Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Simultaneous Thermal Analysis (STA)
2.3. Microscale Combustion Calorimetry
2.4. Modeling
3. Results and Discussion
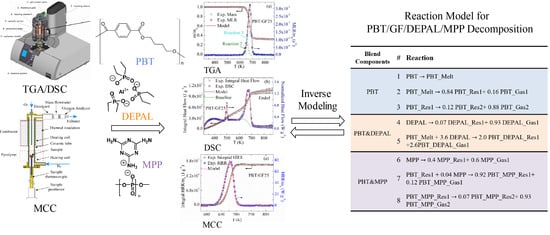
3.1. Overall Approach to Reaction Model Development
3.2. Inverse Modeling of TGA and DSC Data for PBT/GF25
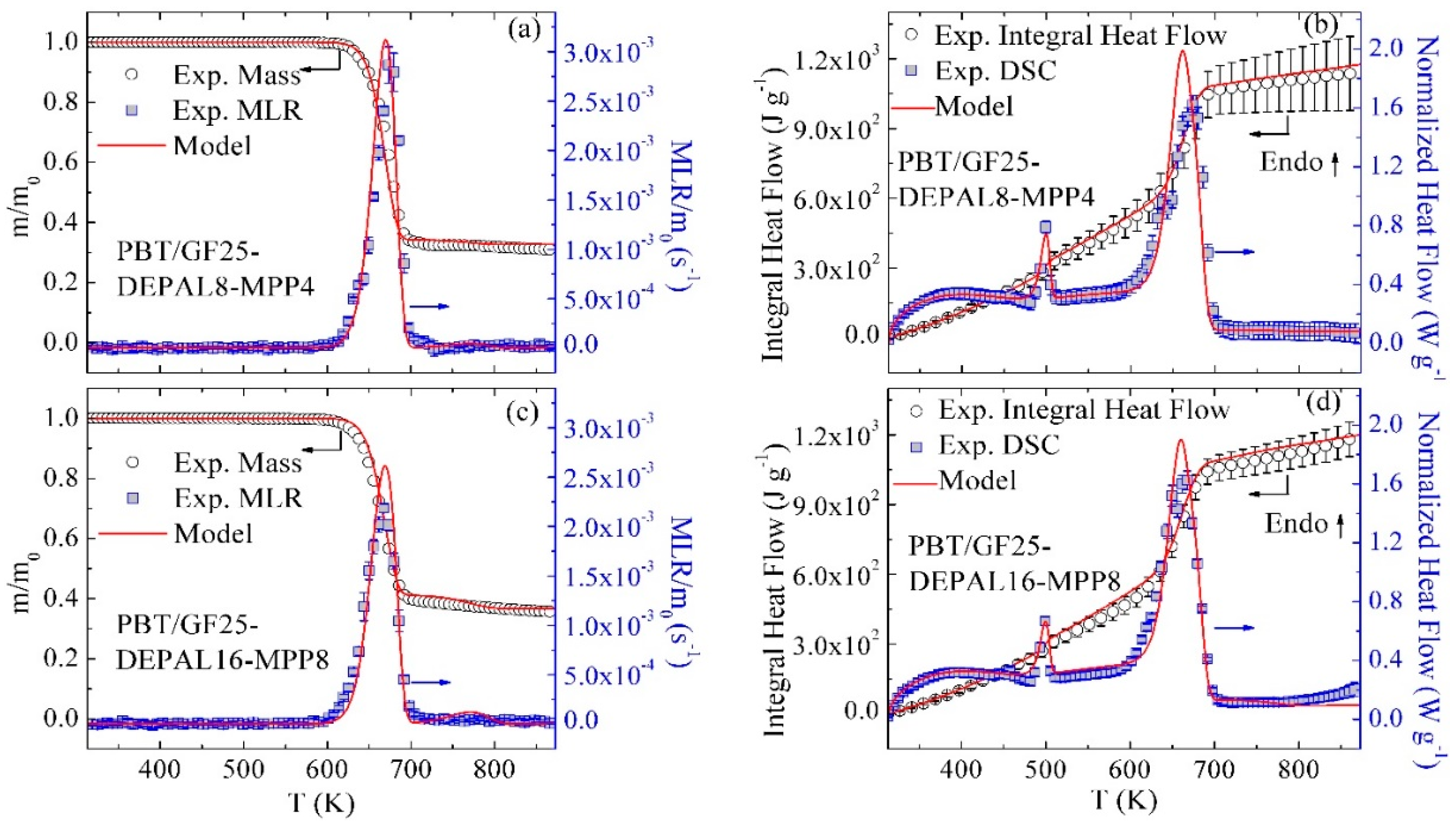
3.3. Inverse Modeling of TGA and DSC Data for PBT/GF/DEPAL
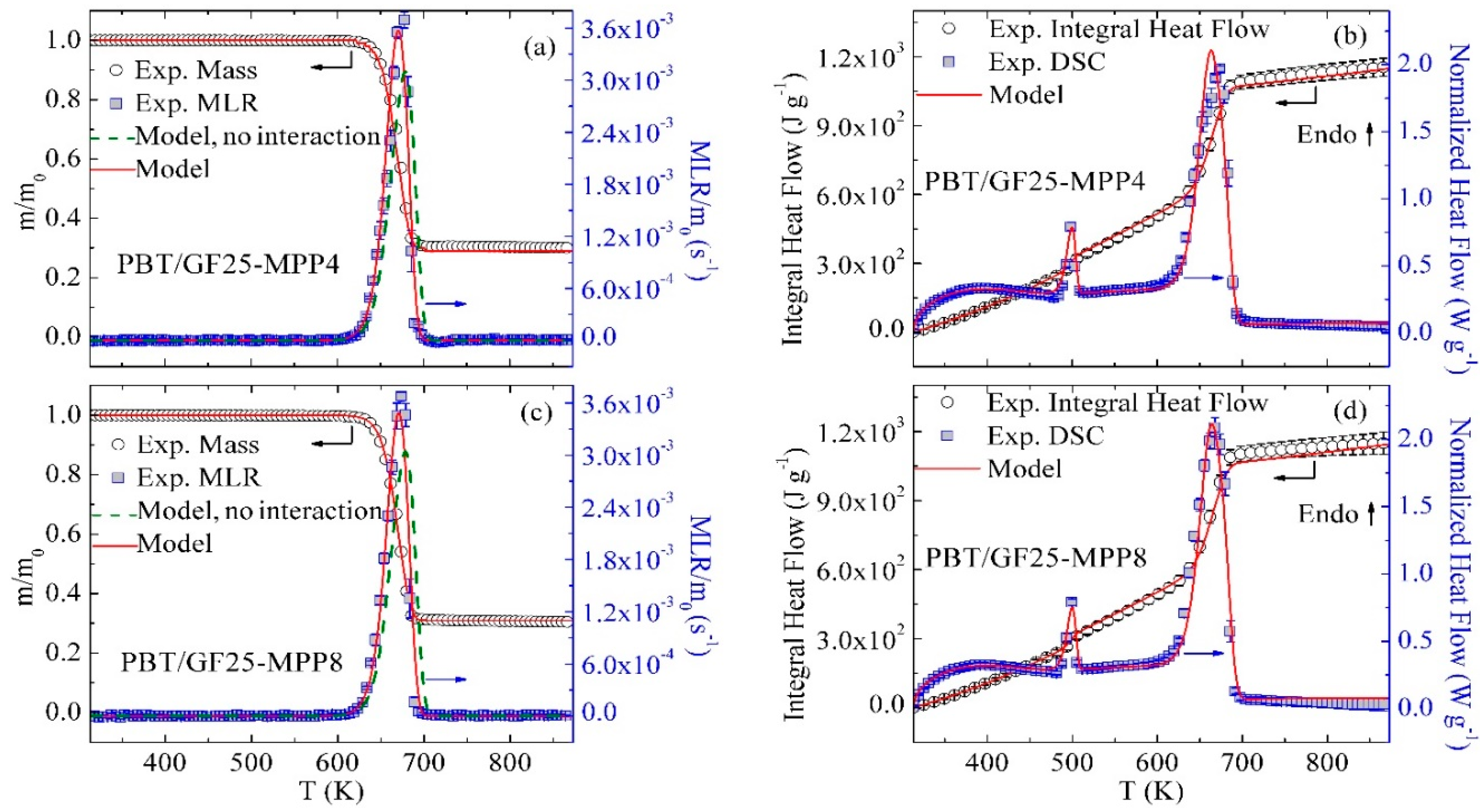
3.4. Inverse Modeling of TGA and DSC Data for PBT/GF/MPP
3.5. Inverse Modeling of MCC Data
3.6. Model Performance at Different Heating Rates
3.7. Modeling of Different Material Compositions
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Di Blasi, C. Modeling and simulation of combustion processes of charring and non-charring solid fuels. Prog. Energy Combust. Sci. 1993, 19, 71–104. [Google Scholar] [CrossRef]
- Kashiwagi, T. Polymer combustion and flammability—Role of the condensed phase. Twenty-Fifth Symp. Combust. 1994, 25, 1423–1437. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Safronava, N.; Lyon, R.E. The effect of variation in polymer properties on the rate of burning. Fire Mater. 2009, 33, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Friedman, H.L. New methods for evaluating kinetic parameters from thermal analysis data. J. Polym. Sci. Part C Polym. Lett. 1969, 7, 41–46. [Google Scholar] [CrossRef]
- Vyazovkin, S.; Sbirrazzuoli, N. Isoconversional kinetic analysis of thermally stimulated processes in polymers. Macromol. Rapid Commun. 2006, 27, 1515–1532. [Google Scholar] [CrossRef]
- Achilias, D.S.; Karabela, M.M.; Sideridou, I.D. Thermal degradation of light-cured dimethacrylate resins: Part I. Isoconversional kinetic analysis. Thermochim. Acta 2008, 472, 74–83. [Google Scholar] [CrossRef]
- Kruse, T.M.; Woo, O.S.; Broadbelt, L.J. Detailed mechanistic modeling of polymer degradation: Application to polystyrene. Chem. Eng. Sci. 2001, 56, 971–979. [Google Scholar] [CrossRef]
- Kruse, T.M.; Wong, H.W.; Broadbelt, L.J. Mechanistic modeling of polymer pyrolysis: Polypropylene. Macromolecules 2003, 36, 9594–9607. [Google Scholar] [CrossRef]
- Woo, O.S.; Broadbelt, L.J. Recovery of high-valued products from styrene-based polymers through coprocessing: Experiments and mechanistic modeling. Catal. Today 1998, 40, 121–140. [Google Scholar] [CrossRef]
- Madras, G.; McCoy, B.J. Distribution kinetics for polymer mixture degradation. Ind. Eng. Chem. Res. 1999, 38, 352–357. [Google Scholar] [CrossRef]
- Kruse, T.M.; Levine, S.E.; Wong, H.W.; Duoss, E.; Lebovitz, A.H.; Torkelson, J.M.; Broadbelt, L.J. Binary mixture pyrolysis of polypropylene and polystyrene: A modeling and experimental study. J. Anal. Appl. Pyrolysis 2005, 73, 342–354. [Google Scholar] [CrossRef]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for non-charring polymers. Combust. Flame 2013, 160, 1287–1297. [Google Scholar] [CrossRef]
- Li, J.; Stoliarov, S.I. Measurement of kinetics and thermodynamics of the thermal degradation for charring polymers. Polym. Degrad. Stab. 2014, 106, 2–15. [Google Scholar] [CrossRef]
- McKinnon, M.B.; Stoliarov, S.I.; Witkowski, A. Development of a pyrolysis model for corrugated cardboard. Combust. Flame 2013, 160, 2595–2607. [Google Scholar] [CrossRef]
- Mckinnon, M.B.; Stoliarov, S.I. Pyrolysis model development for a multilayer floor covering. Materials 2015, 8, 6117–6153. [Google Scholar] [CrossRef] [PubMed]
- McKinnon, M.B.; Ding, Y.; Stoliarov, S.I.; Crowley, S.; Lyon, R.E. Pyrolysis model for a carbon fiber/epoxy structural aerospace composite. J. Fire Sci. 2017, 35, 36–61. [Google Scholar] [CrossRef]
- Ding, Y.; McKinnon, M.B.; Stoliarov, S.I.; Fontaine, G.; Bourbigot, S. Determination of kinetics and thermodynamics of thermal decomposition for polymers containing reactive flame retardants: Application to poly(lactic acid) blended with melamine and ammonium polyphosphate. Polym. Degrad. Stab. 2016, 129, 347–362. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Kwon, K.; Stoliarov, S.I.; Kraemer, R.H. Development of a semi-global reaction mechanism for thermal decomposition of a polymer containing reactive flame retardant. In Proceedings of the 37th International Symposium on Combustion, Dublin, Ireland, 29 July–3 August 2018. [Google Scholar]
- Rein, G.; Lautenberger, C.; Fernandez-Pello, A.C.; Torero, J.L.; Urban, D.L. Application of genetic algorithms and thermogravimetry to determine the kinetics of polyurethane foam in smoldering combustion. Combust. Flame 2006, 146, 95–108. [Google Scholar] [CrossRef] [Green Version]
- Cai, J.; Wu, W.; Liu, R.; Huber, G.W. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013, 15, 1331. [Google Scholar] [CrossRef]
- Gao, F.; Tong, L.; Fang, Z. Effect of a novel phosphorous-nitrogen containing intumescent flame retardant on the fire retardancy and the thermal behaviour of poly(butylene terephthalate). Polym. Degrad. Stab. 2006, 91, 1295–1299. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Sturm, H.; Schartel, B. Flame retardancy mechanisms of metal phosphinates and metal phosphinates in combination with melamine cyanurate in glass-fiber reinforced poly(1,4-butylene terephthalate): The influence of metal cation. Polym. Adv. Technol. 2008, 19, 680–692. [Google Scholar] [CrossRef]
- Horrocks, A.R.; Price, D. Fire Retardant Materials; Woodhead Publishing: Sawston, UK, 2001. [Google Scholar]
- Braun, U.; Schartel, B. Flame retardancy mechanisms of aluminium phosphinate in combination with melamine cyanurate in glass-fibre-reinforced poly(1,4-butylene terephthalate). Macromol. Mater. Eng. 2008, 293, 206–217. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; di Cortemiglia, M.P.L. Overview of fire retardant mechanisms. Polym. Degrad. Stab. 1991, 33, 131–154. [Google Scholar] [CrossRef]
- Xiao, J.; Hu, Y.; Yang, L.; Cai, Y.; Song, L.; Chen, Z.; Fan, W. Fire retardant synergism between melamine and triphenyl phosphate in poly(butylene terephthalate). Polym. Degrad. Stab. 2006, 91, 2093–2100. [Google Scholar] [CrossRef]
- Weil, E.D.; Levchik, S.V. Flame Retardants for Plastics and Textiles: Practical Applications; Carl Hanser Verlag GmbH Co KG: München, Germany, 2015. [Google Scholar]
- Rosato, D.V.; Rosato, M.G. Injection Molding Handbook; Springer: Berlin, Germany, 2012. [Google Scholar]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I.; Safronava, N. Principles and Practice of Microscale Combustion Calorimetry; Federal Aviation Administration: Atlantic City International Airport, NJ, USA, 2013. [Google Scholar]
- Stoliarov, S.I.; Lyon, R.E. Thermo-kinetic model of burning for pyrolyzing materials. Fire Saf. Sci. 2008, 9, 1141–1152. [Google Scholar] [CrossRef]
- Stoliarov, S.I.; Leventon, I.T.; Lyon, R.E. Two-dimensional model of burning for pyrolyzable solids. Fire Mater. 2014, 38, 391–408. [Google Scholar] [CrossRef]
- Duquesne, S.; Fontaine, G.; Cérin-Delaval, O.; Gardelle, B.; Tricot, G.; Bourbigot, S. Study of the thermal degradation of an aluminium phosphinate-aluminium trihydrate combination. Thermochim. Acta 2013, 551, 175–183. [Google Scholar] [CrossRef]
- Lyon, R.E.; Walters, R.N.; Stoliarov, S.I. A thermal analysis method for measuring polymer flammability. J. ASTM Int. 2006, 3, 1–18. [Google Scholar] [CrossRef]
- MatWeb. “E-Glass Fiber, Generic,” MatWeb Material Property Data. Available online: http://matweb.com/search/DataSheet.aspx?MatGUID=d9c18047c49147a2a7c0b0bb1743e812 (accessed on 4 June 2018).
- Han, X.; Zhao, J.; Liu, S.; Yuan, Y. Flame retardancy mechanism of poly(butylene terephthalate)/aluminum diethylphosphinate composites with an epoxy-functional polysiloxane. RSC Adv. 2014, 4, 16551–16560. [Google Scholar] [CrossRef]
- NIST Chemistry WebBook. Available online: http://webbook.nist.gov/chemistry/ (accessed on 4 June 2018).
- Samyn, F.; Bourbigot, S. Thermal decomposition of flame retarded formulations PA6/aluminum phosphinate/melamine polyphosphate/organomodified clay: Interactions between the constituents? Polym. Degrad. Stab. 2012, 97, 2217–2230. [Google Scholar] [CrossRef]
- Braun, U.; Bahr, H.; Schartel, B. Fire retardancy effect of aluminium phosphinate and melamine polyphosphate in glass fibre reinforced polyamide 6. e-Polymers 2010, 10, 1–14. [Google Scholar] [CrossRef]
- Semmes, M.R.; Liu, X.; Mckinnon, M.B.; Stoliarov, S.I.; Witkowski, A. A Model for Oxidative Pyrolysis of Corrugated Cardboard. Fire Saf. Sci. 2014, 11, 111–123. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Gong, J.; Stoliarov, S.I. Development of pyrolysis models for charring polymers. Polym. Degrad. Stab. 2015, 115, 138–152. [Google Scholar] [CrossRef]
- Swann, J.D.; Ding, Y.; McKinnon, M.B.; Stoliarov, S.I. Controlled atmosphere pyrolysis apparatus II (CAPA II): A new tool for analysis of pyrolysis of charring and intumescent polymers. Fire Saf. J. 2017, 91, 130–139. [Google Scholar] [CrossRef]






| Formulation Name | PBT (wt %) Ultradur B4500 | GF (wt %) Glassfiber PPG 3786 | DEPAL (wt %) Exolit OP 1240 | MPP (wt %) Melapur 200 | |
|---|---|---|---|---|---|
| Model Development | PBT/GF25 | 75 | 25 | 0 | 0 |
| PBT/GF25-DEPAL8 | 67 | 25 | 8 | 0 | |
| PBT/GF25-DEPAL16 | 59 | 25 | 16 | 0 | |
| PBT/GF25-MPP4 | 71 | 25 | 0 | 4 | |
| PBT/GF25-MPP8 | 67 | 25 | 0 | 8 | |
| Model Validation | PBT | 100 | 0 | 0 | 0 |
| PBT/GF25-DEPAL8-MPP4 | 63 | 25 | 8 | 4 | |
| PBT/GF25-DEPAL16-MPP8 | 51 | 25 | 16 | 8 |
| Reactive Blend Components | # | Reaction Equation |
|---|---|---|
| PBT | 1 | PBT PBT_Melt |
| 2 | PBT_Melt 0.84 PBT_Res1 + 0.16 PBT_Gas1 | |
| 3 | PBT_Res1 0.12 PBT_Res2 + 0.88 PBT_Gas2 | |
| PBT and DEPAL | 4 | DEPAL 0.07a DEPAL_Res1 + 0.93a DEPAL_Gas1 |
| 5 | PBT_Melt + 3.6 DEPAL 2.0 PBT_DEPAL_Res1 + 2.6 PBT_DEPAL_Gas1 | |
| PBT and MPP | 6 | MPP 0.4 MPP_Res1 + 0.6 MPP_Gas1 |
| 7 | PBT_Res1 + 0.04 MPP 0.92 PBT_MPP_Res1 + 0.12 PBT_MPP_Gas1 | |
| 8 | PBT_MPP_Res1 0.07 PBT_MPP_Res2 + 0.93 PBT_MPP_Gas2 |
| # | A (s−1 or m3·kg−1·s−1) | E (J·mol−1) | h (J·kg−1) | # | A (s−1 or m3·kg−1·s−1) | E (J·mol−1) | h (J·kg−1) |
|---|---|---|---|---|---|---|---|
| 1 | 5 | ||||||
| 2 | 6 | ||||||
| 3 | 7 | ||||||
| 4 | 8 |
| Component | c (J·kg−1·K−1) | Component | c (J·kg−1·K−1) |
|---|---|---|---|
| PBT | DEPAL_Res1 | ||
| PBT_Melt | PBT_DEPAL_Res1 | ||
| PBT_Res1 | MPP | ||
| PBT_Res2 | MPP_Res1 | ||
| GF | () a | PBT_MPP_Res1 | |
| DEPAL | PBT_MPP_Res2 |
| Component | hc (J·kg−1) | Component | hc (J·kg−1) | Component | hc (J·kg−1) |
|---|---|---|---|---|---|
| PBT_Gas1 | PBT_DEPAL_Gas1 | PBT_MPP_Gas1 | |||
| PBT_Gas2 | MPP_Gas1 | PBT_MPP_Gas2 | |||
| DEPAL_Gas1 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, Y.; Stoliarov, S.I.; Kraemer, R.H. Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate. Polymers 2018, 10, 1137. https://doi.org/10.3390/polym10101137
Ding Y, Stoliarov SI, Kraemer RH. Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate. Polymers. 2018; 10(10):1137. https://doi.org/10.3390/polym10101137
Chicago/Turabian StyleDing, Yan, Stanislav I. Stoliarov, and Roland H. Kraemer. 2018. "Development of a Semiglobal Reaction Mechanism for the Thermal Decomposition of a Polymer Containing Reactive Flame Retardants: Application to Glass-Fiber-Reinforced Polybutylene Terephthalate Blended with Aluminum Diethyl Phosphinate and Melamine Polyphosphate" Polymers 10, no. 10: 1137. https://doi.org/10.3390/polym10101137