Single Local Injection of Epigallocatechin Gallate-Modified Gelatin Attenuates Bone Resorption and Orthodontic Tooth Movement in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
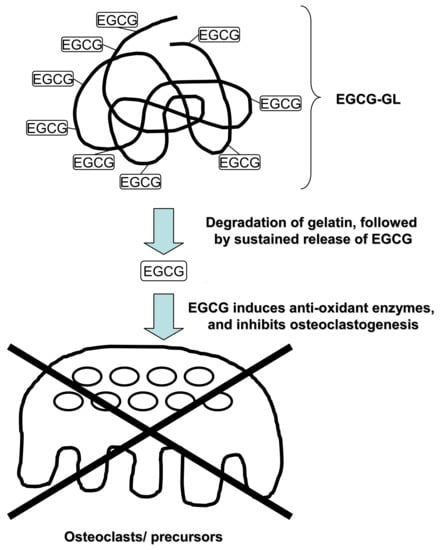
2.2. Preparation of EGCG-GL
2.3. Examination of Prolonged Release of EGCG from EGCG-GL
2.4. Cells and Culturing
2.5. Cytotoxicity Assay of EGCG-GL
2.6. Intracellular ROS Detection
2.7. Real-Time RT-PCR Analysis
2.8. Osteoclastogenesis Assay
2.9. Experimental Animals
2.10. Bone Destruction Model
2.11. Micro-Computed Tomography (microCT) Analysis for Bone Destruction
2.12. OTM
2.13. Immunohistological and Histological Examinations
2.14. Statistical Analysis
3. Results
3.1. EGCG-GL Prolongs EGCG Release
3.2. EGCG-GL Exhibited No Cytotoxicity against RAW 264.7 Cells at the Concentrations We Tested
3.3. EGCG-GL Induces Anti-Oxidant Gene Expression in RAW 264.7 Cells
3.4. EGCG-GL Attenuates RANKL-Mediated Intracellular ROS
3.5. EGCG-GL Inhibits RANKL-Mediated Osteoclastogenesis
3.6. Local Single EGCG-GL Injection Inhibited LPS-Mediated Oxidative Stress in Mice
3.7. Local Single EGCG-GL Injection Ameliorates LPS-Induced Bone Destruction in Mice
3.8. Local Single EGCG-GL Injection Inhibits the Rate of OTM via Attenuation of Osteoclastogenesis
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Roodman, G.D. Advances in bone biology: The osteoclast. Endocr. Rev. 1996, 17, 308–332. [Google Scholar] [PubMed]
- Lacey, D.L.; Timms, E.; Tan, H.L.; Kelley, M.J.; Dunstan, C.R.; Burgess, T.; Elliott, R.; Colombero, A.; Elliott, G.; Scully, S.; et al. Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93, 165–176. [Google Scholar] [CrossRef]
- Taubman, M.A.; Valverde, P.; Han, X.; Kawai, T. Immune response: The key to bone resorption in periodontal disease. J. Periodontol. 2005, 76, 2033–2041. [Google Scholar] [CrossRef] [PubMed]
- Filvaroff, E.; Derynck, R. Bone remodelling: A signalling system for osteoclast regulation. Curr. Biol. CB 1998, 8, R679–R682. [Google Scholar] [CrossRef]
- Storey, E. The nature of tooth movement. Am. J. Orthod. 1973, 63, 292–314. [Google Scholar] [CrossRef]
- Davidovitch, Z.; Nicolay, O.F.; Ngan, P.W.; Shanfeld, J.L. Neurotransmitters, cytokines, and the control of alveolar bone remodeling in orthodontics. Dent. Clin. N. Am. 1988, 32, 411–435. [Google Scholar]
- Brudvik, P.; Rygh, P. Multi-nucleated cells remove the main hyalinized tissue and start resorption of adjacent root surfaces. Eur. J. Orthod. 1994, 16, 265–273. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, Y.; Tanaka, T.; Kuwahara, Y. New findings in the degenerating tissues of the periodontal ligament during experimental tooth movement. Am. J. Orthod. Dent. Orthop. 1996, 109, 348–354. [Google Scholar] [CrossRef]
- Nakamura, Y.; Noda, K.; Shimpo, S.; Oikawa, T.; Kawasaki, K.; Hirashita, A. Phosphatidylinositol-dependent bond between alkaline phosphatase and collagen fibers in the periodontal ligament of rat molars. Histochem. Cell Biol. 2004, 121, 39–45. [Google Scholar] [CrossRef]
- Yashiro, Y.; Nomura, Y.; Kanazashi, M.; Noda, K.; Hanada, N.; Nakamura, Y. Function of chemokine (cxc motif) ligand 12 in periodontal ligament fibroblasts. PLoS ONE 2014, 9, e95676. [Google Scholar] [CrossRef]
- Shiotani, A.; Shibasaki, Y.; Sasaki, T. Localization of receptor activator of nfkappab ligand, RANKL, in periodontal tissues during experimental movement of rat molars. J. Electron Microsc. 2001, 50, 365–369. [Google Scholar] [CrossRef]
- Oshiro, T.; Shiotani, A.; Shibasaki, Y.; Sasaki, T. Osteoclast induction in periodontal tissue during experimental movement of incisors in osteoprotegerin-deficient mice. Anat. Rec. 2002, 266, 218–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishijima, Y.; Yamaguchi, M.; Kojima, T.; Aihara, N.; Nakajima, R.; Kasai, K. Levels of RANKL and OPG in gingival crevicular fluid during orthodontic tooth movement and effect of compression force on releases from periodontal ligament cells in vitro. Orthod. Craniofac. Res. 2006, 9, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Takahashi, T.; Yamaguchi, M.; Kasai, K. Effects of aging on RANKL and OPG levels in gingival crevicular fluid during orthodontic tooth movement. Orthod. Craniofac. Res. 2006, 9, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Chiba, M.; Arai, K.; Takahashi, I.; Haruyama, N.; Nishimura, M.; Mitani, H. Local RANKL gene transfer to the periodontal tissue accelerates orthodontic tooth movement. Gene Ther. 2006, 13, 678–685. [Google Scholar] [CrossRef] [Green Version]
- Nishimura, M.; Chiba, M.; Ohashi, T.; Sato, M.; Shimizu, Y.; Igarashi, K.; Mitani, H. Periodontal tissue activation by vibration: Intermittent stimulation by resonance vibration accelerates experimental tooth movement in rats. Am. J. Orthod. Dent. Orthop. 2008, 133, 572–583. [Google Scholar] [CrossRef] [PubMed]
- Dunn, M.D.; Park, C.H.; Kostenuik, P.J.; Kapila, S.; Giannobile, W.V. Local delivery of osteoprotegerin inhibits mechanically mediated bone modeling in orthodontic tooth movement. Bone 2007, 41, 446–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanzaki, H.; Chiba, M.; Takahashi, I.; Haruyama, N.; Nishimura, M.; Mitani, H. Local OPG gene transfer to periodontal tissue inhibits orthodontic tooth movement. J. Dent. Res. 2004, 83, 920–925. [Google Scholar] [CrossRef] [PubMed]
- Bax, B.E.; Alam, A.S.; Banerji, B.; Bax, C.M.; Bevis, P.J.; Stevens, C.R.; Moonga, B.S.; Blake, D.R.; Zaidi, M. Stimulation of osteoclastic bone resorption by hydrogen peroxide. Biochem. Biophys. Res. Commun. 1992, 183, 1153–1158. [Google Scholar] [CrossRef]
- Kanzaki, H.; Shinohara, F.; Kajiya, M.; Fukaya, S.; Miyamoto, Y.; Nakamura, Y. Nuclear nrf2 induction by protein transduction attenuates osteoclastogenesis. Free Radic. Biol. Med. 2014, 77, 239–248. [Google Scholar] [CrossRef]
- Kanzaki, H.; Shinohara, F.; Itohiya-Kasuya, K.; Ishikawa, M.; Nakamura, Y. Nrf2 activation attenuates both orthodontic tooth movement and relapse. J. Dent. Res. 2015, 94, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Gambari, L.; Lisignoli, G.; Cattini, L.; Manferdini, C.; Facchini, A.; Grassi, F. Sodium hydrosulfide inhibits the differentiation of osteoclast progenitor cells via nrf2-dependent mechanism. Pharmacol. Res. 2014, 87, 99–112. [Google Scholar] [CrossRef] [PubMed]
- Kanzaki, H.; Shinohara, F.; Itohiya, K.; Yamaguchi, Y.; Katsumata, Y.; Matsuzawa, M.; Fukaya, S.; Miyamoto, Y.; Wada, S.; Nakamura, Y. RANKL induces bach1 nuclear import and attenuates nrf2-mediated antioxidant enzymes, thereby augmenting intracellular reactive oxygen species signaling and osteoclastogenesis in mice. FASEB J. 2017, 31, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Kanzaki, H.; Katsumata, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; Nakamura, Y. Dimethyl fumarate inhibits osteoclasts via attenuation of reactive oxygen species signalling by augmented antioxidation. J Cell Mol. Med. 2018, 22, 1138–1147. [Google Scholar] [CrossRef]
- Honda, Y.; Tanaka, T.; Tokuda, T.; Kashiwagi, T.; Kaida, K.; Hieda, A.; Umezaki, Y.; Hashimoto, Y.; Imai, K.; Matsumoto, N.; et al. Local controlled release of polyphenol conjugated with gelatin facilitates bone formation. Int. J. Mol. Sci. 2015, 16, 14143–14157. [Google Scholar] [CrossRef] [PubMed]
- Ayon Haro, E.R.; Ukai, T.; Yokoyama, M.; Kishimoto, T.; Yoshinaga, Y.; Hara, Y. Locally administered interferon-gamma accelerates lipopolysaccharide-induced osteoclastogenesis independent of immunohistological RANKL upregulation. J. Period. Res. 2011, 46, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Hsu, H.Y.; Wen, M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [PubMed]
- Alam, J.; Stewart, D.; Touchard, C.; Boinapally, S.; Choi, A.M.K.; Cook, J.L. Nrf2, a cap’n’collar transcription factor, regulates induction of the heme oxygenase-1 gene. J. Biol. Chem. 1999, 274, 26071–26078. [Google Scholar] [CrossRef] [PubMed]
- Wild, A.C.; Gipp, J.J.; Mulcahy, T. Overlapping antioxidant response element and pma response element sequences mediate basal and beta-naphthoflavone-induced expression of the human gamma-glutamylcysteine synthetase catalytic subunit gene. Biochem. J. 1998, 332 Pt 2, 373–381. [Google Scholar] [CrossRef]
- Na, H.K.; Surh, Y.J. Modulation of nrf2-mediated antioxidant and detoxifying enzyme induction by the green tea polyphenol egcg. Food Chem. Toxicol. 2008, 46, 1271–1278. [Google Scholar] [CrossRef] [PubMed]
- Tominari, T.; Matsumoto, C.; Watanabe, K.; Hirata, M.; Grundler, F.M.; Miyaura, C.; Inada, M. Epigallocatechin gallate (egcg) suppresses lipopolysaccharide-induced inflammatory bone resorption, and protects against alveolar bone loss in mice. FEBS Open Bio 2015, 5, 522–527. [Google Scholar] [CrossRef]
- Sakai, G.; Otsuka, T.; Fujita, K.; Kainuma, S.; Kuroyanagi, G.; Kawabata, T.; Matsushima-Nishiwaki, R.; Kozawa, O.; Tokuda, H. Amplification by (-)-epigallocatechin gallate of prostaglandin f2alphastimulated synthesis of osteoprotegerin in osteoblasts. Mol. Med. Rep. 2017, 16, 6376–6381. [Google Scholar] [CrossRef] [PubMed]
- Kuroyanagi, G.; Tokuda, H.; Yamamoto, N.; Kainuma, S.; Fujita, K.; Ohguchi, R.; Kawabata, T.; Sakai, G.; Matsushima-Nishiwaki, R.; Harada, A.; et al. (-)-Epigallocatechin gallate synergistically potentiates prostaglandin e2-stimulated osteoprotegerin synthesis in osteoblasts. Prostag. Other Lipid Med. 2017, 128–129, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Takeda, Y.; Li, P.; Huang, A.; Sasayama, S.; Hara, E.; Uemura, N.; Ueda, M.; Hashimoto, M.; Arita, K.; et al. Epigallocatechin gallate-modified gelatin sponges treated by vacuum heating as a novel scaffold for bone tissue engineering. Molecules 2018, 23, 876. [Google Scholar] [CrossRef] [PubMed]
- Lauer-Fields, J.L.; Fields, G.B. Triple-helical peptide analysis of collagenolytic protease activity. Biol. Chem. 2002, 383, 1095–1105. [Google Scholar] [CrossRef]
- Zitka, O.; Kukacka, J.; Krizkova, S.; Huska, D.; Adam, V.; Masarik, M.; Prusa, R.; Kizek, R. Matrix metalloproteinases. Curr. Med. Chem. 2010, 17, 3751–3768. [Google Scholar] [CrossRef] [PubMed]
- Narimiya, T.; Wada, S.; Kanzaki, H.; Ishikawa, M.; Tsuge, A.; Yamaguchi, Y.; Nakamura, Y. Orthodontic tensile strain induces angiogenesis via type iv collagen degradation by matrix metalloproteinase-12. J. Period. Res. 2017, 52, 842–852. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, C.C.; Lin, H.P.; Shih, W.A.; Hsieh, W.L.; Lai, C.H.; Takeuchi, Y.; Chen, Y.W. A functional chitosan membrane with grafted epigallocatechin-3-gallate and lovastatin enhances periodontal tissue regeneration in dogs. Carbohydr. Polym. 2016, 151, 790–802. [Google Scholar] [CrossRef]
- Thein-Han, W.W.; Saikhun, J.; Pholpramoo, C.; Misra, R.D.; Kitiyanant, Y. Chitosan-gelatin scaffolds for tissue engineering: Physico-chemical properties and biological response of buffalo embryonic stem cells and transfectant of gfp-buffalo embryonic stem cells. Acta Biomater. 2009, 5, 3453–3466. [Google Scholar] [CrossRef]
- Gomez-Mascaraque, L.G.; Sanchez, G.; Lopez-Rubio, A. Impact of molecular weight on the formation of electrosprayed chitosan microcapsules as delivery vehicles for bioactive compounds. Carbohydr. Polym. 2016, 150, 121–130. [Google Scholar] [CrossRef] [Green Version]
- Shin, Y.C.; Yang, W.J.; Lee, J.H.; Oh, J.W.; Kim, T.W.; Park, J.C.; Hyon, S.H.; Han, D.W. Plga nanofiber membranes loaded with epigallocatechin-3-o-gallate are beneficial to prevention of postsurgical adhesions. Int. J. Nanomed. 2014, 9, 4067–4078. [Google Scholar]
- Shafiei, S.S.; Solati-Hashjin, M.; Samadikuchaksaraei, A.; Kalantarinejad, R.; Asadi-Eydivand, M.; Abu Osman, N.A. Epigallocatechin gallate/layered double hydroxide nanohybrids: Preparation, characterization, and in vitro anti-tumor study. PLoS ONE 2015, 10, e0136530. [Google Scholar] [CrossRef] [PubMed]








© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Katsumata, Y.; Kanzaki, H.; Honda, Y.; Tanaka, T.; Yamaguchi, Y.; Itohiya, K.; Fukaya, S.; Miyamoto, Y.; Narimiya, T.; Wada, S.; et al. Single Local Injection of Epigallocatechin Gallate-Modified Gelatin Attenuates Bone Resorption and Orthodontic Tooth Movement in Mice. Polymers 2018, 10, 1384. https://doi.org/10.3390/polym10121384
Katsumata Y, Kanzaki H, Honda Y, Tanaka T, Yamaguchi Y, Itohiya K, Fukaya S, Miyamoto Y, Narimiya T, Wada S, et al. Single Local Injection of Epigallocatechin Gallate-Modified Gelatin Attenuates Bone Resorption and Orthodontic Tooth Movement in Mice. Polymers. 2018; 10(12):1384. https://doi.org/10.3390/polym10121384
Chicago/Turabian StyleKatsumata, Yuta, Hiroyuki Kanzaki, Yoshitomo Honda, Tomonari Tanaka, Yuuki Yamaguchi, Kanako Itohiya, Sari Fukaya, Yutaka Miyamoto, Tsuyoshi Narimiya, Satoshi Wada, and et al. 2018. "Single Local Injection of Epigallocatechin Gallate-Modified Gelatin Attenuates Bone Resorption and Orthodontic Tooth Movement in Mice" Polymers 10, no. 12: 1384. https://doi.org/10.3390/polym10121384