Effect of Domain Structure of Segmented Poly(urethane-imide) Membranes with Polycaprolactone Soft Blocks on Dehydration of n-Propanol via Pervaporation
Abstract
:1. Introduction
2. Materials and Methods
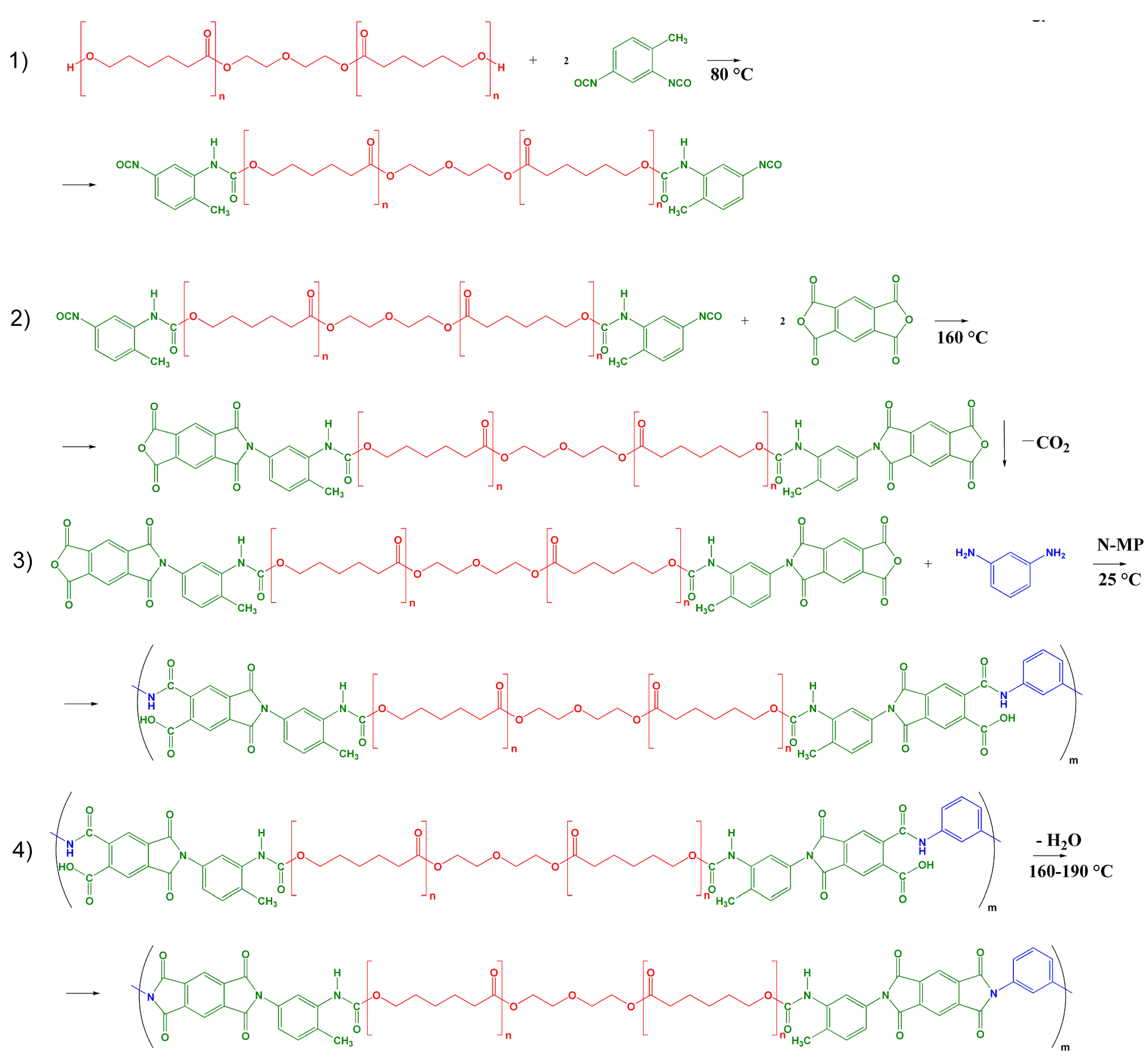
2.1. Materials and Preparation of Segmented PUIs
2.2. Characterization Methods
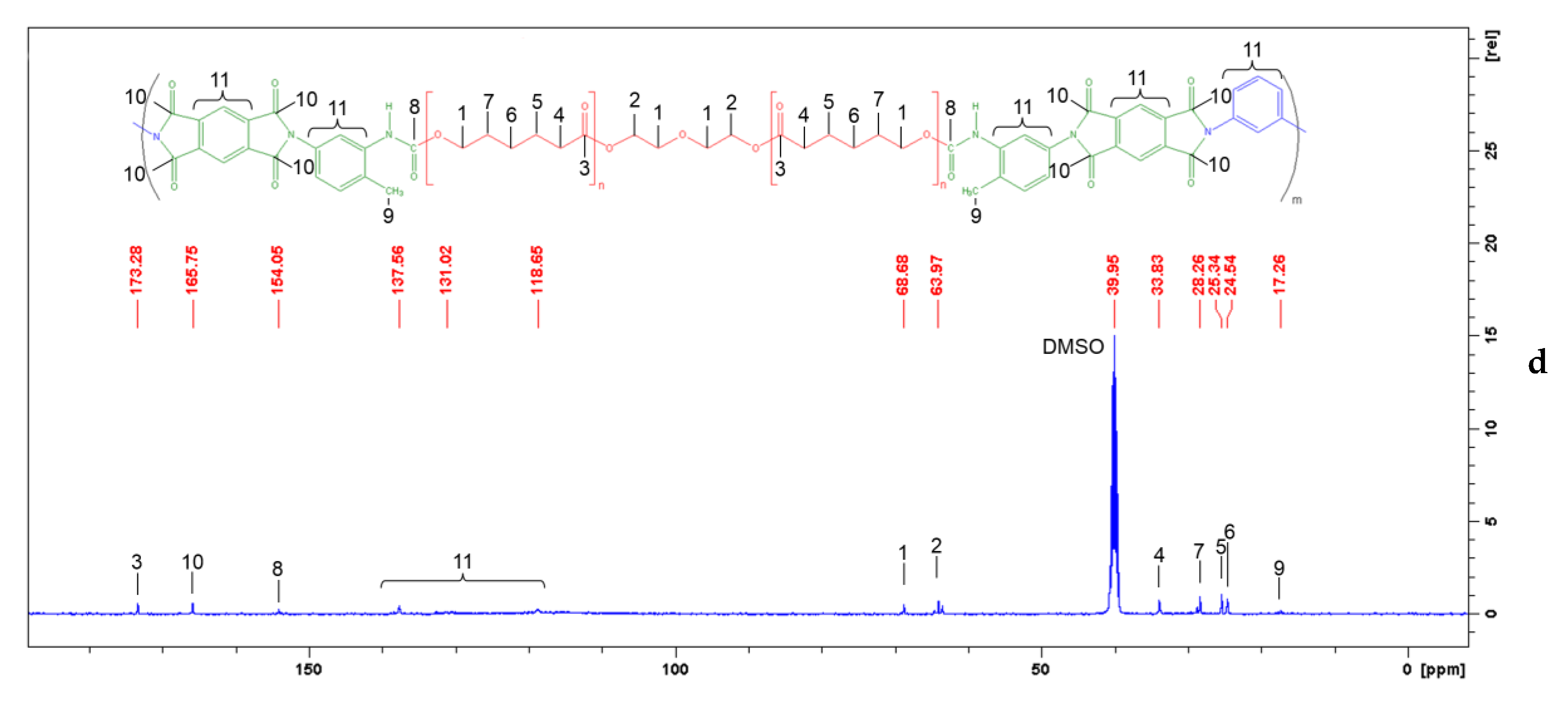
2.2.1. FTIR and NMR Spectroscopy Investigations
2.2.2. Microscopic Investigation
2.2.3. Small-Angle and Wide-Angle X-ray Diffraction Study
2.2.4. Pervaporation Experiments
2.2.5. Simulation Details
3. Results and Discussion
3.1. FTIR and NMR Spectroscopy of Segmented PUIs
3.2. Investigation of Morphology with AFM
3.3. SAXS and WAXD Investigations
3.4. Pervaporation Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Li, F.; Wan, W.; Lai, J.; Liu, F.; Qi, H.; Li, X.; You, X. Investigations on the Polyimides Derived from Unfunctionalized Symmetric Cyclopentyl-Containing Alicyclic Cardo-Type Dianhydride. J. Appl. Polym. Sci. 2015, 42670. [Google Scholar] [CrossRef]
- Huang, X.; Chen, B.; Mei, M.; Li, H.; Liu, C.; Wei, C. Synthesis and Characterization of Organosoluble, Thermal Stable and Hydrophobic Polyimides Derived from 4-(4-(1-Pyrrolidinyl)phenyl)-2,6-bis(4-(4-Aminophenoxy)phenyl)pyridine. Polymers 2017, 9, 484. [Google Scholar] [CrossRef]
- Yao, J.; Wang, C.; Tian, C.; Zhao, X.; Zhou, H.; Wang, D.; Chen, C. Highly Optical Transparency and Thermally Stable Polyimides Containing Pyridine and Phenyl Pendant. Des. Monomers Polym. 2017, 20, 449–457. [Google Scholar] [CrossRef] [PubMed]
- Thomas, P.J.; Hellevang, J.O. A High Response Polyimide Fiber Optic Sensor for Distributed Humidity Measurements. Sens. Actuators B Chem. 2018, 270, 417–423. [Google Scholar] [CrossRef]
- Xu, H.-Y.; Yang, H.-X.; Tao, L.-M.; Lin Fan, S.; Yang, H.-Y. Preparation and Properties of Glass Cloth-Reinforced Polyimide Composites with Improved Impact Toughness for Microelectronics Packaging Substrates. J. Appl. Polym. Sci. 2010, 117, 1173–1183. [Google Scholar] [CrossRef]
- Lanč, M.; Sysel, P.; Šoltys, M.; Štěpánek, F.; Fónod, K.; Klepić, M.; Vopička, O.; Lhotka, M.; Ulbrich, P.; Friess, K. Synthesis, Preparation and Characterization of Novel Hyperbranched 6FDA-TTM Based Polyimide Membranes for Effective CO2 Separation: Effect of Embedded Mesoporous Silica Particles and Siloxane Linkages. Polymer 2018, 144, 33–42. [Google Scholar] [CrossRef]
- Luo, S.; Stevens, K.A.; Park, J.S.; Moon, J.D.; Liu, Q.; Freeman, B.D.; Guo, R. Highly CO2-Selective Gas Separation Membranes Based on Segmented Copolymers of Poly(Ethylene Oxide) Reinforced with Pentiptycene-Containing Polyimide Hard Segments. ACS Appl. Mater. Interfaces 2016, 8, 2306–2317. [Google Scholar] [CrossRef] [PubMed]
- Kudasheva, A.; Sorribas, S.; Zornoza, B.; Téllez, C.; Coronas, J. Pervaporation of Water/ethanol Mixtures through Polyimide Based Mixed Matrix Membranes Containing ZIF-8, Ordered Mesoporous Silica and ZIF-8-Silica Core-Shell Spheres. J. Chem. Technol. Biotechnol. 2015, 90, 669–677. [Google Scholar] [CrossRef]
- Xu, S.; Liu, L.; Wang, Y. Network Cross-Linking of Polyimide Membranes for Pervaporation Dehydration. Sep. Purif. Technol. 2017, 185, 215–226. [Google Scholar] [CrossRef]
- Lin, M.-F.; Shu, Y.-C.; Tsen, W.-C.; Chuang, F.-S. Synthesis of Polyurethane-Imide (PU-Imide) Copolymers with Different Dianhydrides and Their Properties. Polym. Int. 1999, 48, 433–445. [Google Scholar] [CrossRef]
- Yudin, V.E.; Bugrov, A.N.; Didenko, A.L.; Smirnova, V.E.; Gofman, I.V.; Kononova, S.V.; Kremnev, R.V.; Popova, E.N.; Svetlichnyi, V.M.; Kudryavtsev, V.V. Composites of Multiblock (Segmented) Aliphatic Poly(ester Imide) with Zirconia Nanoparticles: Synthesis, Mechanical Properties, and Pervaporation Behavior. Polym. Sci. Ser. B 2014, 56, 919–926. [Google Scholar] [CrossRef]
- Woodruff, M.A.; Hutmacher, D.W. The Return of a Forgotten Polymer–Polycaprolactone in the 21st Century. Prog. Polym. Sci. 2010, 35, 1217–1256. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.; Cai, T.; Jin, Q.; Ji, J. Design and Fabrication of Functional Polycaprolactone. E-Polymers 2015, 15, 3–13. [Google Scholar] [CrossRef]
- Meleshko, T.K.; Kashina, A.V.; Saprykina, N.N.; Kostyuk, S.V.; Vasilenko, I.V.; Nikishev, P.A.; Yakimanskii, A.V. Synthesis and Morphology of Polycaprolactone–block-Polyimide–block-Polycaprolactone Triblock Copolymers for Film Separation Membranes. Russ. J. Appl. Chem. 2017, 90, 602–612. [Google Scholar] [CrossRef]
- Iqbal, N.; Tripathi, M.; Parthasarathy, S.; Kumar, D.; Roy, P.K. Tuning the Properties of Segmented Polyurea by Regulating Soft-Segment Length. J. Appl. Polym. Sci. 2018, 135, 1–8. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Emelina, O.Y.; Vorotyntsev, I.V.; Davletbaev, R.S.; Grebennikova, E.S.; Petukhov, A.N.; Akhmetshina, A.I.; Sazanova, T.S. Synthesis and Properties of Novel Polyurethanes Based on Amino Ethers of Boric Acid for Gas Separation Membranes. RSC Adv. 2015, 5, 65674–65683. [Google Scholar] [CrossRef]
- Davletbaeva, I.M.; Nurgaliyeva, G.R.; Akhmetshina, A.I.; Davletbaev, R.S.; Atlaskin, A.A.; Sazanova, T.S.; Efimov, S.V.; Klochkov, V.V.; Vorotyntsev, I.V. Porous Polyurethanes Based on Hyperbranched Amino Ethers of Boric Acid. RSC Adv. 2016, 6, 111109–111119. [Google Scholar] [CrossRef]
- Solimando, X.; Babin, J.; Arnal-Herault, C.; Wang, M.; Barth, D.; Roizard, D.; Doillon-Halmenschlager, J.R.; Ponçot, M.; Royaud, I.; Alcouffe, P.; et al. Highly Selective Multi-Block Poly(ether-Urea-Imide)s for CO2/N2 Separation: Structure-Morphology-Properties Relationships. Polymer 2017, 131, 56–67. [Google Scholar] [CrossRef]
- Swolfs, Y.; Bertels, E.; Verpoest, I.; Goderis, B. Linking the Morphology of a High Hard Segment Content Polyurethane to Its Thermal Behaviour and Mechanical Properties. Polymer 2015, 81, 1–11. [Google Scholar] [CrossRef]
- Hossieny, N.; Shaayegan, V.; Ameli, A.; Saniei, M.; Park, C.B. Characterization of Hard-Segment Crystalline Phase of Thermoplastic Polyurethane in the Presence of Butane and Glycerol Monosterate and Its Impact on Mechanical Property and Microcellular Morphology. Polymer 2017, 112, 208–218. [Google Scholar] [CrossRef]
- Xu, W.; Zhang, R.; Liu, W.; Zhu, J.; Dong, X.; Guo, H.; Hu, G.H. A Multiscale Investigation on the Mechanism of Shape Recovery for IPDI to PPDI Hard Segment Substitution in Polyurethane. Macromolecules 2016, 49, 5931–5944. [Google Scholar] [CrossRef]
- Fernández-D’Arlas, B.; Balko, J.; Baumann, R.P.; Pöselt, E.; Dabbous, R.; Eling, B.; Thurn-Albrecht, T.; Müller, A.J. Tailoring the Morphology and Melting Points of Segmented Thermoplastic Polyurethanes by Self-Nucleation. Macromolecules 2016, 49, 7952–7964. [Google Scholar] [CrossRef]
- Yanagihara, Y.; Osaka, N.; Murayama, S.; Saito, H. Thermal Annealing Behavior and Structure Development of Crystalline Hard Segment Domain in a Melt-Quenched Thermoplastic Polyurethane. Polymer 2013, 54, 2183–2189. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Bobrova, N.V.; Pientka, Z.; Elyashevich, G.K. High-Conductivity Polypyrrole Layers Supported on Porous Polyethylene Films. Polym. Sci. Ser. B 2005, 47, 215–219. [Google Scholar]
- Yilgör, I.; Yilgör, E.; Wilkes, G.L. Critical Parameters in Designing Segmented Polyurethanes and Their Effect on Morphology and Properties: A Comprehensive Review. Polymer 2015, 58, A1–A36. [Google Scholar] [CrossRef]
- Wu, X.; Shi, S.; Yu, Z.; Russell, T.P.; Wang, D. AFM Nanomechanical Mapping and Nanothermal Analysis Reveal Enhanced Crystallization at the Surface of a Semicrystalline Polymer. Polymer 2018, 146, 188–195. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Geydt, P.; Bugrov, A.N.; Ovaska, S.S.; Lahderanta, E.; Toikka, A.M. Structure and Transport Properties of Mixed-Matrix Membranes Based on Polyimides with ZrO2 Nanostars. Polymers 2016, 8, 403. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Bugrov, A.N.; Geydt, P.; Popova, E.N.; Lahderanta, E.; Svetlichnyi, V.M.; Toikka, A.M. Structure of Composite Based on Polyheteroarylene Matrix and ZrO2 Nanostars Investigated by Quantitative Nanomechanical Mapping. Polymers 2017, 9, 268. [Google Scholar] [CrossRef]
- Smirnov, M.A.; Sokolova, M.P.; Geydt, P.; Smirnov, N.N.; Bobrova, N.V.; Toikka, A.M.; Lahderanta, E. Dual Doped Electroactive Hydrogelic Fibrous Mat with High Areal Capacitance. Mater. Lett. 2017, 199, 192–195. [Google Scholar] [CrossRef]
- Wang, D.; Russell, T.P. Advances in Atomic Force Microscopy for Probing Polymer Structure and Properties. Macromolecules 2018, 51, 3–24. [Google Scholar] [CrossRef]
- Sokolova, M.P.; Smirnov, M.A.; Samarov, A.A.; Bobrova, N.V.; Vorobiov, V.K.; Popova, E.N.; Filippova, E.; Geydt, P.; Lahderanta, E.; Toikka, A.M. Plasticizing of Chitosan Films with Deep Eutectic Mixture of Malonic Acid and Choline Chloride. Carbohydr. Polym. 2018, 197, 548–557. [Google Scholar] [CrossRef] [PubMed]
- Magonov, S.N.; Reneker, D.H. Characterization of Polymer Surfaces With Atomic Force Microscopy. Annu. Rev. Mater. Sci. 1997, 27, 175–222. [Google Scholar] [CrossRef]
- He, C.; Shi, S.; Wu, X.; Russell, T.P.; Wang, D. Atomic Force Microscopy Nanomechanical Mapping Visualizes Interfacial Broadening between Networks Due to Chemical Exchange Reactions. J. Am. Chem. Soc. 2018, 140, 6793–6796. [Google Scholar] [CrossRef] [PubMed]
- McLean, R.S.; Sauer, B.B. Tapping-Mode AFM Studies Using Phase Detection for Resolution of Nanophases in Segmented Polyurethanes and Other Block Copolymers. Macromolecules 1997, 30, 8314–8317. [Google Scholar] [CrossRef]
- Ren, L.; Shah, P.N.; Faust, R. Morphology and Tensile Properties of Model Thermoplastic Polyurethanes with MDI/butanediol Based Monodisperse Hard Segments. J. Polym. Sci. Part B Polym. Phys. 2016, 54, 2485–2493. [Google Scholar] [CrossRef]
- Cappella, B. Mechanical Properties and Adhesion of a Micro Structured Polymer Blend. Polymers 2011, 3, 1091–1106. [Google Scholar] [CrossRef] [Green Version]
- Didenko, A.L.; Yudin, V.E.; Smirnova, V.E.; Gofman, I.V.; Popova, E.N.; Elokhovskii, V.Y.; Svetlichnyi, V.M.; Kudryavtsev, V.V. Modification of the Thermoplastic Polyheteroarylenes with Aliphatic Polyethers and Polyesters: Synthesis and Dynamic Mechanical Properties. J. Int. Sci. Publ. Mater. Methods Technol. Vol. 2014, 8, 31–40. [Google Scholar]
- Qin, X.; Yang, X.; Wang, X.; Wang, M. Synthesis and Characterization of Poly(imide-Urethane) Based on Novel Chain-Extender Containing Both Imide and Sulphone Functions. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 4469–4477. [Google Scholar] [CrossRef]
- Gnanarajan, T.P.; Iyer, N.P.; Nasar, A.S.; Radhakrishnan, G. Preparation and Properties of Poly(urethane-Imide)s Derived from Amine-Blocked-Polyurethane Prepolymer and Pyromellitic Dianhydride. Eur. Polym. J. 2002, 38, 487–495. [Google Scholar] [CrossRef]
- Mândru, M.; Ciobanu, C.; Vlad, S.; Butnaru, M.; Lebrun, L.; Popa, M. Characteristics of Polyurethane-Based Sustained Release Membranes for Drug Delivery. Cent. Eur. J. Chem. Charact. 2013, 11, 542–553. [Google Scholar] [CrossRef]
- Veerapandian, S.; Sultan Nasar, A. Amine- and Blocked Isocyanate-Terminated Polyurethane Dendrimers: Integrated Synthesis, Photophysical Properties and Application in a Heat Curable System. RSC Adv. 2015, 3799–3806. [Google Scholar] [CrossRef]
- Hui, B.; Ye, L. Highly Heat-Resistant Silicon-Containing Polyurethane-Imide Copolymers: Synthesis and Thermal Mechanical Stability. Eur. Polym. J. 2017, 91, 337–353. [Google Scholar] [CrossRef]
- Shih, T.; Yang, J.; Jia, H.; Chen, J. Synthesis and Properties of Biodegradable Segmented Poly-ε-Caprolactone. J. Med. Biol. Eng. 2013, 34, 238–242. [Google Scholar] [CrossRef]
- Avci, A.; Sirin, K. Thermal, Fluorescence, and Electrochemical Characteristics of Novel Poly(urethane-Imide)s. Des. Monomers Polym. 2014, 17, 380–389. [Google Scholar] [CrossRef]
- Okamoto, H. Preparation and Properties of Imide-Containing Elastic Polymers from Elastic Polyureas and Pyromellitic. Dep. Appl. Chem. 2000, 38, 715–723. [Google Scholar]
- Wang, S.; Lu, L.; Gruetzmacher, J.A.; Currier, B.L.; Yaszemski, M.J. A Biodegradable and Cross-Linkable Multiblock Copolymer Consisting of Poly(propylene Fumarate) and Poly(ε-Caprolactone): Synthesis, Characterization, and Physical Properties. Macromolecules 2005, 38, 7358–7370. [Google Scholar] [CrossRef]
- Debye, P.; Bueche, A.M. Scattering by an Inhomogeneous Solid. J. Appl. Phys. 1949, 20, 518–525. [Google Scholar] [CrossRef]
- Kinning, D.J.; Thomas, E.L. Hard-Sphere Interactions Between Spherical Domains in Diblock Copolymers. Macromolecules 1984, 17, 1712–1718. [Google Scholar] [CrossRef]
- Svergun, D.I.; Feigin, L.A.; Schedrin, B.M. The Solution of the One-dimensional Sign Problem for Fourier Transforms. Prog. Polym. Sci. 1984, 40, 137–142. [Google Scholar] [CrossRef]
- López-Calzada, G.; Hernandez-Martínez, A.R.; Cruz-Soto, M.; Ramírez-Cardona, M.; Rangel, D.; Molina, G.A.; Luna-Barcenas, G.; Estevez, M. Development of Meniscus Substitutes Using a Mixture of Biocompatible Polymers and Extra Cellular Matrix Components by Electrospinning. Mater. Sci. Eng. C 2016, 61, 893–905. [Google Scholar] [CrossRef] [PubMed]
- Afsar, F.; Saljoughi, E.; Mousavi, S.M. Poly(Caprolactone)/poly(Ethylene Glycol) Pervaporation Blend Membranes: Synthesis, Characterization, and Performance. Polym. Adv. Technol. 2018, 29, 2467–2476. [Google Scholar] [CrossRef]
- Rahal, A.; Mas, A.; Elharfi, A.; Arcana, A.; Schue, F. Membranes de Pervaporation En Polyvalerolactone et Polycaprolactone Testees Pout La Deshydration de L’ethanol. Eur. Polym. J. 1998, 34, 45–50. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, C.; Li, J. Experimental Study on Physical Properties and Pervaporation Performances of Polyimide Membranes. Chem. Eng. Sci. 2007, 62, 2466–2473. [Google Scholar] [CrossRef]







| Parameters | Samples | |
|---|---|---|
| PUI-530 | PUI-2000 | |
| A1 | 2.847(5) × 10−4 | 5.280(6) × 10−4 |
| ν | 0.3350(6) | 0.3926(4) |
| a, nm | 2.733(2) | 2.562(1) |
| RHS, nm | 3.595(7) | 3.367(3) |
| φ | 6.17(2) × 10−2 | 8.84(1) × 10−2 |
| A2 | 1.496(1) × 10−2 | 2.533(1) × 10−2 |
| ξ, nm | 5.31(1) × 10−2 | 5.36(1) × 10−2 |
| D, nm | 14.67(3) | 12.18(1) |
| L1 | 5.466(4) | 5.124(2) |
| L2 | 1.831(4) | 2.012(2) |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sokolova, M.P.; Bugrov, A.N.; Smirnov, M.A.; Smirnov, A.V.; Lahderanta, E.; Svetlichnyi, V.M.; Toikka, A.M. Effect of Domain Structure of Segmented Poly(urethane-imide) Membranes with Polycaprolactone Soft Blocks on Dehydration of n-Propanol via Pervaporation. Polymers 2018, 10, 1222. https://doi.org/10.3390/polym10111222
Sokolova MP, Bugrov AN, Smirnov MA, Smirnov AV, Lahderanta E, Svetlichnyi VM, Toikka AM. Effect of Domain Structure of Segmented Poly(urethane-imide) Membranes with Polycaprolactone Soft Blocks on Dehydration of n-Propanol via Pervaporation. Polymers. 2018; 10(11):1222. https://doi.org/10.3390/polym10111222
Chicago/Turabian StyleSokolova, Maria P., Alexander N. Bugrov, Michael A. Smirnov, Alexander V. Smirnov, Erkki Lahderanta, Valentin M. Svetlichnyi, and Alexander M. Toikka. 2018. "Effect of Domain Structure of Segmented Poly(urethane-imide) Membranes with Polycaprolactone Soft Blocks on Dehydration of n-Propanol via Pervaporation" Polymers 10, no. 11: 1222. https://doi.org/10.3390/polym10111222







