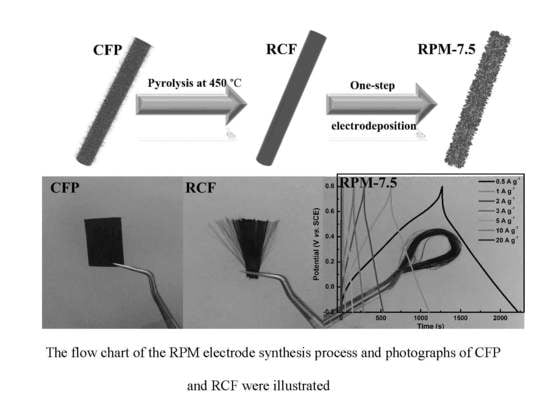
Recycled Carbon Fiber-Supported Polyaniline/Manganese Dioxide Prepared via One-Step Electrodeposition for Flexible Supercapacitor Integrated Electrodes
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Recovery of Carbon Fiber from CFP
2.3. Synthesis of RCF/PANI, RCF/MnO2 and RCF/PANI/MnO2
2.4. Materials Characterization
2.5. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of Materials
3.2. Electrochemical Measurements
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Simon, P.; Gogotsi, Y. Materials for electrochemical capacitors. Nat. Mater. 2008, 7, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Holdren, J. Energy and sustainability. Science 2007, 315, 737. [Google Scholar] [CrossRef] [PubMed]
- Jin, K.; Zhang, W.; Wang, Y.; Guo, X.; Chen, Z.; Li, L.; Zhang, Y.; Wang, Z.; Chen, J.; Sun, L.; et al. In-situ hybridization of polyaniline nanofibers on functionalized reduced graphene oxide films for high-performance supercapacitor. Electrochim. Acta 2018, 285, 221–229. [Google Scholar] [CrossRef]
- Arunachalam, V.; Fleischer, E. The global energy landscape and materials innovation. MRS Bull. 2008, 33, 264–288. [Google Scholar] [CrossRef]
- Wang, H.; Dai, H. ChemInform Abstract: Strongly Coupled Inorganic-Nano-Carbon Hybrid Materials for Energy Storage. Chem. Soc. Rev. 2013, 42, 3088–3113. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Zhou, Y.; Ge, Z.; Zhao, C.; Qian, X. Facile construction of MoS2/RCF electrode for high-performance supercapacitor. Carbon 2018, 127, 699–706. [Google Scholar] [CrossRef]
- Pan, S.; Lin, H.; Deng, J.; Chen, P.; Chen, X.; Yang, Z. Novel wearable energy devices based on aligned carbon nanotube fiber textiles. Adv. Energy Mater. 2015, 5, 1401438. [Google Scholar] [CrossRef]
- Yang, Z.; Deng, J.; Chen, X.; Ren, J.; Peng, H. A highly stretchable, fiber-shaped supercapacitor. Angew. Chem. Int. Ed. 2013, 52, 13453–13457. [Google Scholar] [CrossRef] [PubMed]
- Hatzell, K.; Fan, L.; Beidaghi, M.; Boota, M.; Pomerantseva, E.; Kumbur, E.; Gogotsi, Y. Composite Manganese Oxide Percolating Networks As a Suspension Electrode for an Asymmetric Flow Capacitor. ACS Appl. Mater. Interfaces 2014, 6, 8886–8893. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ge, Z.; Zhao, C.; Dong, J.; Shitian, M.; Ma, W. Solvothermal recovery of CFs from thermoset polymer-based CFRPs. J. Reinf. Plast. Compos. 2015, 34, 1673–1683. [Google Scholar] [CrossRef]
- Song, B.; Li, L.; Lin, Z.; Wu, Z.; Moon, K.; Wong, C. Water-dispersible graphene/polyaniline composites for flexible micro-supercapacitors with high energy densities. Nano Energy 2015, 16, 470–478. [Google Scholar] [CrossRef]
- Smolin, Y.; Aken, K.; Boota, M.; Soroush, M.; Gogotsi, Y.; Lau, K. Engineering Ultrathin Polyaniline in Micro/Mesoporous Carbon Supercapacitor Electrodes Using Oxidative Chemical Vapor Deposition. Adv. Mater. Interfaces 2017, 4, 1601201. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, Z.; Zheng, L.; Teng, F.; Hu, L.; Fang, X. A Novel Sustainable Flour Derived Hierarchical Nitrogen-Doped Porous Carbon/Polyaniline Electrode for Advanced Asymmetric Supercapacitors. Adv. Energy Mater. 2016, 6, 1601111. [Google Scholar] [CrossRef]
- Zhou, D.; Che, B.; Lu, X. Rapid one-pot electrodeposition of polyaniline/manganese dioxide hybrids: A facile approach to stable high-performance anodic electrochromic materials. J. Mater. Chem. C 2017, 5, 1758–1766. [Google Scholar] [CrossRef]
- Sun, L.; Liu, X. Electrodepositions and capacitive properties of hybrid films of polyaniline and manganese dioxide with fibrous morphologies. Eur. Polym. J. 2008, 44, 219–224. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, C. Extremely facile synthesis of manganese dioxide-polyaniline nano-reticulation with enhanced electrochemical properties. J. Alloys Compd. 2016, 677, 281–287. [Google Scholar] [CrossRef]
- Kim, M.; Kim, Y.; Lee, K.; Jeong, S.; Lee, E.; Baeck, S. Electrochemical improvement due to alignment of carbon nanofibers fabricated by electrospinning as an electrode for supercapacitor. Carbon 2016, 99, 607–618. [Google Scholar] [CrossRef]
- Tzeng, S.; Hung, K.; Ko, T. Growth of carbon nanofibers on activated carbon fiber fabrics. Carbon 2006, 44, 859–865. [Google Scholar] [CrossRef]
- Yu, P.; Li, Y.; Zhao, X.; Wu, L.; Zhang, Q. In situ growth of ordered polyaniline nanowires on surfactant stabilized exfoliated graphene as high-performance supercapacitor electrodes. Synth. Met. 2013, 185–186, 89–95. [Google Scholar] [CrossRef]
- Du, W.; Wang, X.; Ju, X.; Xu, K.; Gao, M.; Zhang, X. Carbonized Enteromorpha prolifera with porous architecture and its polyaniline composites as high-performance electrode materials for supercapacitors. J. Electroanal. Chem. 2017, 802, 15–21. [Google Scholar] [CrossRef]
- Cong, H.; Ren, X.; Wang, P.; Yu, S. Flexible graphene-polyaniline composite paper for high-performance supercapacitor. Energy Environ. Sci. 2013, 6, 1185–1191. [Google Scholar] [CrossRef]
- Ni, W.; Wang, D.; Huang, Z.; Zhao, J.; Cui, G. Fabrication of nanocomposite electrode with MnO2 nanoparticles distributed in polyaniline for electrochemical capacitors. Mater. Chem. Phys. 2010, 124, 1151–1154. [Google Scholar] [CrossRef]
- Li, Z.; Mi, Y.; Liu, X.; Liu, S.; Yang, S.; Wang, J. Flexible graphene/MnO2 composite papers for supercapacitor electrodes. J. Mater. Chem. 2011, 21, 14706–14711. [Google Scholar] [CrossRef]
- Li, D.; Li, Y.; Feng, Y.; Hu, W.; Feng, W. Hierarchical graphene oxide/polyaniline nanocomposites prepared by interfacial electrochemical polymerization for flexible solid-state supercapacitors. J. Mater. Chem. A 2015, 3, 2135–2143. [Google Scholar] [CrossRef]
- Boota, M.; Paranthaman, M.; Naskar, A.; Li, Y.; Akato, K.; Gogotsi, Y. Waste Tire Derived Carbon–Polymer Composite Paper as Pseudocapacitive Electrode with Long Cycle Life. ChemSusChem 2015, 8, 3576–3581. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Si, L.; Dunn, B.; Ma, J.; Lu, X. Covalently Bonded Polyaniline−TiO2 Hybrids: A Facile Approach to Highly Stable Anodic Electrochromic Materials with Low Oxidation Potentials. Chem. Mater. 2009, 22, 255–260. [Google Scholar] [CrossRef]
- Ling, H.; Liu, L.; Lee, P.; Mandler, D.; Lu, X. Layer-by-Layer Assembly of PEDOT: PSS and WO3 Nanoparticles: Enhanced Electrochromic Coloration Efficiency and Mechanism Studies by Scanning Electrochemic. Electrochim. Acta 2015, 174, 57–65. [Google Scholar] [CrossRef]
- Wang, D.; Fang, G.; Xue, T.; Ma, J.; Geng, G. A melt route for the synthesis of activated carbon derived from carton box for high performance symmetric supercapacitor applications. J. Power Sources 2016, 307, 401–409. [Google Scholar] [CrossRef]
- Yang, M.; Hong, S.; Choi, B. Hierarchical core/shell structure of MnO2@polyaniline composites grown on carbon fiber paper for application in pseudocapacitors. Phys. Chem. Chem. Phys. 2015, 17, 29874–29879. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Yue, C.; Sk, M.; Jena, R. Development of 3D Urchin-Shaped Coaxial Manganese Dioxide@Polyaniline (MnO2@PANI) Composite and Self-Assembled 3D Pillared Graphene Foam for Asymmetric All-Solid-State Flexible Supercapacitor Application. ACS Appl. Mater. Interfaces 2017, 9, 15350–15363. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Shahryari, E. Synthesis, Characterization and Electrochemical Study of Graphene Oxide-Multi Walled Carbon Nanotube-Manganese Oxide-Polyaniline Electrode as Supercapacitor. J. Mater. Sci. Technol. 2016, 32, 763–773. [Google Scholar] [CrossRef]
- Wang, L.; Ouyang, Y.; Jiao, X.; Xia, X.; Lei, W.; Hao, Q. Polyaniline-assisted growth of MnO2 ultrathin nanosheets on graphene and porous graphene for asymmetric supercapacitor with enhanced energy density. Chem. Eng. J. 2018, 334, 1–9. [Google Scholar] [CrossRef]
- Chen, W.; Tao, X.; Wei, D.; Wang, H.; Qiang, Y.; Li, Y. High-performance supercapacitor based on active carbon–MnO2–polyaniline composite. J. Mater. Sci. Mater. Electron. 2016, 27, 1357–1362. [Google Scholar] [CrossRef]
- He, Y.; Du, S.; Li, H.; Cheng, Q.; Pavlinek, V.; Saha, P. MnO2/polyaniline hybrid nanostructures on carbon cloth for supercapacitor electrodes. J. Solid State. Electrochem. 2016, 20, 1459–1467. [Google Scholar] [CrossRef]
- Liu, M.; Gan, L.; Xiong, W.; Xu, Z.; Zhu, D.; Chen, L. Development of MnO2/porous carbon microspheres with a partially graphitic structure for high performance supercapacitor electrodes. J. Mater. Chem. A 2014, 2, 2555–2562. [Google Scholar] [CrossRef]
- Xiong, W.; Liu, M.; Gan, L.; Lv, Y.; Li, Y.; Yang, L.; Xu, Z.; Hao, Z.; Liu, H.; Chen, L. A novel synthesis of mesoporous carbon microspheres for supercapacitor electrodes. J. Power Sources 2011, 196, 10461–10464. [Google Scholar] [CrossRef]
- Cong, S.; Tian, Y.; Li, Q.; Zhao, Z.; Geng, F. Single-Crystalline Tungsten Oxide Quantum Dots for Fast Pseudocapacitor and Electrochromic Applications. Adv. Mater. 2014, 26, 4260–4267. [Google Scholar] [CrossRef] [PubMed]






| No. | Name | Electrolyte | Specific Capacitance (F·g−1) | Scan Rate/Current Density | References |
|---|---|---|---|---|---|
| 1 | MnO2/PANI nano-reticulation composites | 1 M Na2SO4 | 425 | 0.1 A·g−1 | [16] |
| 2 | MnO2@PANI on carbon fiber paper | 1 M Na2SO4 | 437 | 1 A·g−1 | [29] |
| 3 | GO/MWCNT/PANI/MnO2 | 0.5 M Na2SO4 | 173.00 (mF·cm−2) | 4 mA·cm−2 | [31] |
| 4 | PANI-assisted graphene@MnO2 | 1 M Na2SO4 | 245 | 0.5 A·g−1 | [32] |
| 5 | AC/MnO2/PANI | 1 M Na2SO4 | 245 | 1 A·g−1 | [33] |
| 6 | carbon cloth/MnO2/PANI | 1 M Na2SO4 | 1.56 (F·cm−2) | 10 mV·s−1 | [34] |
| 7 | RPM-7.5 | 1 M Na2SO4 | 475 | 1 A·g−1 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Wei, H.; Du, W.; Sun, X.; Kang, L.; Zhang, Y.; Zhao, X.; Jiang, F. Recycled Carbon Fiber-Supported Polyaniline/Manganese Dioxide Prepared via One-Step Electrodeposition for Flexible Supercapacitor Integrated Electrodes. Polymers 2018, 10, 1152. https://doi.org/10.3390/polym10101152
Wang X, Wei H, Du W, Sun X, Kang L, Zhang Y, Zhao X, Jiang F. Recycled Carbon Fiber-Supported Polyaniline/Manganese Dioxide Prepared via One-Step Electrodeposition for Flexible Supercapacitor Integrated Electrodes. Polymers. 2018; 10(10):1152. https://doi.org/10.3390/polym10101152
Chicago/Turabian StyleWang, Xiaoning, Hongli Wei, Wei Du, Xueqin Sun, Litao Kang, Yuping Zhang, Xiangjin Zhao, and Fuyi Jiang. 2018. "Recycled Carbon Fiber-Supported Polyaniline/Manganese Dioxide Prepared via One-Step Electrodeposition for Flexible Supercapacitor Integrated Electrodes" Polymers 10, no. 10: 1152. https://doi.org/10.3390/polym10101152
APA StyleWang, X., Wei, H., Du, W., Sun, X., Kang, L., Zhang, Y., Zhao, X., & Jiang, F. (2018). Recycled Carbon Fiber-Supported Polyaniline/Manganese Dioxide Prepared via One-Step Electrodeposition for Flexible Supercapacitor Integrated Electrodes. Polymers, 10(10), 1152. https://doi.org/10.3390/polym10101152