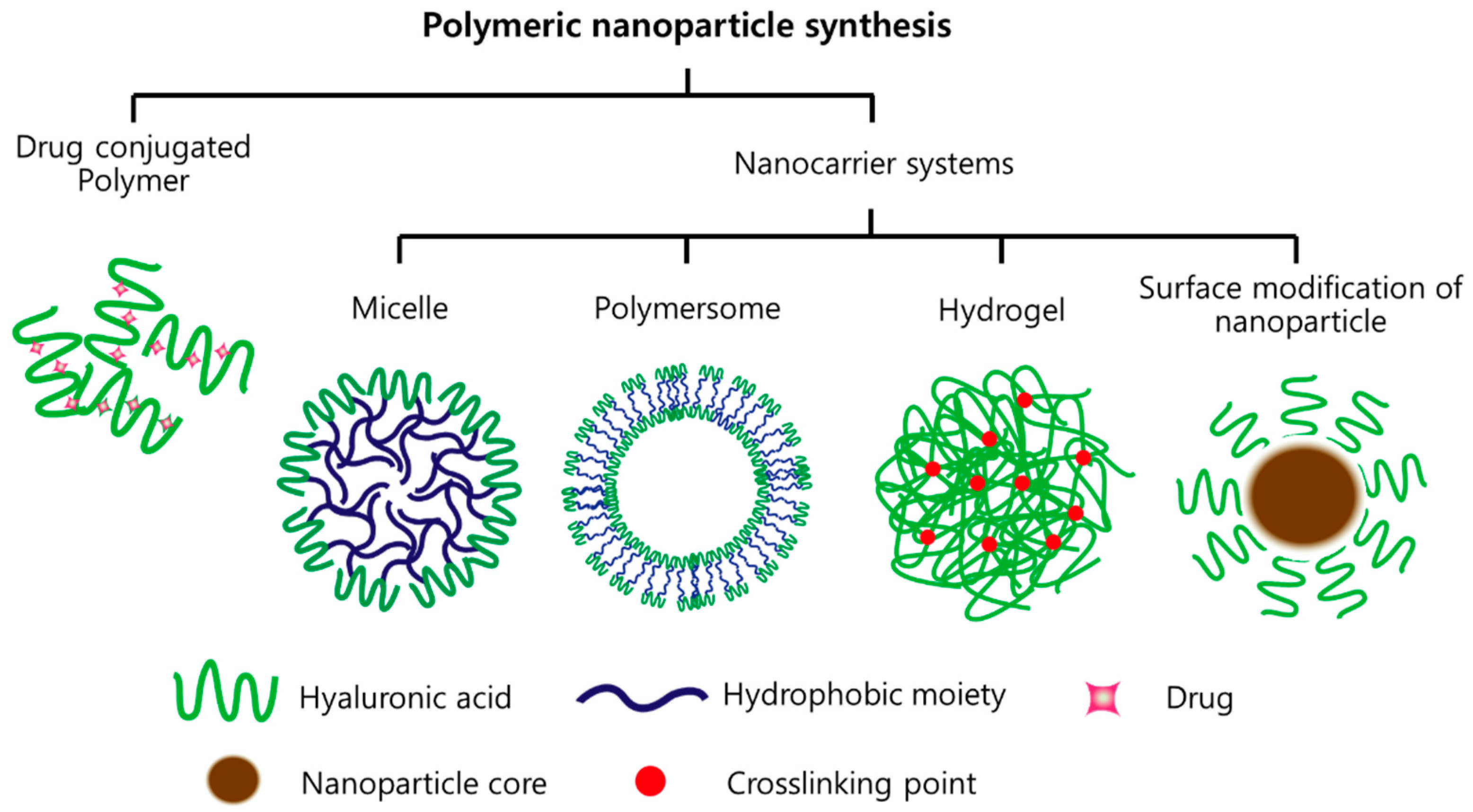
2. HA-Based Nanomaterials
2.1. Drug-Conjugated HA
2.2. Micelles
2.3. Polymersome
2.4. Hydrogels
2.5. Inorganic NPs
3. Degradation of HA
4. Cancer Therapy via HA-Based Nanomaterials
4.1. Chemotherapeutic Agents
4.2. Gene Delivery
4.3. Immunotherapy
4.4. Combination Therapy
5. Summary and Prospective Outlook
Funding
Conflicts of Interest
References
- Wickens, J.M.; Alsaab, H.O.; Kesharwani, P.; Bhise, K.; Amin, M.C.I.M.; Tekade, R.K.; Gupta, U.; Iyer, A.K. Recent advances in hyluronic acid-decorated nanocarriers for targeted cancer therapy. Drug Discov. Today 2017, 22, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Mattheolabakis, G.; Milane, L.; Singh, A.; Amiji, M.M. Hyaluronic acid targeting of CD44 for cancer therapy: From receptor biology to nanomedicine. J. Drug Target. 2015, 23, 605–618. [Google Scholar] [CrossRef] [PubMed]
- Lapcik, L.; Lapcik, L.; De Smedt, S.; Demeester, J.; Chabrecek, P. Hyaluronan: Preparation, structure, properties, and applications. Chem. Rev. 1998, 98, 2663–2684. [Google Scholar] [CrossRef]
- Cai, Z.X.; Zhang, H.B.; Wei, Y.; Gong, F.S. Hyaluronan-Inorganic Nanohybrid Materials for Biomedical Applications. Biomacromolecules 2017, 18, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.Z.; Xu, C.L.; Wen, L.Q.; Han, M.K.; Xiao, B.; Zhou, J.; Zhang, Y.; Zhang, Z.; Viennois, E.; Merlin, D. A Hyaluronidase-Responsive Nanoparticle-Based Drug Delivery System for Targeting Colon Cancer Cells. Cancer Res. 2016, 76, 7208–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dosio, F.; Arpicco, S.; Stella, B.; Fattal, E. Hyaluronic acid for anticancer drug and nucleic acid delivery. Adv. Drug Deliv. Rev. 2016, 97, 204–236. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, U.; Maeda, H.; Jain, R.K.; Sevick-Muraca, E.M.; Zamboni, W.; Farokhzad, O.C.; Barry, S.T.; Gabizon, A.; Grodzinski, P.; Blakey, D.C. Challenges and Key Considerations of the Enhanced Permeability and Retention Effect for Nanomedicine Drug Delivery in Oncology. Cancer Res. 2013, 73, 2412–2417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattheolabakis, G.; Rigas, B.; Constantinides, P.P. Nanodelivery strategies in cancer chemotherapy: Biological rationale and pharmaceutical perspectives. Nanomedicine 2012, 7, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Lokeshwar, V.B.; Mirza, S.; Jordan, A. Targeting Hyaluronic Acid Family for Cancer Chemoprevention and Therapy. Adv. Cancer Res. 2014, 123, 35–65. [Google Scholar] [PubMed] [Green Version]
- Zamboni, F.; Keays, M.; Hayes, S.; Albadarin, A.B.; Walker, G.M.; Kiely, P.A.; Collins, M.N. Enhanced cell viability in hyaluronic acid coated poly(lactic-co-glycolic acid) porous scaffolds within microfluidic channels. Int. J. Pharm. 2017, 532, 595–602. [Google Scholar] [CrossRef] [PubMed]
- Souness, A.; Zamboni, F.; Walker, G.M.; Collins, M.N. Influence of scaffold design on 3D printed cell constructs. J. Biomed. Mater. Res. B Appl. Biomater. 2018, 106, 533–545. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Saravanakumar, G.; Park, J.H.; Park, K. Hyaluronic acid-based nanocarriers for intracellular targeting: Interfacial interactions with proteins in cancer. Colloids Surf. B 2012, 99, 82–94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Y.; Prestwich, G.D. Synthesis and selective cytotoxicity of a hyaluronic acid-antitumor bioconjugate. Bioconjug. Chem. 1999, 10, 755–763. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Ziebell, M.R.; Prestwich, G.D. A hyaluronic acid-taxol antitumor bioconjugate targeted to cancer cells. Biomacromolecules 2000, 1, 208–218. [Google Scholar] [CrossRef] [PubMed]
- Leonelli, F.; La Bella, A.; Francescangeli, A.; Joudioux, R.; Capodilupo, A.L.; Quagliariello, M.; Migneco, L.M.; Bettolo, R.M.; Crescenzi, V.; De Luca, G.; et al. A new and simply available class of hydrosoluble bioconjugates by coupling paclitaxel to hyaluronic acid through a 4-hydroxybutanoic acid derived linker. Helv. Chim. Acta 2005, 88, 154–159. [Google Scholar] [CrossRef]
- Li, H.; Liu, Y.C.; Shu, X.Z.; Gray, S.D.; Prestwich, G.D. Synthesis and biological evaluation of a cross-linked hyaluronan-mitomycin C hydrogel. Biomacromolecules 2004, 5, 895–902. [Google Scholar] [CrossRef] [PubMed]
- Montagner, I.M.; Merlo, A.; Zuccolotto, G.; Renier, D.; Campisi, M.; Pasut, G.; Zanovello, P.; Rosato, A. Peritoneal tumor carcinomatosis: Pharmacological targeting with hyaluronan-based bioconjugates overcomes therapeutic indications of current drugs. PLoS ONE 2014, 9, e112240. [Google Scholar] [CrossRef] [PubMed]
- Banzato, A.; Bobisse, S.; Rondina, M.; Renier, D.; Bettella, F.; Esposito, G.; Quintieri, L.; Meléndez-Alafort, L.; Mazzi, U.; Zanovello, P.; et al. A paclitaxel-hyaluronan bioconjugate targeting ovarian cancer affords a potent in vivo therapeutic activity. Clin. Cancer Res. 2008, 14, 3598–3606. [Google Scholar] [CrossRef] [PubMed]
- Jaracz, S.; Chen, J.; Kuznetsova, L.V.; Ojima, L. Recent advances in tumor-targeting anticancer drug conjugates. Bioorg. Med. Chem. 2005, 13, 5043–5054. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Bernshaw, N.J.; Lu, Z.R.; Kopecek, J.; Prestwich, G.D. Targeted delivery of doxorubicin by HPMA copolymer-hyaluronan bioconjugates. Pharm. Res. 2002, 19, 396–402. [Google Scholar] [CrossRef] [PubMed]
- Schante, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Mero, A.; Campisi, M. Hyaluronic Acid Bioconjugates for the Delivery of Bioactive Molecules. Polymers 2014, 6, 346–369. [Google Scholar] [CrossRef] [Green Version]
- Prestwich, G.D.; Marecak, D.M.; Marecek, J.F.; Vercruysse, K.P.; Ziebell, M.R. Controlled chemical modification of hyaluronic acid: Synthesis, applications, and biodegradation of hydrazide derivatives. J. Control. Release 1998, 53, 93–103. [Google Scholar] [CrossRef]
- Jordan, M.A.; Wilson, L. Microtubules as a target for anticancer drugs. Nat. Rev. Cancer 2004, 4, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Kessinger, C.W.; Sumer, B.D.; Gao, J. Multifunctional Micellar Nanomedicine for Cancer Therapy. Exp. Biol. Med. 2009, 234, 123–131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Mongayt, D.; Torchilin, V.P. Polymeric micelles for delivery of poorly soluble drugs: Preparation and anticancer activity in vitro of paclitaxel incorporated into mixed micelles based on poly(ethylene glycol)-lipid conjugate and positively charged lipids. J. Drug Target. 2005, 13, 73–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Husseini, G.A.; Pitt, W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008, 60, 1137–1152. [Google Scholar] [CrossRef] [PubMed]
- Degim, I.T.; Celebi, N. Controlled delivery of peptides and proteins. Curr. Pharm. Des. 2007, 13, 99–117. [Google Scholar] [CrossRef] [PubMed]
- Khan, D.R.; Rezler, E.M.; Lauer-Fields, J.; Fields, G.B. Effects of drug hydrophobicity on liposomal stability. Chem. Biol. Drug Des. 2008, 71, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Bermudez, H.; Brannan, A.K.; Hammer, D.A.; Bates, F.S.; Discher, D.E. Molecular weight dependence of polymersome membrane structure, elasticity, and stability. Macromolecules 2002, 35, 8203–8208. [Google Scholar] [CrossRef]
- Discher, B.M.; Won, Y.Y.; Ege, D.S.; Lee, J.C.M.; Bates, F.S.; Discher, D.E.; Hammer, D.A. Polymersomes: Tough vesicles made from diblock copolymers. Science 1999, 284, 1143–1146. [Google Scholar] [CrossRef] [PubMed]
- Kamat, N.P.; Lee, M.H.; Lee, D.; Hammer, D.A. Micropipette aspiration of double emulsion-templated polymersomes. Soft Matter 2011, 7, 9863–9866. [Google Scholar] [CrossRef]
- Upadhyay, K.K.; Le Meins, J.F.; Misra, A.; Voisin, P.; Bouchaud, V.; Ibarboure, E.; Schatz, C.; Lecommandoux, S. Biomimetic Doxorubicin Loaded Polymersomes from Hyaluronan-block-Poly(gamma-benzyl glutamate) Copolymers. Biomacromolecules 2009, 10, 2802–2808. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, K.K.; Bhatt, A.N.; Mishra, A.K.; Dwarakanath, B.S.; Jain, S.; Schatz, C.; Le Meins, J.F.; Farooque, A.; Chandraiah, G.; Jain, A.K.; et al. The intracellular drug delivery and anti tumor activity of doxorubicin loaded poly(gamma-benzyl l-glutamate)-b-hyaluronan polymersomes. Biomaterials 2010, 31, 2882–2892. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Highley, C.B.; Prestwich, G.D.; Burdick, J.A. Recent advances in hyaluronic acid hydrogels for biomedical applications. Curr. Opin. Biotechnol. 2016, 40, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Jha, A.K.; Harrington, D.A.; Farach-Carson, M.C.; Jia, X.Q. Hyaluronic acid-based hydrogels: From a natural polysaccharide to complex networks. Soft Matter 2012, 8, 3280–3294. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.K.; Drumright, R.; Siegwart, D.J.; Matyjaszewski, K. The development of microgels/nanogels for drug delivery applications. Prog. Polym. Sci. 2008, 33, 448–477. [Google Scholar] [CrossRef]
- Arpicco, S.; Milla, P.; Stella, B.; Dosio, F. Hyaluronic Acid Conjugates as Vectors for the Active Targeting of Drugs, Genes and Nanocomposites in Cancer Treatment. Molecules 2014, 19, 3193–3230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.E.; Kim, A.Y.; Saravanakumar, G.; Koo, H.; Kwon, I.C.; Choi, K.; Park, J.H.; Kim, K. Hyaluronidase-Sensitive SPIONs for MR/Optical Dual Imaging Nanoprobes. Macromol. Res. 2011, 19, 861–867. [Google Scholar] [CrossRef]
- Lu, J.; Liong, M.; Li, Z.X.; Zink, J.I.; Tamanoi, F. Biocompatibility, Biodistribution, and Drug-Delivery Efficiency of Mesoporous Silica Nanoparticles for Cancer Therapy in Animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, M.; Chen, H.R.; Chen, Y.; Zhang, K.; Wang, X.; Cui, X.Z.; Shi, J.L. Hyaluronic acid-conjugated mesoporous silica nanoparticles: Excellent colloidal dispersity in physiological fluids and targeting efficacy. J. Mater. Chem. 2012, 22, 5615–5621. [Google Scholar] [CrossRef]
- Akhavan, O.; Ghaderi, E.; Aghayee, S.; Fereydooni, Y.; Talebi, A. The use of a glucose-reduced graphene oxide suspension for photothermal cancer therapy. J. Mater. Chem. 2012, 22, 13773–13781. [Google Scholar] [CrossRef]
- Song, E.Q.; Han, W.Y.; Li, C.; Cheng, D.; Li, L.R.; Liu, L.C.; Zhu, G.Z.; Song, Y.; Tan, W.H. Hyaluronic Acid-Decorated Graphene Oxide Nanohybrids as Nanocarriers for Targeted and pH-Responsive Anticancer Drug Delivery. ACS Appl. Mater. Interfaces 2014, 6, 11882–11890. [Google Scholar] [CrossRef] [PubMed]
- Pellegrino, T.; Manna, L.; Kudera, S.; Liedl, T.; Koktysh, D.; Rogach, A.L.; Keller, S.; Rädler, J.; Natile, G.; Parak, W.J. Hydrophobic nanocrystals coated with an amphiphilic polymer shell: A general route to water soluble nanocrystals. Nano Lett. 2004, 4, 703–707. [Google Scholar] [CrossRef]
- Stern, R.; Jedrzejas, M.J. Hyaluronidases: Their genomics, structures, and mechanisms of action. Chem. Rev. 2006, 106, 818–839. [Google Scholar] [CrossRef] [PubMed]
- Necas, J.; Bartosikova, L.; Brauner, P.; Kolar, J. Hyaluronic acid (hyaluronan): A review. Vet. Med. 2008, 53, 397–411. [Google Scholar] [CrossRef]
- Volpi, N.; Schiller, J.; Stern, R.; Soltes, L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Curr. Med. Chem. 2009, 16, 1718–1745. [Google Scholar] [CrossRef] [PubMed]
- Valachova, K.; Topol’ska, D.; Mendichi, R.; Collins, M.N.; Sasinkova, V.; Soltes, L. Hydrogen peroxide generation by the Weissberger biogenic oxidative system during hyaluronan degradation. Carbohydr. Polym. 2016, 148, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Valachova, K.; Banasova, M.; Topol’ska, D.; Sasinkova, V.; Juranek, I.; Collins, M.N.; Šoltés, L. Influence of tiopronin, captopril and levamisole therapeutics on the oxidative degradation of hyaluronan. Carbohydr. Polym. 2015, 134, 516–523. [Google Scholar] [CrossRef] [PubMed]
- Jahn, M.; Baynes, J.W.; Spiteller, G. The reaction of hyaluronic acid and its monomers, glucuronic acid and N-acetylglucosamine, with reactive oxygen species. Carbohydr. Res. 1999, 321, 228–234. [Google Scholar] [CrossRef]
- Fraser, J.R.; Laurent, T.C.; Pertoft, H.; Baxter, E. Plasma clearance, tissue distribution and metabolism of hyaluronic acid injected intravenously in the rabbit. Biochem. J. 1981, 200, 415–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeda, H.; Wu, J.; Sawa, T.; Matsumura, Y.; Hori, K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: A review. J. Control. Release 2000, 65, 271–284. [Google Scholar] [CrossRef]
- Lee, H.; Lee, K.; Park, T.G. Hyaluronic acid-paclitaxel conjugate micelles: Synthesis, characterization, and antitumor activity. Bioconjug. Chem. 2008, 19, 1319–1325. [Google Scholar] [CrossRef] [PubMed]
- Cai, S.A.; Thati, S.; Bagby, T.R.; Diab, H.M.; Davies, N.M.; Cohen, M.S.; Forrest, M.L. Localized doxorubicin chemotherapy with a biopolymeric nanocarrier improves survival and reduces toxicity in xenografts of human breast cancer. J. Control. Release 2010, 146, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.G.; Moon, M.; Lee, S.; Jeong, Y.Y. Paclitaxel loaded hyaluronic acid nanoparticles for targeted cancer therapy: In vitro and in vivo analysis. Int. J. Biol. Macromol. 2015, 72, 510–518. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Huo, M.R.; Wang, J.; Zhou, J.P.; Mohammad, J.M.; Zhang, Y.L.; Zhu, Q.N.; Waddad, A.Y.; Zhang, Q. Redox-sensitive micelles self-assembled from amphiphilic hyaluronic acid-deoxycholic acid conjugates for targeted intracellular delivery of paclitaxel. Biomaterials 2012, 33, 2310–2320. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.Y.; Jeon, E.J.; Yoon, H.Y.; Lee, B.S.; Na, J.H.; Min, K.H.; Kim, S.Y.; Myung, S.J.; Lee, S.; Chen, X.; et al. Theranostic nanoparticles based on PEGylated hyaluronic acid for the diagnosis, therapy and monitoring of colon cancer. Biomaterials 2012, 33, 6186–6193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paliwal, S.R.; Paliwal, R.; Agrawal, G.P.; Vyas, S.P. Hyaluronic acid modified pH-sensitive liposomes for targeted intracellular delivery of doxorubicin. J. Liposome Res. 2016, 26, 276–287. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, G.; Kim, M.R.; Mohammed, S.I.; Yeo, Y. Hyaluronic acid-based hydrogel for regional delivery of paclitaxel to intraperitoneal tumors. J. Control. Release 2012, 158, 386–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.M.; Yang, X.J.; Yang, P.; Gao, F.N. Hyaluronic acid-conjugated silica nanoparticles for breast cancer therapy. Inorg. Nano-Met. Chem. 2017, 47, 777–782. [Google Scholar] [CrossRef]
- Fu, C.P.; Yang, R.M.; Wang, L.; Li, N.N.; Qi, M.; Xu, X.D.; Wei, X.H.; Jiang, X.Q.; Zhang, L.M. Surface functionalization of superparamagnetic nanoparticles by an acid-liable polysaccharidebased prodrug for combinatorial monitoring and chemotherapy of hepatocellular carcinoma. RSC Adv. 2017, 7, 41919–41928. [Google Scholar] [CrossRef]
- Ganesh, S.; Iyer, A.K.; Gattacceca, F.; Morrissey, D.V.; Amiji, M.M. In vivo biodistribution of siRNA and cisplatin administered using CD44-targeted hyaluronic acid nanoparticles. J. Control. Release 2013, 172, 699–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ganesh, S.; Iyer, A.K.; Morrissey, D.V.; Amiji, M.M. Hyaluronic acid based self-assembling nanosystems for CD44 target mediated siRNA delivery to solid tumors. Biomaterials 2013, 34, 3489–3502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, G.; Park, K.; Kim, J.; Kim, K.S.; Oh, E.J.; Kang, H.; Han, S.E.; Oh, Y.K.; Park, T.G.; Kwang Hahn, S. Hyaluronic acid-polyethyleneimine conjugate for target specific intracellular delivery of siRNA. Biopolymers 2008, 89, 635–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Mok, H.; Lee, S.; Oh, Y.K.; Park, T.G. Target-specific intracellular delivery of siRNA using degradable hyaluronic acid nanogels. J. Control. Release 2007, 119, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Y.Y.; Yu, D.M. Lung cancer gene therapy: Transferrin and hyaluronic acid dual ligand-decorated novel lipid carriers for targeted gene delivery. Oncol. Rep. 2017, 37, 937–944. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.S.; Lee, J.E.; Byun, E.; Kim, N.W.; Lee, K.; Lee, H.; Sim, S.J.; Lee, D.S.; Jeong, J.H. Target-specific delivery of siRNA by stabilized calcium phosphate nanoparticles using dopa-hyaluronic acid conjugate. J. Control. Release 2014, 192, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.H.; Wang, Y.H.; Zhang, L.; Huang, L. Nanoparticle-Delivered Transforming Growth Factor-beta siRNA Enhances Vaccination against Advanced Melanoma by Modifying Tumor Microenvironment. ACS Nano 2014, 8, 3636–3645. [Google Scholar] [CrossRef] [PubMed]
- Zamboni, F.; Vieira, S.; Reis, R.L.; Oliveira, J.M.; Collins, M.N. The potential of hyaluronic acid in immunoprotection and immunomodulation: Chemistry, processing and function. Prog. Mater. Sci. 2018, 97, 97–122. [Google Scholar] [CrossRef]
- Lee, Y.H.; Yoon, H.Y.; Shin, J.M.; Saravanakumar, G.; Noh, K.H.; Song, K.H.; Jeon, J.H.; Kim, D.W.; Lee, K.M.; Kim, K.; et al. A polymeric conjugate foreignizing tumor cells for targeted immunotherapy in vivo. J. Control. Release 2015, 199, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.M.; Oh, S.J.; Kwon, S.; Deepagan, V.G.; Lee, M.; Song, S.H.; Lee, H.J.; Kim, S.; Song, K.H.; Kim, T.W.; et al. A PEGylated hyaluronic acid conjugate for targeted cancer immunotherapy. J. Control. Release 2017, 267, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Ueda, K.; Akiba, J.; Ogasawara, S.; Todoroki, K.; Nakayama, M.; Sumi, A.; Kusano, H.; Sanada, S.; Suekane, S.; Xu, K.; et al. Growth inhibitory effect of an injectable hyaluronic acid-tyramine hydrogels incorporating human natural interferon-alpha and sorafenib on renal cell carcinoma cells. Acta Biomater. 2016, 29, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Shin, W.J.; Noh, H.J.; Noh, Y.W.; Kim, S.; Um, S.H.; Lim, Y.T. Hyaluronic acid-supported combination of water insoluble immunostimulatory compounds for anti-cancer immunotherapy. Carbohydr. Polym. 2017, 155, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Jia, F.; Liu, X.P.; Li, L.H.; Mallapragada, S.; Narasimhan, B.; Wang, Q. Multifunctional nanoparticles for targeted delivery of immune activating and cancer therapeutic agents. J. Control. Release 2013, 172, 1020–1034. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Qiao, L.N.; Zhang, S.P.; Wan, G.Y.; Chen, B.W.; Zhou, P.; Zhang, N.; Wang, Y. Dual pH-responsive multifunctional nanoparticles for targeted treatment of breast cancer by combining immunotherapy and chemotherapy. Acta Biomater. 2018, 66, 310–324. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Cho, H.J.; Yi, E.; Kim, D.D.; Jheon, S. Hypocrellin B and paclitaxel-encapsulated hyaluronic acid-ceramide nanoparticles for targeted photodynamic therapy in lung cancer. J. Photochem. Photobiol. B 2016, 158, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Xu, C.; Zhao, X.; Lin, C.; Yang, X.; Xin, X.; Zhang, L.; Qin, C.; Han, X.; Yang, L.; et al. Nanoplatform Assembled from a CD44-Targeted Prodrug and Smart Liposomes for Dual Targeting of Tumor Microenvironment and Cancer Cells. ACS Nano 2018, 12, 1519–1536. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.W.; Cao, M.J.; Zhang, J.K.; Hu, K.L.; Yin, Z.X.; Zhou, Z.X.; Xiao, X.; Yang, Y.; Sheng, W.; Wu, Y.; et al. Hyaluronic acid-chitosan nanoparticles for co-delivery of M1R-34a and doxorubicin in therapy against triple negative breast cancer. Biomaterials 2014, 35, 4333–4344. [Google Scholar] [CrossRef] [PubMed]
- Khatun, Z.; Nurunnabi, M.; Nafiujjaman, M.; Reeck, G.R.; Khan, H.A.; Cho, K.J.; Lee, Y.K. A hyaluronic acid nanogel for photo-chemo theranostics of lung cancer with simultaneous light-responsive controlled release of doxorubicin. Nanoscale 2015, 7, 10680–10689. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Z.; Chen, Z.W.; Liu, Z.; Shi, P.; Dong, K.; Ju, E.G.; Ren, J.S.; Qu, X.G. A multi-stimuli responsive gold nanocage-hyaluronic platform for targeted photothermal and chemotherapy. Biomaterials 2014, 35, 9678–9688. [Google Scholar] [CrossRef] [PubMed]



| Formulation | Component | Status | Indication | Ref. |
|---|---|---|---|---|
| Drug conjugation | Paclitaxel-HA | In vitro | HCT-116 MCF-7 | [54] |
| Doxorubicin-HA | In vivo | MDA-MB-468LN (human breast cancer) | [55] | |
| Micelle | 5B-cholanic acid-HA, paclitaxel | In vivo | SCC7 (squamous cell carcinoma) | [56] |
| Deoxycholic acid-HA, paclitaxel | In vivo | MDA-MB-231 (breast adenocarcinoma) | [57] | |
| 5B-cholanic acid-HA-PEG, irinotecan | In vivo | HT29 (human colorectal adenocarcinoma) | [58] | |
| Polymersome | DSPE-PEG-HA, doxorubicin | In vivo | MCF7 (human breast adenocarcinoma) | [59] |
| Hydrogel | paclitaxel | In vivo | SKOV-3 (human ovarian cancer) | [60] |
| Nanoparticle (surface modification) | Si nanoparticle, paclitaxel | In vivo | MCF-7 (human breast adenocarcinoma) | [61] |
| SPION-HA, doxorubicin | In vivo | HepG2 (hepatocellular carcinoma) | [62] |
| Formulation | Component | Status | Indication | Ref. |
|---|---|---|---|---|
| Complex (electric interaction) | Polyethyleneimine-HA, siRNA | In vivo | MDA-MB 468, A549, B16F10 (CD44 expressed cancer) | [64] |
| Polyethyleneimine-HA, siRNA | In vitro | B16F1 (murine melanoma), HEK-293 (human embryonic kidney) | [65] | |
| Gene conjugation | siRNA-HA | In vitro | HCT-116 (human colon carcinoma) | [66] |
| Polymersome | DSPE-PEG-HA, pDNA | In vitro | A549 (human lung adenocarcinoma) | [67] |
| Nanoparticle (surface modification) | Cancium phosphate-HA, siRNA | In vivo | HT29 (human colorectal adenocarcinoma) | [68] |
| Formulation | Component | Status | Indication | Ref. |
|---|---|---|---|---|
| Drug (direct) conjugation | HA-ovalbumin | In vivo | TC-1 (murine cervical cancer) | [71] |
| Micelle | PEG-pep-HA, ovalbumin | In vivo | TC-1 (murine cervical cancer) | [72] |
| Polymersome | DSPE-PEG-HA, siRNA (for TGF-β) | In vivo | B16F10 (melanoma) | [69] |
| Hydrogel complex | HA-tyramine, IFN-α, sorafenib | In vivo | ACHN (human renal adenocarcinoma) | [73] |
| Monophosphoryl lipid, QS21, R837 + HA | In vivo | EG7-OVA tumor (mouse lymphocyte) | [74] |
| Formulation | Component | Status | Indication | Ref. |
|---|---|---|---|---|
| Drug conjugation | R848 (immuno), HA-doxorubicin (chemo) | In vivo | 4T1 (mammary carcinoma) | [76] |
| Micelle | Hypocrellin B (PDT), paclitaxel (chemo), HA-ceramide | In vivo | A549 (human lung adenocarcinoma) | [77] |
| Polymersome | Marimastat (TME), HA-paclitaxel (chemo) | In vivo | 4T1 (mammary carcinoma) | [78] |
| Hydrogel | Doxorubicin (chemo), mRNA (gene), HA-chitosan | In vivo | MDA-MB-231 (human breast cancer) | [79] |
| Graphene (PTT), doxorubicin (chemo), HA-disulfide | In vivo | A549 (human lung adenocarcinoma) | [80] | |
| Nanoparticle | Gold nanoparticle (PTT), doxorubicin (chemo), HA-dopamine | In vivo | MDA-MB-231 (human breast cancer) | [81] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Moon, M.J.; Kim, D.Y.; Heo, S.H.; Jeong, Y.Y. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers 2018, 10, 1133. https://doi.org/10.3390/polym10101133
Kim JH, Moon MJ, Kim DY, Heo SH, Jeong YY. Hyaluronic Acid-Based Nanomaterials for Cancer Therapy. Polymers. 2018; 10(10):1133. https://doi.org/10.3390/polym10101133
Chicago/Turabian StyleKim, Jin Hong, Myeong Ju Moon, Dong Yi Kim, Suk Hee Heo, and Yong Yeon Jeong. 2018. "Hyaluronic Acid-Based Nanomaterials for Cancer Therapy" Polymers 10, no. 10: 1133. https://doi.org/10.3390/polym10101133





