Functional Supramolecular of Inclusion Complex of Herbicide Fluroxypyr with HPβCD
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Inclusion Complex
2.3. Preparation of Physical Mixture
2.4. Characterisation of Fluroxypyr-HPβCD Inclusion Complex
2.4.1. Phase Solubility Studies
2.4.2. Fourier Transform Infrared Spectroscopy (FT-IR)
2.4.3. Scanning Electron Microscopy (SEM)
2.4.4. Thermal Gravity Analysis (TGA)
2.4.5. X-ray Diffraction (XRD)
2.5. Biological Activity Assay
3. Results
3.1. Phase Solubility Studies
3.2. Fourier Transform Infrared Spectroscopy (FT-IR)
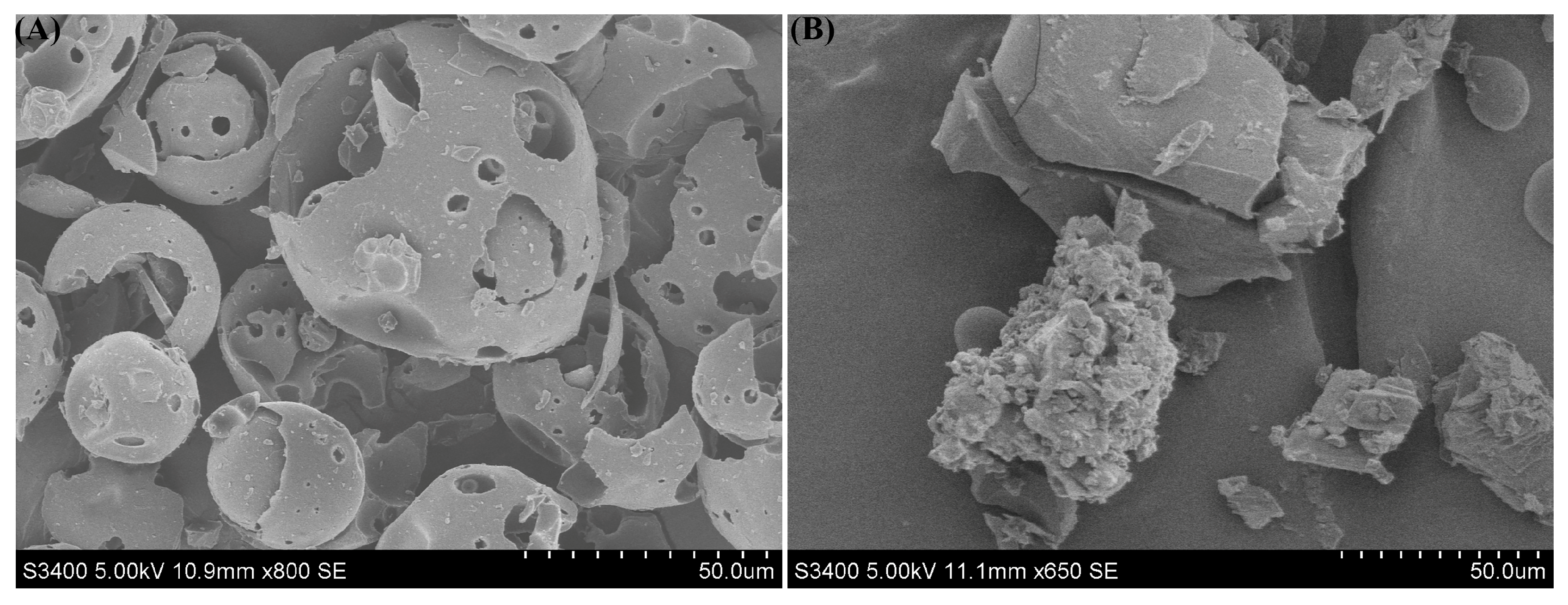
3.3. The Results of Scanning Electron Microscopy (SEM)
3.4. Thermal Gravity Analysis (TGA)
3.5. X-ray Diffraction (XRD)
3.6. The Result of Biological Activity Assay
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Wu, G.L.; Cui, J.; Tao, L.; Yang, H. Fluroxypyr triggers oxidative damage by producing superoxide and hydrogen peroxide in rice (Oryza sativa). Ecotoxicology 2010, 19, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Yang, H. Fluroxypyr biodegradation in soils by multiple factors. Environ. Monit. Assess. 2011, 175, 227–238. [Google Scholar] [CrossRef] [PubMed]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of cyclodextrins and drug/cyclodextrin complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [PubMed]
- Woldum, H.S.; Larsen, K.L.; Madsen, F. Cyclodextrin controlled release of poorly water-soluble drugs from hydrogels. Drug Deliv. 2008, 15, 69–80. [Google Scholar] [CrossRef] [PubMed]
- Reguera, J.; Alonso, M.; Testera, A.M.; López, I.M.; Martín, S.; Rodríguez-Cabello, J.C. Effect of modified α, β, and γ-cyclodextrins on the thermo-responsive behavior of the elastin-like polymer, poly(VPGVG). Carbohydr. Polym. 2004, 57, 293–297. [Google Scholar] [CrossRef]
- Kfoury, M.; Auezova, L.; Greige-Gerges, H.; Fourmentin, S. Development of a total organic carbon method for the quantitative determination of solubility enhancement by cyclodextrins: Application to essential oils. Anal. Chim. Acta 2016, 918, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Carlotti, M.E.; Sapino, S.; Ugazio, E.; Caron, G. On the complexation of quercetin with methyl-β-cyclodextrin: Photostability and antioxidant studies. J. Incl. Phenom. Macrocycl. Chem. 2010, 70, 81–90. [Google Scholar] [CrossRef]
- Hadian, Z.; Maleki, M.; Abdi, K.; Atyabi, F.; Mohammadi, A.; Khaksar, R. Preparation and characterization of nanoparticle β-cyclodextrin: Geraniol inclusion complexes. Iran J. Pharm. Res. 2018, 17, 39–51. [Google Scholar] [PubMed]
- Da Silva, E.S.; Burrows, H.D.; Wong-Wah-Chung, P.; Sarakha, M. β-Cyclodextrin as a photostabilizer of the plant growth regulator 2-(1-naphthyl) acetamide in aqueous solution. J. Incl. Phenom. Macrocycl. Chem. 2013, 79, 329–336. [Google Scholar] [CrossRef]
- Iacovino, R.; Caso, J.V.; Rapuano, F.; Russo, A.; Isidori, M.; Lavorgna, M.; Malgieri, G.; Isernia, C. Physicochemical characterization and cytotoxic activity evaluation of hydroxymethylferrocene: Beta-cyclodextrin inclusion complex. Molecules 2012, 17, 6056–6070. [Google Scholar] [CrossRef] [PubMed]
- Karathanos, V.T.; Mourtzinos, I.; Yannakopoulou, K.; Andrikopoulos, N.K. Study of the solubility, antioxidant activity and structure of inclusion complex of vanillin with β-cyclodextrin. Food Chem. 2007, 101, 652–658. [Google Scholar] [CrossRef]
- Higuchi, T.; Connors, K.A. Phase solubility techniques. Adv. Anal. Chem. Instrum. 1965, 4, 117–212. [Google Scholar]
- Rekharsky, M.V.; Inoue, Y. Complexation thermodynamics of cyclodextrins. Chem. Rev. 1998, 98, 1875–1918. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Hreinsdottir, D.; Masson, M. Evaluation of cyclodextrin solubilization of drugs. Int. J. Pharm. 2005, 302, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Mura, P.; Adragna, E.; Rabasco, A.M.; Moyano, J.R.; Perez-Martinez, J.I.; Arias, M.J.; Gines, J.M. Effects of the host cavity size and the preparation method on the physicochemical properties of ibuproxam-cyclodextrin systems. Drug Dev. Ind. Pharm. 1999, 25, 279–287. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Qiu, L.; Gao, J.; Jin, Y. Preparation, characterization and in vivo evaluation of formulation of baicalein with hydroxypropyl-beta-cyclodextrin. Int. J. Pharm. 2006, 312, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.O., III; Mahaguna, V.; Sriwongjanya, M. Characterization of an inclusion complex of cholesterol and hydroxypropyl-β-cyclodextrin. Eur. J. Pharm. Biopharm. 1998, 46, 355–360. [Google Scholar] [CrossRef]
- Moyano, J.R.; Ginés, J.M.; Arias, M.J.; Rabasco, A.M. Study of the dissolution characteristics of oxazepam via complexation with β-cyclodextrin. Int. J. Pharm. 1995, 114, 95–102. [Google Scholar] [CrossRef]
- Figueiras, A.; Ribeiro, L.; Vieira, M.T.; Veiga, F. Preparation and physicochemical characterization of omeprazole:methyl-beta-cyclodextrin inclusion complex in solid state. J. Incl. Phenom. Macrocycl. Chem. 2007, 57, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Mukne, A.P.; Nagarsenker, M. Triamterene-β-cyclodextrin systems: Preparation, characterization and in vivo evaluation. AAPS PharmSciTech 2004, 5, 142. [Google Scholar]







| Drugs | The Average Root Length (cm) | The Average Plant Height (cm) | The Average Fresh Weight (g) |
|---|---|---|---|
| fluroxypyr | 0.80 ± 0.10 b | 3.53 ± 0.13 b | 0.20 ± 0.08 b |
| physical mixture | 0.76 ± 0.12 b | 3.66 ± 0.11 b | 0.21 ± 0.07 b |
| inclusion complex | 0.70 ± 0.10 b | 3.00 ± 0.08 c | 0.17 ± 0.05 c |
| water | 1.60 ± 0.13 a | 7.23 ± 0.10 a | 0.47 ± 0.05 a |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, S.; Bie, C.; Liu, Y.; Zhang, T.; Fu, Y.; Ye, F. Functional Supramolecular of Inclusion Complex of Herbicide Fluroxypyr with HPβCD. Polymers 2018, 10, 1294. https://doi.org/10.3390/polym10121294
Gao S, Bie C, Liu Y, Zhang T, Fu Y, Ye F. Functional Supramolecular of Inclusion Complex of Herbicide Fluroxypyr with HPβCD. Polymers. 2018; 10(12):1294. https://doi.org/10.3390/polym10121294
Chicago/Turabian StyleGao, Shuang, Chao Bie, Yanyan Liu, Tianyu Zhang, Ying Fu, and Fei Ye. 2018. "Functional Supramolecular of Inclusion Complex of Herbicide Fluroxypyr with HPβCD" Polymers 10, no. 12: 1294. https://doi.org/10.3390/polym10121294






