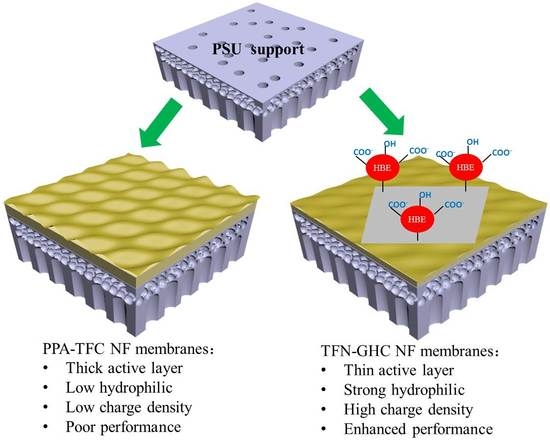
A Novel Thin-Film Nanocomposite Nanofiltration Membrane by Incorporating 3D Hyperbranched Polymer Functionalized 2D Graphene Oxide
Abstract
:1. Introduction
2. Experimental
2.1. Materials
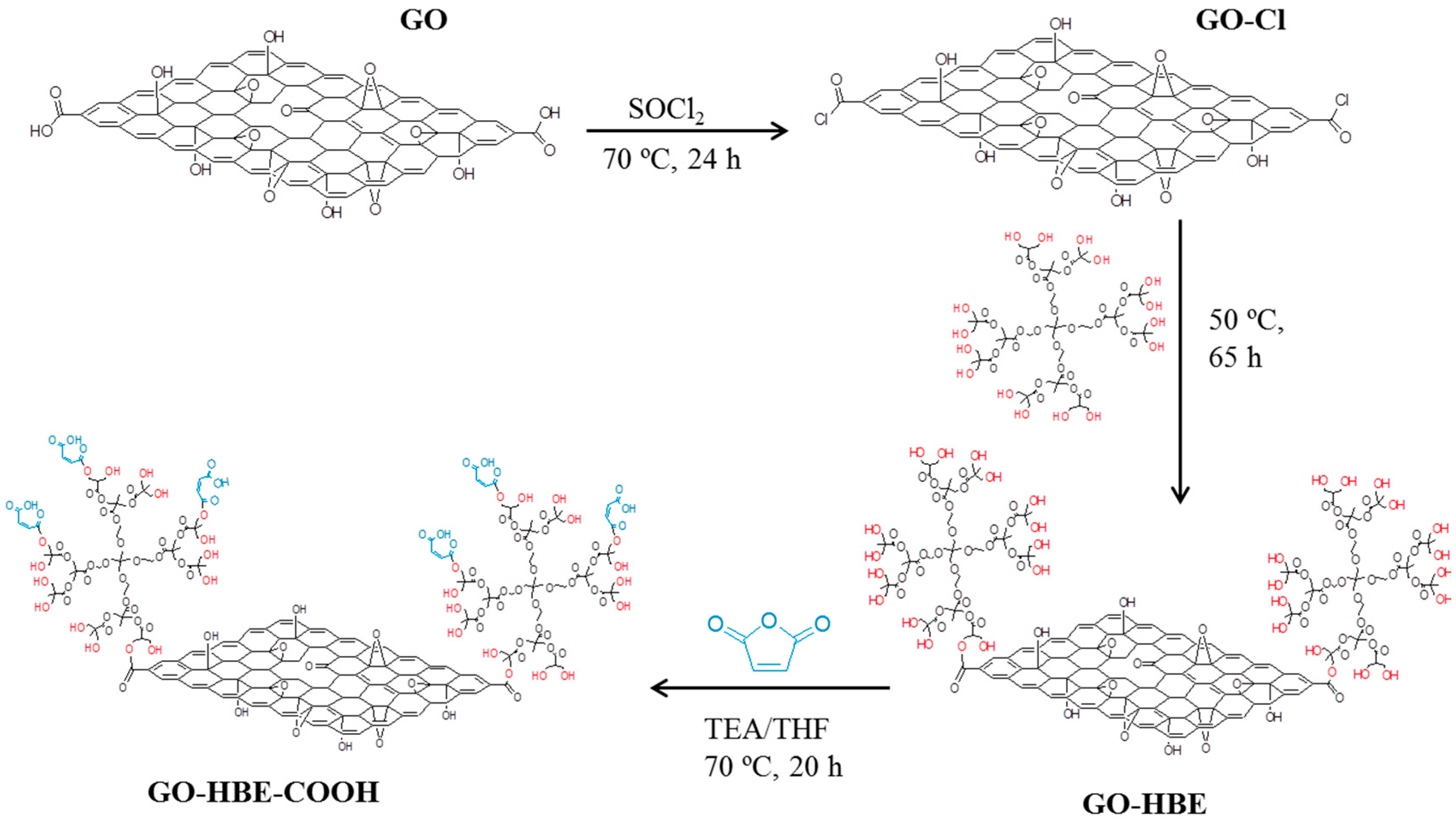
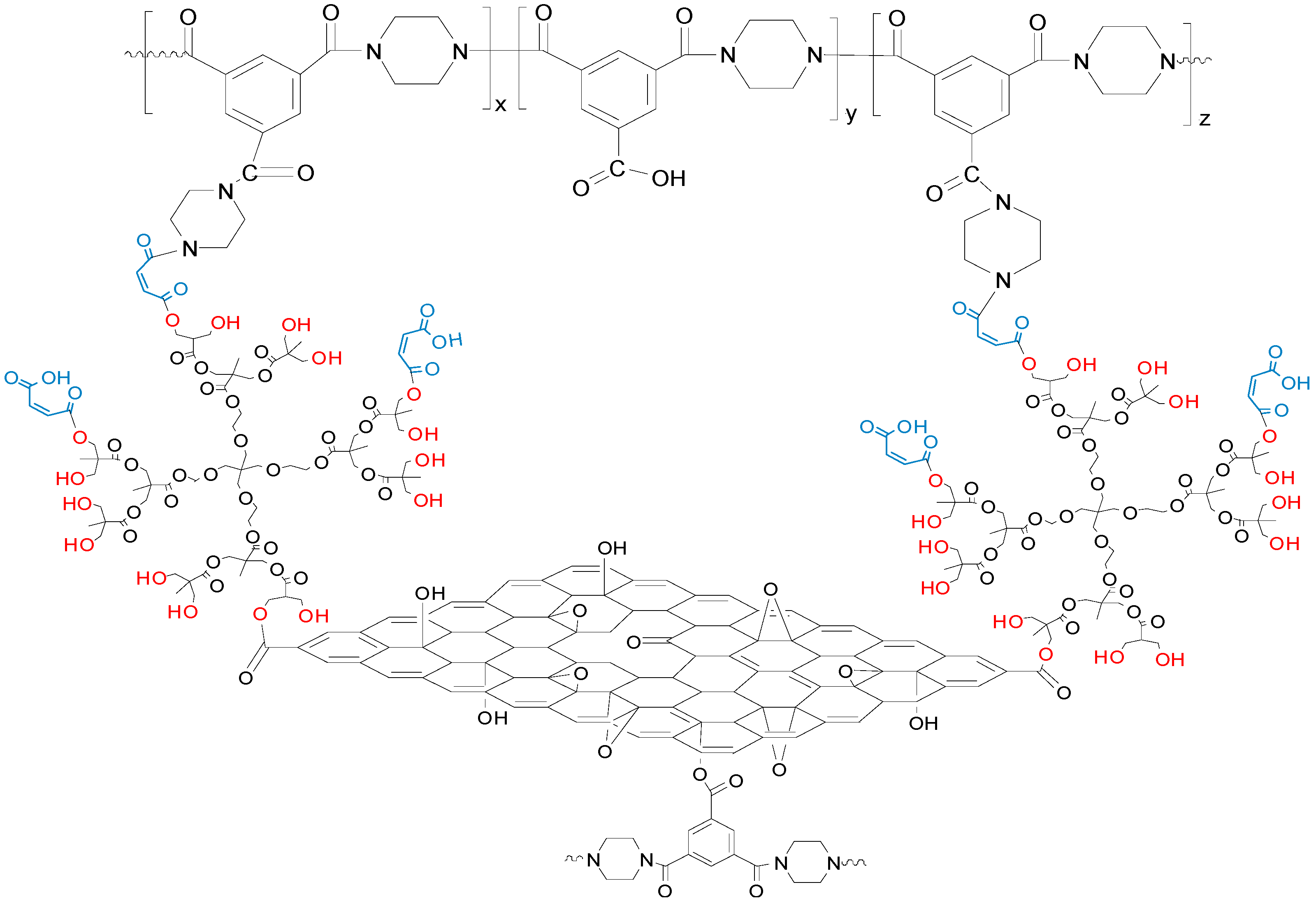
2.2. Preparation of GO and GO-HBE-COOH
2.3. Preparation of Composite NF Membrane
2.4. Characterization of GO and GO-HBE-COOH
2.5. Characterization of Composite NF Membranes
2.6. Membrane Performance
3. Results and Discussion
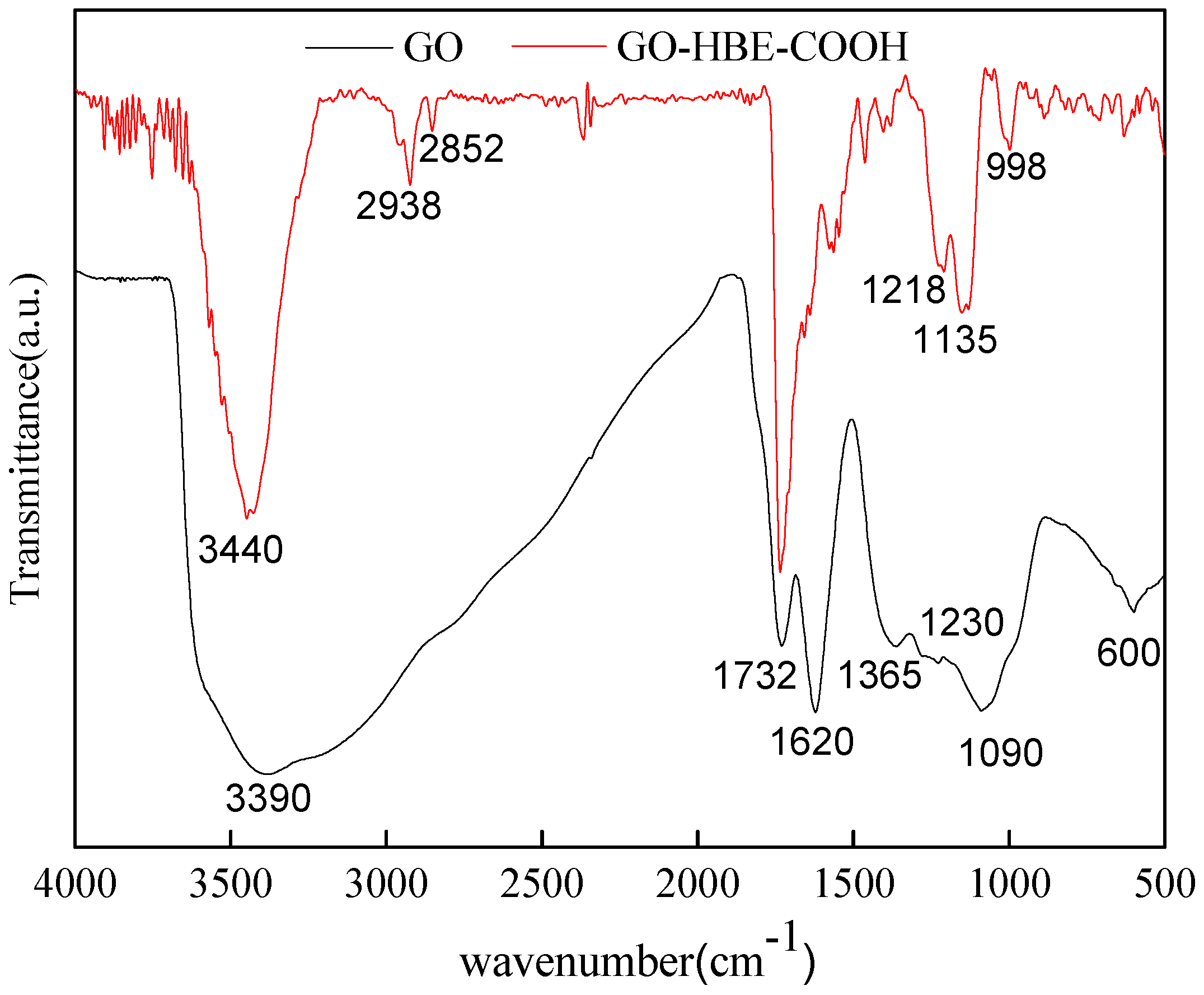
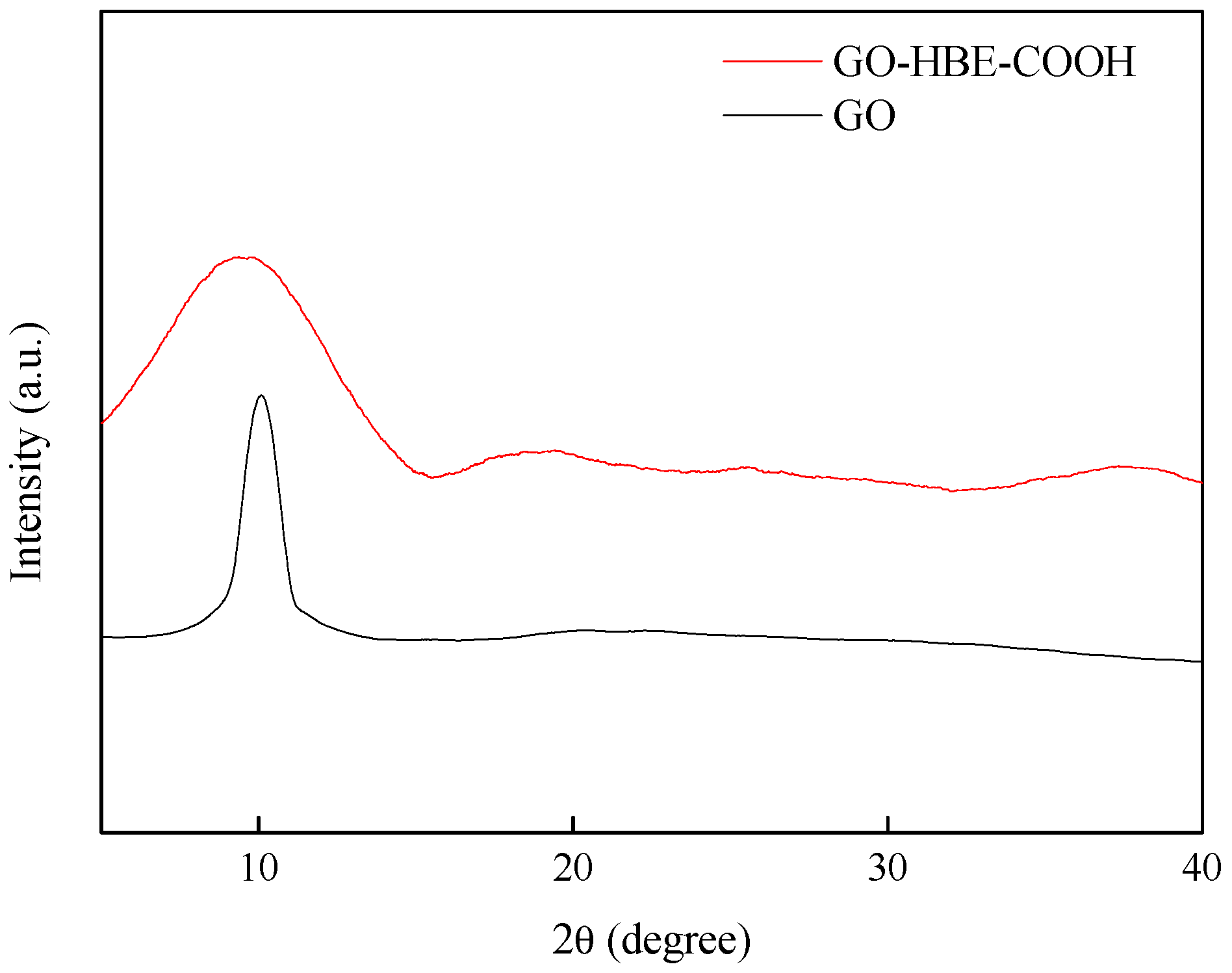
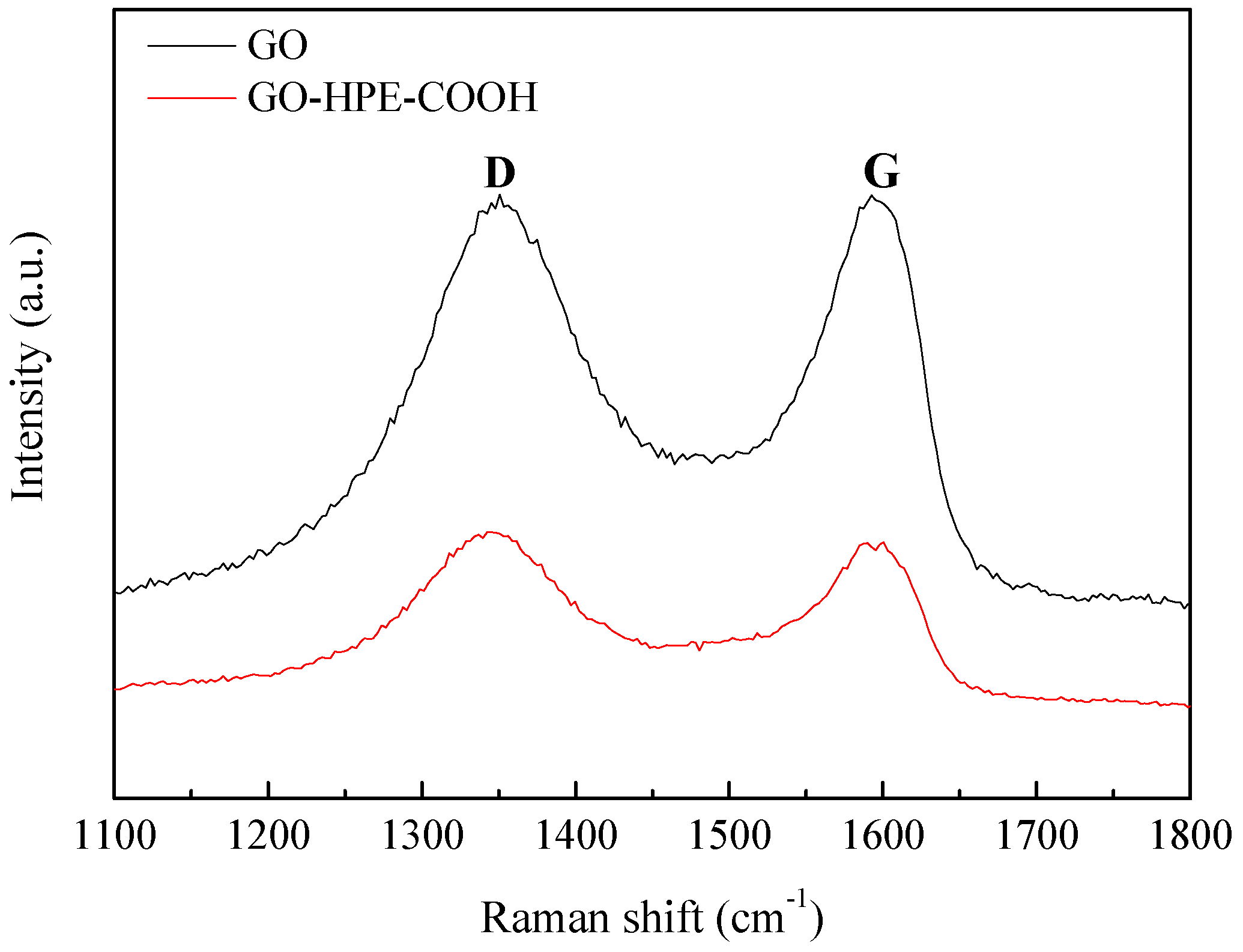
3.1. Characterizations of GO and GO-HBE-COOH
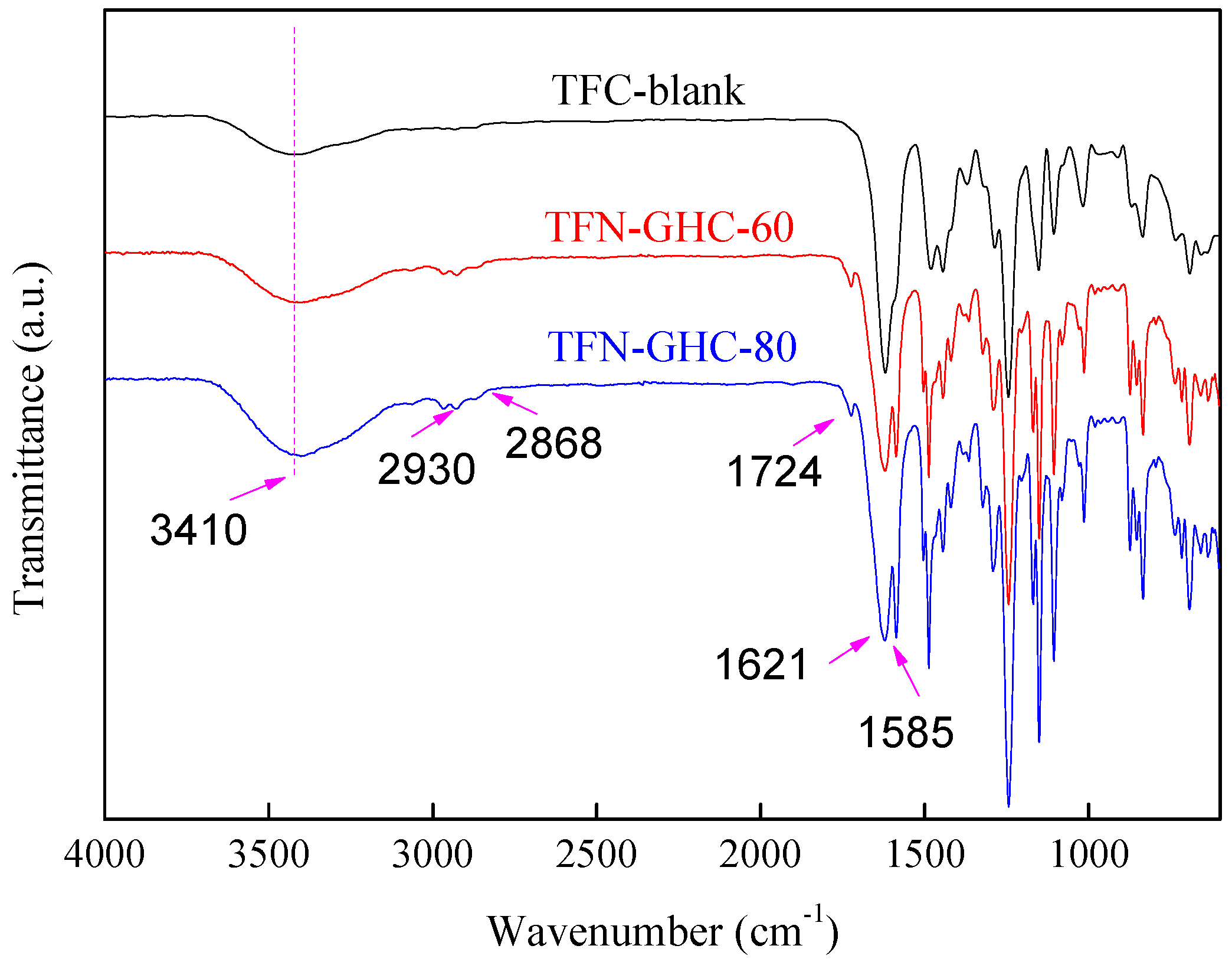
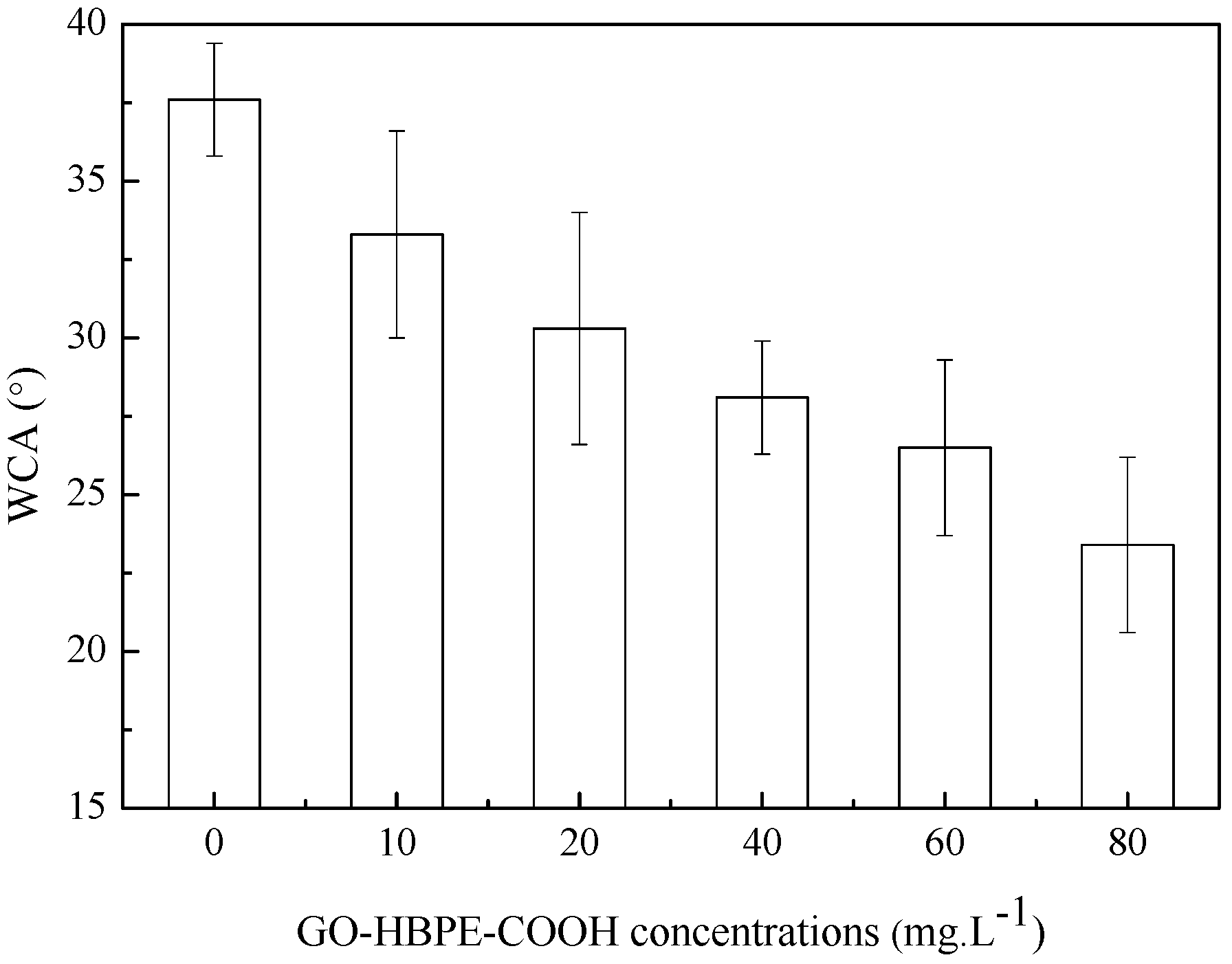
3.2. Characterization of Composite NF Membranes
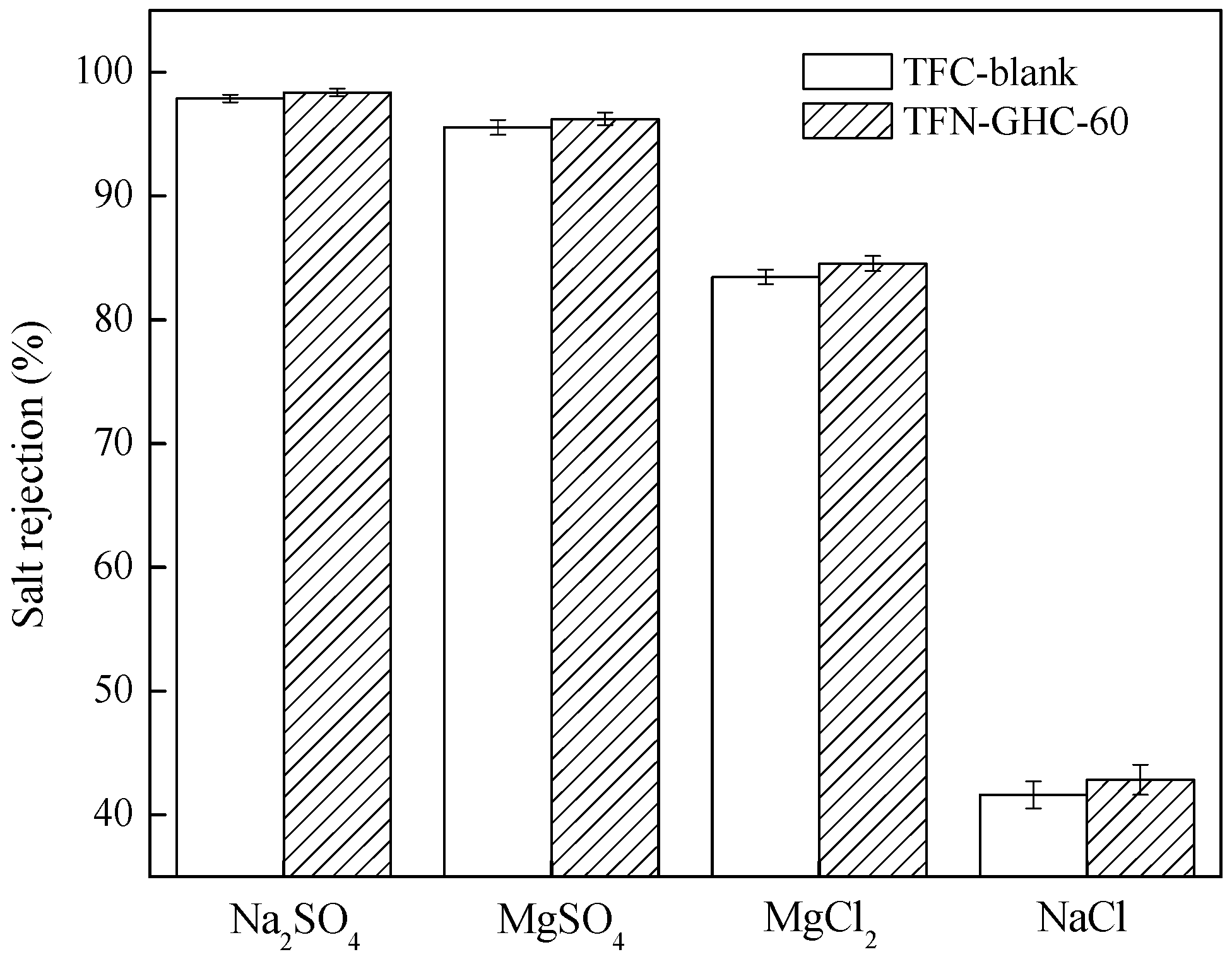
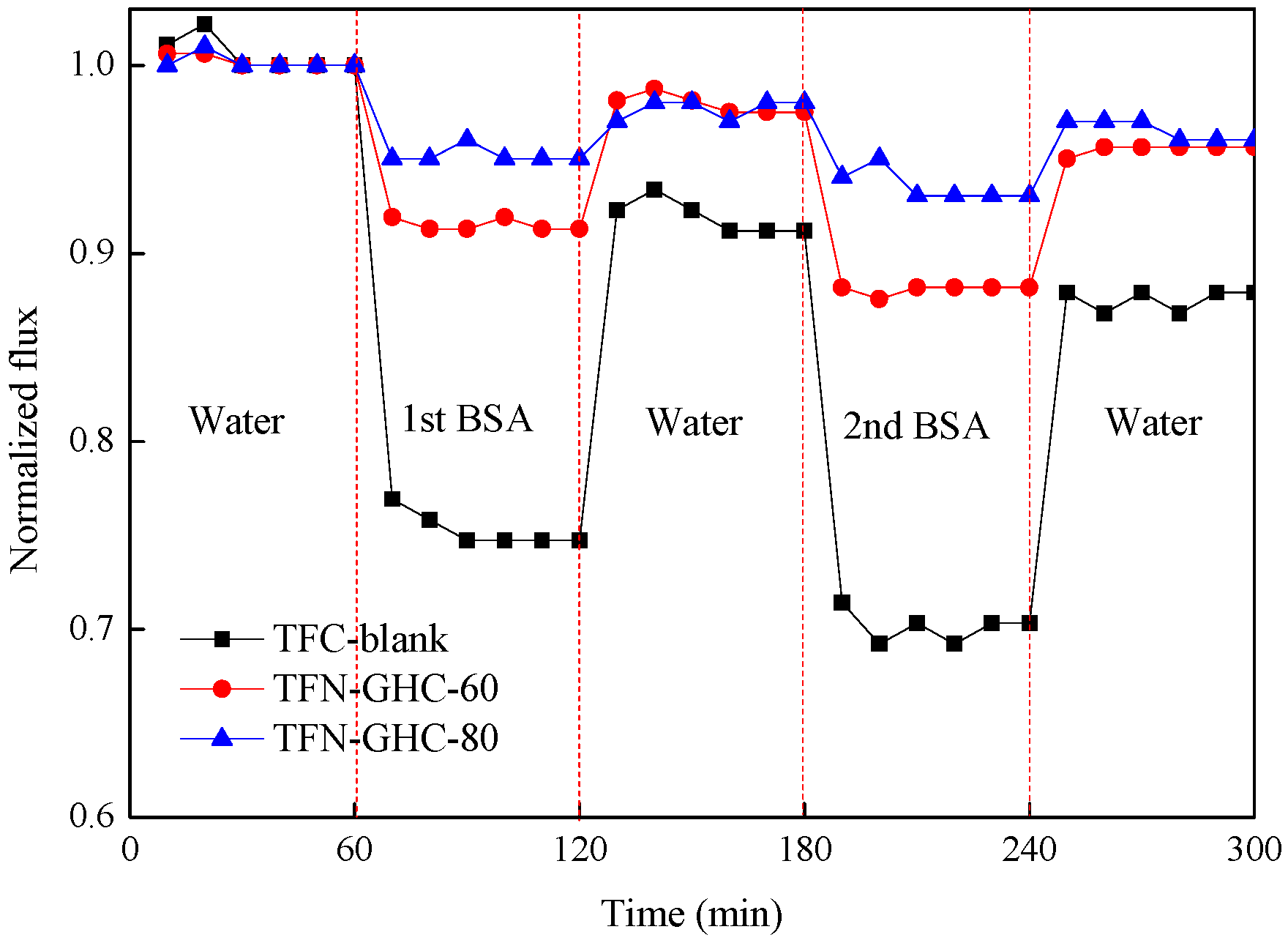
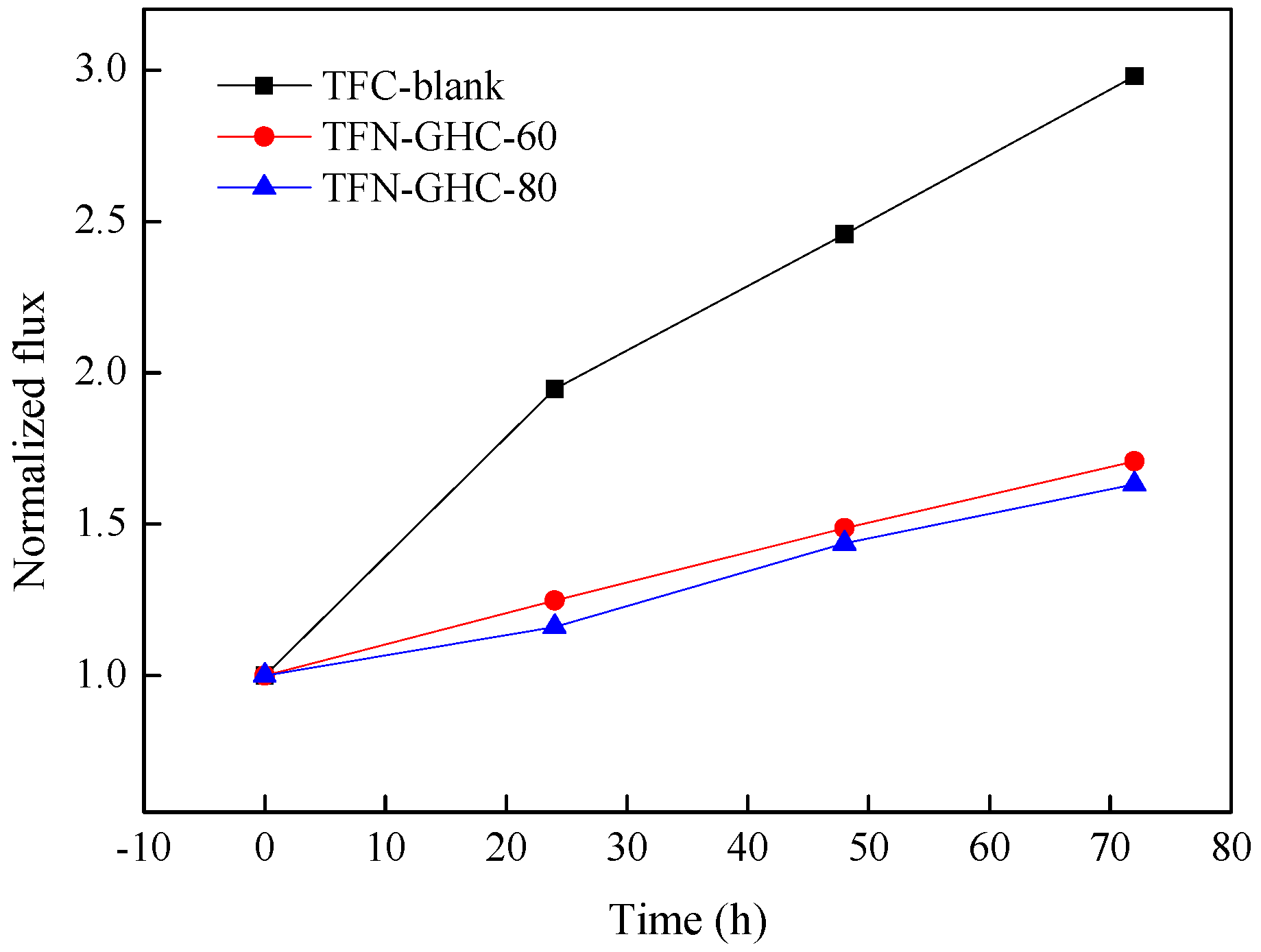
3.3. Membrane Performance
3.4. Mechanisms
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mohammad, A.W.; Teow, Y.H.; Ang, W.L.; Chung, Y.T.; Oatley-Radcliffe, D.L.; Hilal, N. Nanofiltration membranes review: Recent advances and future prospects. Desalination 2015, 356, 226–254. [Google Scholar] [CrossRef]
- Xu, G.-R.; Xu, J.-M.; Feng, H.-J.; Zhao, H.-L.; Wu, S.-B. Tailoring structures and performance of polyamide thin film composite (PA-TFC) desalination membranes via sublayers adjustment—A review. Desalination 2017, 417, 19–35. [Google Scholar] [CrossRef]
- Lau, W.J.; Gray, S.; Matsuura, T.; Emadzadeh, D.; Chen, J.P.; Ismail, A.F. A review on polyamide thin film nanocomposite (TFN) membranes: History, applications, challenges and approaches. Water Res. 2015, 80, 306–324. [Google Scholar] [CrossRef] [PubMed]
- Ismail, A.F.; Padaki, M.; Hilal, N.; Matsuura, T.; Lau, W.J. Thin film composite membrane—Recent development and future potential. Desalination 2015, 356, 140–148. [Google Scholar] [CrossRef]
- Yin, J.; Zhu, G.C.; Deng, B.L. Graphene oxide (GO) enhanced polyamide (PA) thin-film nanocomposite (TFN) membrane for water purification. Desalination 2016, 379, 93–101. [Google Scholar] [CrossRef]
- Lai, G.S.; Lau, W.J.; Goh, P.S.; Ismail, A.F.; Yusof, N.; Tan, Y.H. Graphene oxide incorporated thin film nanocomposite nanofiltration membrane for enhanced salt removal performance. Desalination 2016, 387, 14–24. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, C.W.; Wang, T.; Wu, Z.; Li, X.; Li, J.S. Graphene oxide polypiperazine-amide nanofiltration membrane for improving flux and anti-fouling in water purification. RSC Adv. 2016, 6, 82174–82185. [Google Scholar] [CrossRef] [Green Version]
- Bano, S.; Mahmood, A.; Kim, S.J.; Lee, K.H. Graphene oxide modified polyamide nanofiltration membrane with improved flux and antifouling properties. J. Mater. Chem. A 2015, 3, 2065–2071. [Google Scholar] [CrossRef]
- Xia, S.J.; Yao, L.J.; Zhao, Y.; Li, N.N.; Zheng, Y. Preparation of graphene oxide modified polyamide thin film composite membranes with improved hydrophilicity for natural organic matter removal. Chem. Eng. J. 2015, 280, 720–727. [Google Scholar] [CrossRef]
- Ali, M.E.A.; Wang, L.Y.; Wang, X.Y.; Feng, X.S. Thin film composite membranes embedded with graphene oxide for water desalination. Desalination 2016, 386, 67–76. [Google Scholar] [CrossRef]
- Kim, F.; Cote, L.J.; Huang, J. Graphene oxide: Surface activity and two-dimensional assembly. Adv. Mater. 2010, 22, 1954–1958. [Google Scholar] [CrossRef] [PubMed]
- Cote, L.J.; Kim, J.; Tung, V.C.; Luo, J.Y.; Kim, F.; Huang, J.X. Graphene oxide as surfactant sheets. Pure Appl. Chem. 2011, 83, 95–110. [Google Scholar] [CrossRef]
- Hung, W.S.; An, Q.F.; De Guzman, M.; Lin, H.Y.; Huang, S.H.; Liu, W.R.; Hu, C.C.; Lee, K.R.; Lai, J.Y. Pressure-assisted self-assembly technique for fabricating composite membranes consisting of highly ordered selective laminate layers of amphiphilic graphene oxide. Carbon 2014, 68, 670–677. [Google Scholar] [CrossRef]
- Xie, Q.L.; Shao, W.Y.; Zhang, S.S.; Hong, Z.; Wang, Q.Q.; Zeng, B.R. Enhancing the performance of thin-film nanocomposite nanofiltration membranes using MAH-modified GO nanosheets. RSC Adv. 2017, 7, 54898–54910. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.C.; Li, S.P.; Weng, Z.L.; Gao, C. Hyperbranched polymers: Advances from synthesis to applications. Chem. Soc. Rev. 2015, 44, 4091–4130. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Yan, D. Hyperbranched polymers: From synthesis to applications. Prog. Polym. Sci. 2004, 29, 183–275. [Google Scholar] [CrossRef]
- Qiu, Z.L.; Kong, X.; Yuan, J.J.; Shen, Y.J.; Zhu, B.K.; Zhu, L.P.; Yao, Z.K.; Tang, C.Y. Cross-linked PVC/hyperbranched polyester composite hollow fiber membranes for dye removal. React. Funct. Polym. 2018, 122, 51–59. [Google Scholar] [CrossRef]
- Schulze, A.; Went, M.; Prager, A. Membrane functionalization with hyperbranched polymers. Materials 2016, 9, 706. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.Z.; Ong, X.; Yang, J.; Zhang, G.L.; Chen, J.Y.; Wang, J.D. Structure influence of hyperbranched polyester on structure and properties of synthesized nanofiltration membranes. J. Membr. Sci. 2013, 440, 67–76. [Google Scholar] [CrossRef]
- Kong, X.; Zhang, Y.; Zeng, S.Y.; Zhu, B.K.; Zhu, L.P.; Fang, L.F.; Matsuyama, H. Incorporating hyperbranched polyester into cross-linked polyamide layer to enhance both permeability and selectivity of nanofiltration membrane. J. Membr. Sci. 2016, 518, 141–149. [Google Scholar] [CrossRef]
- Wei, X.Z.; Yang, J.; Zhang, G.L. Preparation and characterization of nanofiltration membranes synthesized by hyperbranched polyester and terephthaloyl chloride (TPC). Polym. Polym. Compos. 2012, 20, 261–270. [Google Scholar] [CrossRef]
- Wei, X.Z.; Zhu, L.P.; Deng, H.Y.; Xu, Y.Y.; Zhu, B.K.; Huang, Z.M. New type of nanofiltration membrane based on crosslinked hyperbranched polymers. J. Membr. Sci. 2008, 323, 278–287. [Google Scholar] [CrossRef]
- Wei, X.Z.; Yang, J.; Xu, Y.Y.; Zhu, B.K.; Zhang, G.L. Preparation and characterization of low-pressure nanofiltration membranes and the application in the separation process of dye molecules. J. Porous Mater. 2012, 19, 721–731. [Google Scholar] [CrossRef]
- Zhao, F.Y.; Mi, Y.F.; An, Q.F.; Gao, C.J. Preparation and applications of positively charged polyethyleneimine nanofiltration membrane. Prog. Chem. 2016, 28, 541–551. [Google Scholar]
- Chiang, Y.C.; Hsub, Y.Z.; Ruaan, R.C.; Chuang, C.J.; Tung, K.L. Nanofiltration membranes synthesized from hyperbranched polyethyleneimine. J. Membr. Sci. 2009, 326, 19–26. [Google Scholar] [CrossRef]
- Wei, X.Z.; Hong, J.L.; Zhu, S.S.; Chen, J.Y.; Lv, B.S. Structure-performance study of polyamide composite nanofiltration membranes prepared with polyethyleneimine. J. Mater. Sci. 2017, 52, 11701–11714. [Google Scholar] [CrossRef]
- Sun, S.P.; Hatton, T.A.; Chung, T.S. Hyperbranched polyethyleneimine induced cross-linking of polyamide-imide nanofiltration hollow fiber membranes for effective removal of ciprofloxacin. Environ. Sci. Technol. 2011, 45, 4003–4009. [Google Scholar] [CrossRef] [PubMed]
- Misdan, N.; Lau, W.J.; Ismail, A.F.; Matsuura, T. Formation of thin film composite nanofiltration membrane: Effect of polysulfone substrate characteristics. Desalination 2013, 329, 9–18. [Google Scholar] [CrossRef]
- Wang, D.X.; Su, M.; Yu, Z.Y.; Wang, X.L.; Ando, M.; Shintani, T. Separation performance of a nanofiltration membrane influenced by species and concentration of ions. Desalination 2005, 175, 219–225. [Google Scholar] [CrossRef]
- Xie, Q.L.; Zhang, S.S.; Xiao, Z.Y.; Hu, X.F.; Hong, Z.; Yi, R.Z.; Shao, W.Y.; Wang, Q.Q. Preparation and characterization of novel alkali-resistant nanofiltration membranes with enhanced permeation and antifouling properties: The effects of functionalized graphene nanosheets. RSC Adv. 2017, 7, 18755–18764. [Google Scholar] [CrossRef]
- Wu, H.Q.; Tang, B.B.; Wu, P.Y. Development of novel SiO2-GO nanohybrid/polysulfone membrane with enhanced performance. J. Membr. Sci. 2014, 451, 94–102. [Google Scholar] [CrossRef]
- Chae, H.R.; Lee, J.; Lee, C.H.; Kim, I.C.; Park, P.K. Graphene oxide-embedded thin-film composite reverse osmosis membrane with high flux, anti-biofouling, and chlorine resistance. J. Membr. Sci. 2015, 483, 128–135. [Google Scholar] [CrossRef]
- Li, Y.; He, G.; Wang, S.; Yu, S.; Pan, F.; Wu, H.; Jiang, Z. Recent advances in the fabrication of advanced composite membranes. J. Mater. Chem. A 2013, 1, 10058–10077. [Google Scholar] [CrossRef]
- Garcia-Aleman, J.; Dickson, J.M. Permeation of mixed-salt solutions with commercial and pore-filled nanofiltration membranes: Membrane charge inversion phenomena. J. Membr. Sci. 2004, 239, 163–172. [Google Scholar] [CrossRef]
- Glater, J.; Hong, S.K.; Elimelech, M. The search for a chlorine-resistant reverse-osmosis membrane. Desalination 1994, 95, 325–345. [Google Scholar] [CrossRef]
- Tang, Y.J.; Xu, Z.L.; Xue, S.M.; Wei, Y.M.; Yang, H. A chlorine-tolerant nanofiltration membrane prepared by the mixed diamine monomers of PIP and BHTTM. J. Membr. Sci. 2016, 498, 374–384. [Google Scholar] [CrossRef]
- Yuan, B.H.; Bao, C.L.; Song, L.; Hong, N.N.; Liew, K.M.; Hu, Y. Preparation of functionalized graphene oxide/polypropylene nanocomposite with significantly improved thermal stability and studies on the crystallization behavior and mechanical properties. Chem. Eng. J. 2014, 237, 411–420. [Google Scholar] [CrossRef]
- Shao, F.F.; Dong, L.F.; Dong, H.Z.; Zhang, Q.; Zhao, M.; Yu, L.Y.; Pang, B.L.; Chen, Y.J. Graphene oxide modified polyamide reverse osmosis membranes with enhanced chlorine resistance. J. Membr. Sci. 2017, 525, 9–17. [Google Scholar] [CrossRef]
- Choi, W.; Choi, J.; Bang, J.; Lee, J.H. Layer-by-layer assembly of graphene oxide nanosheets on polyamide membranes for durable reverse-osmosis applications. ACS Appl. Mater. Interfaces 2013, 5, 12510–12519. [Google Scholar] [CrossRef] [PubMed]
- Matthews, T.D.; Yan, H.; Cahill, D.G.; Coronell, O.; Mariñas, B.J. Growth dynamics of interfacially polymerized polyamide layers by diffuse reflectance spectroscopy and rutherford backscattering spectrometry. J. Membr. Sci. 2013, 429, 71–80. [Google Scholar] [CrossRef]






| Ions | z | D (10−3 mm2·s−1) | rs (nm) |
|---|---|---|---|
| Na+ | 1 | 1.333 | 0.183 |
| Mg+ | 2 | 0.706 | 0.345 |
| Cl− | −1 | 2.032 | 0.120 |
| SO42− | −2 | 1.065 | 0.229 |
| Membrane ID | Roughness | ||
|---|---|---|---|
| Ra (nm) | Rq (nm) | Rz (nm) | |
| TFC-blank | 23.2 | 30.9 | 90.9 |
| TFN-GHC-10 | 21.9 | 28.0 | 83.1 |
| TFN-GHC-20 | 19.5 | 26.6 | 91.2 |
| TFN-GHC-40 | 16.0 | 20.0 | 69.4 |
| TFN-GHC-60 | 13.8 | 17.9 | 54.1 |
| TFN-GHC-80 | 26.7 | 39.8 | 93.5 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, Q.; Zhang, S.; Ma, H.; Shao, W.; Gong, X.; Hong, Z. A Novel Thin-Film Nanocomposite Nanofiltration Membrane by Incorporating 3D Hyperbranched Polymer Functionalized 2D Graphene Oxide. Polymers 2018, 10, 1253. https://doi.org/10.3390/polym10111253
Xie Q, Zhang S, Ma H, Shao W, Gong X, Hong Z. A Novel Thin-Film Nanocomposite Nanofiltration Membrane by Incorporating 3D Hyperbranched Polymer Functionalized 2D Graphene Oxide. Polymers. 2018; 10(11):1253. https://doi.org/10.3390/polym10111253
Chicago/Turabian StyleXie, Quanling, Shishen Zhang, Hanjun Ma, Wenyao Shao, Xiao Gong, and Zhuan Hong. 2018. "A Novel Thin-Film Nanocomposite Nanofiltration Membrane by Incorporating 3D Hyperbranched Polymer Functionalized 2D Graphene Oxide" Polymers 10, no. 11: 1253. https://doi.org/10.3390/polym10111253