Polyelectrolyte-Coated Gold Nanoparticles: The Effect of Salt and Polyelectrolyte Concentration on Colloidal Stability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Polyelectrolyte (PE) Concentration
2.2. Salt Concentration
2.3. Solvent Stability
3. Results and Discussion
3.1. Effect of PE Concentration
3.2. Effect of Salt Concentration
3.3. Effect of Solvent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Siafaka, P.; Üstündağ Okur, N.; Karavas, E.; Bikiaris, D. Surface Modified Multifunctional and Stimuli Responsive Nanoparticles for Drug Targeting: Current Status and Uses. Int. J. Mol. Sci. 2016, 17, 1440. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Vishakante, G.D.; Siddaramaiah, H. Gold nanoparticles: A paradigm shift in biomedical applications. Adv. Colloid Interface Sci. 2013, 199–200, 44–58. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.C.; Caldas, M.; Pattekari, P.; Fontes Ribeiro, C.; Ribeiro, A.J.; Lvov, Y.; Veiga, F. Chapter 16-Layer-by-Layer coated drug-core nanoparticles as versatile delivery platforms A2-Grumezescu, Alexandru Mihai. In Design and Development of New Nanocarriers; William Andrew Publishing: New York, NY, USA, 2018; pp. 595–635. [Google Scholar] [CrossRef]
- Richardson, J.J.; Björnmalm, M.; Caruso, F. Technology-driven layer-by-layer assembly of nanofilms. Science 2015, 348. [Google Scholar] [CrossRef]
- Harris, C.M.; Miller, S.G.; Andresen, K.; Thompson, L.B. Quantitative measurement of sodium polystyrene sulfonate adsorption onto CTAB capped gold nanoparticles reveals hard and soft coronas. J. Colloid Interface Sci. 2018, 510, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Hirsjärvi, S.; Peltonen, L.; Hirvonen, J. Layer-by-layer polyelectrolyte coating of low molecular weight poly(lactic acid) nanoparticles. Colloids Surf. B Biointerfaces 2006, 49, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Chen, K.; Xu, Y.; Yu, X.; Wang, W.; Li, L.; Guo, X. Protein immobilization and separation using anionic/cationic spherical polyelectrolyte brushes based on charge anisotropy. Soft Matter 2013, 9, 11276–11287. [Google Scholar] [CrossRef]
- Wandrey, C.; Hernández-Barajas, J.; Hunkeler, D. Diallyldimethylammonium Chloride and its Polymers. In Radical Polymerisation Polyelectrolytes; Capek, I., Hernfández-Barajas, J., Hunkeler, D., Reddinger, J.L., Reynolds, J.R., Wandrey, C., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 123–183. [Google Scholar] [CrossRef]
- Gittins, D.I.; Caruso, F. Tailoring the Polyelectrolyte Coating of Metal Nanoparticles. J. Phys. Chem. B 2001, 105, 6846–6852. [Google Scholar] [CrossRef]
- Hierrezuelo, J.; Sadeghpour, A.; Szilagyi, I.; Vaccaro, A.; Borkovec, M. Electrostatic Stabilization of Charged Colloidal Particles with Adsorbed Polyelectrolytes of Opposite Charge. Langmuir ACS J. Surf. Colloids 2010, 26, 15109–15111. [Google Scholar] [CrossRef]
- Polte, J. Fundamental growth principles of colloidal metal nanoparticles—A new perspective. CrystEngComm 2015, 17, 6809–6830. [Google Scholar] [CrossRef]
- Egger, C.C.; Anderson, M.W.; Tiddy, G.J.T.; Casci, J.L. In situ NMR and XRD studies of the growth mechanism of SBA-1. Phys. Chem. Chem. Phys. 2005, 7, 1845–1855. [Google Scholar] [CrossRef]
- Moore, T.L.; Rodriguez-Lorenzo, L.; Hirsch, V.; Balog, S.; Urban, D.; Jud, C.; Rothen-Rutishauser, B.; Lattuada, M.; Petri-Fink, A. Nanoparticle colloidal stability in cell culture media and impact on cellular interactions. Chem. Soc. Rev. 2015, 44, 6287–6305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, K.; Besseling, N.A.M. Formation of polyelectrolyte multilayers: Ionic strengths and growth regimes. Soft Matter 2016, 12, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Ruckenstein, E. The Bridging Force between Colloidal Particles in a Polyelectrolyte Solution. Langmuir ACS J. Surf. Colloids 2012, 28, 16300–16305. [Google Scholar] [CrossRef] [PubMed]
- Schneider, G.; Decher, G. Functional Core/Shell Nanoparticles via Layer-by-Layer Assembly. Investigation of the Experimental Parameters for Controlling Particle Aggregation and for Enhancing Dispersion Stability. Langmuir ACS J. Surf. Colloids 2008, 24, 1778–1789. [Google Scholar] [CrossRef] [PubMed]
- Bizmark, N.; Ioannidis, M.A. Effects of Ionic Strength on the Colloidal Stability and Interfacial Assembly of Hydrophobic Ethyl Cellulose Nanoparticles. Langmuir ACS J. Surf. Colloids 2015, 31, 9282–9289. [Google Scholar] [CrossRef] [PubMed]
- Benoit, D.N.; Zhu, H.; Lilierose, M.H.; Verm, R.A.; Ali, N.; Morrison, A.N.; Fortner, J.D.; Avendano, C.; Colvin, V.L. Measuring the Grafting Density of Nanoparticles in Solution by Analytical Ultracentrifugation and Total Organic Carbon Analysis. Anal. Chem. 2012, 84, 9238–9245. [Google Scholar] [CrossRef] [PubMed]
- Notley, S.M.; Norgren, M. Adsorption of a strong polyelectrolyte to model lignin surfaces. Biomacromolecules 2008, 9, 2081–2086. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.; Lee, Y.; Lee, C.-H.; Lee, K.; Kim, Y.; Choi, H.; Ryu, P.-D.; Lee, S.Y.; Joo, S.-W. Selective Aggregation Mechanism of Unmodified Gold Nanoparticles in Detection of Single Nucleotide Polymorphism. J. Phys. Chem. C 2008, 112, 8629–8633. [Google Scholar] [CrossRef]
- Mengarelli, V.; Auvray, L.; Pastré, D.; Zeghal, M. Charge inversion, condensation and decondensation of DNA and polystyrene sulfonate by polyethylenimine. Eur. Phys. J. E 2011, 34, 127. [Google Scholar] [CrossRef] [PubMed]
- Kleimann, J.; Gehin-Delval, C.; Auweter, H.; Borkovec, M. Super-stoichiometric charge neutralization in particle-polyelectrolyte systems. Langmuir ACS J. Surf. Colloids 2005, 21, 3688–3698. [Google Scholar] [CrossRef] [PubMed]
- Quinsaat, J.E.Q.; Nuesch, F.A.; Hofmann, H.; Opris, D.M. Dielectric properties of silver nanoparticles coated with silica shells of different thicknesses. RSC Adv. 2013, 3, 6964–6971. [Google Scholar] [CrossRef]
- Schwarz, S.; Buchhammer, H.M.; Lunkwitz, K.; Jacobasch, H.J. Polyelectrolyte adsorption on charged surfaces: Study by electrokinetic measurements. Colloids Surf. A Physicochem. Eng. Asp. 1998, 140, 377–384. [Google Scholar] [CrossRef]
- Alkilany, A.M.; Thompson, L.B.; Murphy, C.J. Polyelectrolyte Coating Provides a Facile Route to Suspend Gold Nanorods in Polar Organic Solvents and Hydrophobic Polymers. ACS Appl. Mater. Interfaces 2010, 2, 3417–3421. [Google Scholar] [CrossRef] [PubMed]
- Shih, Y.-C.; Ke, C.-Y.; Yu, C.-J.; Lu, C.-Y.; Tseng, W.-L. Combined Tween 20-Stabilized Gold Nanoparticles and Reduced Graphite Oxide–Fe3O4 Nanoparticle Composites for Rapid and Efficient Removal of Mercury Species from a Complex Matrix. ACS Appl. Mater. Interfaces 2014, 6, 17437–17445. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.-Y.; Yu, C.-J.; Lin, Y.-H.; Tseng, W.-L. Colorimetric Sensing of Silver(I) and Mercury(II) Ions Based on an Assembly of Tween 20-Stabilized Gold Nanoparticles. Anal. Chem. 2010, 82, 6830–6837. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, K.D.; de Vellis, J. Preparation of separate astroglial and oligodendroglial cell cultures from rat cerebral tissue. J. Cell Biol. 1980, 85, 890–902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tuomola, E.M.; Salminen, S.J. Adhesion of some probiotic and dairy Lactobacillus strains to Caco-2 cell cultures. Int. J. Food Microbiol. 1998, 41, 45–51. [Google Scholar] [CrossRef]

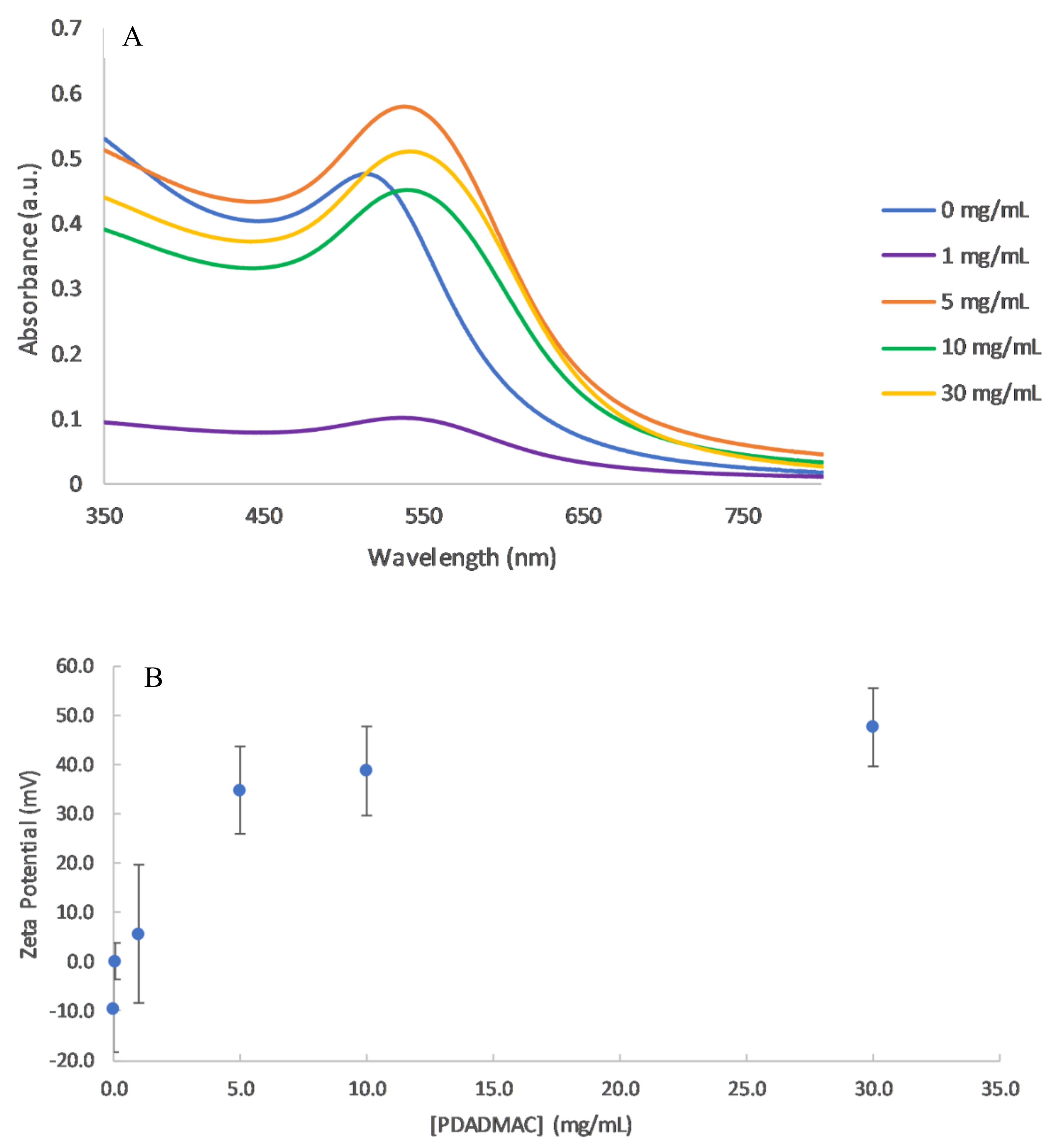

| PDADMAC Concentration (mg/mL) | PC/NP Ratio | SPR Maximum Absorbance Peak (nm) | Absorbance (a.u.) | Zeta Potential (mV) |
|---|---|---|---|---|
| 0 | 0 | 513 ± 0.3 | 0.48 ± 0.2 | −9.6 ± 8.9 |
| 0.1 | 21.5 | - * | - * | 0.3 ± 3.7 |
| 1.0 | 215 | 537 ± 1.5 | 0.10 ± 0.09 | 5.7 ± 14 |
| 5.0 | 1075 | 539 ± 0.47 | 0.58 ± 0.04 | 35 ± 8.9 |
| 10 | 2150 | 540 ± 2.5 | 0.45 ± 0.07 | 39 ± 9.1 |
| 30 | 6450 | 542 ± 0.94 | 0.51 ± 0.08 | 48 ± 7.9 |
| Salt Concentration (M) | Average SPR Peak (nm) | Average Absorbance (a.u.) |
|---|---|---|
| 0 | 543 ± 1.2 | 0.69 ± 0.04 |
| 0.001 | 539 ± 0.47 | 0.58 ± 0.04 |
| 0.05 | 540 ± 3.7 | 0.17 ± 0.05 |
| 0.1 | * | * |
| 0.5 | * | * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fuller, M.; Kӧper, I. Polyelectrolyte-Coated Gold Nanoparticles: The Effect of Salt and Polyelectrolyte Concentration on Colloidal Stability. Polymers 2018, 10, 1336. https://doi.org/10.3390/polym10121336
Fuller M, Kӧper I. Polyelectrolyte-Coated Gold Nanoparticles: The Effect of Salt and Polyelectrolyte Concentration on Colloidal Stability. Polymers. 2018; 10(12):1336. https://doi.org/10.3390/polym10121336
Chicago/Turabian StyleFuller, Melanie, and Ingo Kӧper. 2018. "Polyelectrolyte-Coated Gold Nanoparticles: The Effect of Salt and Polyelectrolyte Concentration on Colloidal Stability" Polymers 10, no. 12: 1336. https://doi.org/10.3390/polym10121336