A Novel Self-Binding Composite Separator Based on Poly(tetrafluoroethylene) Coating for Li-Ion Batteries
Abstract
:1. Introduction
2. Materials and Methods
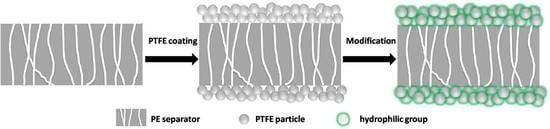
2.1. Fabrication of the Composite Separator
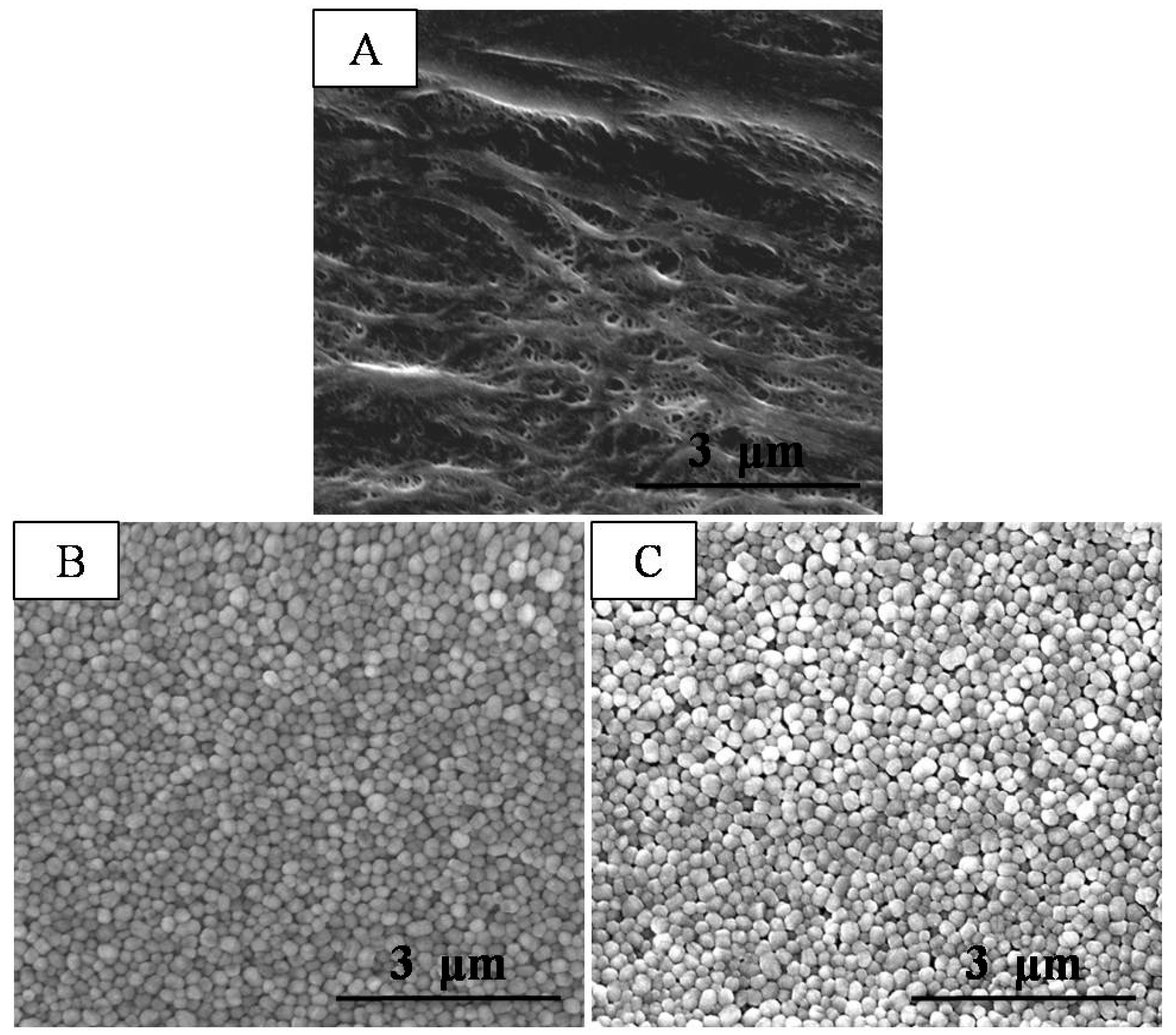
2.2. Characterization of the Composite Separator
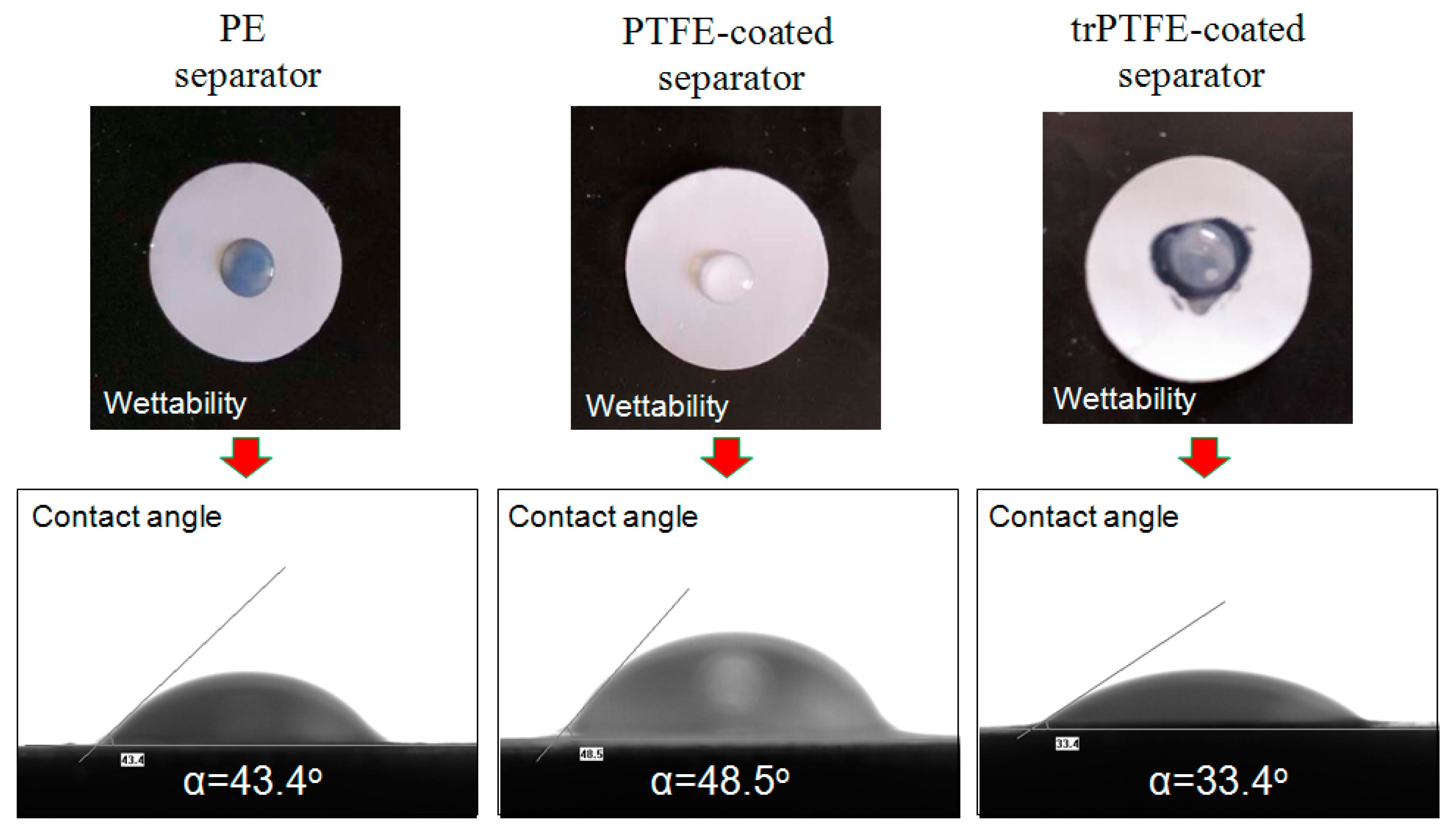
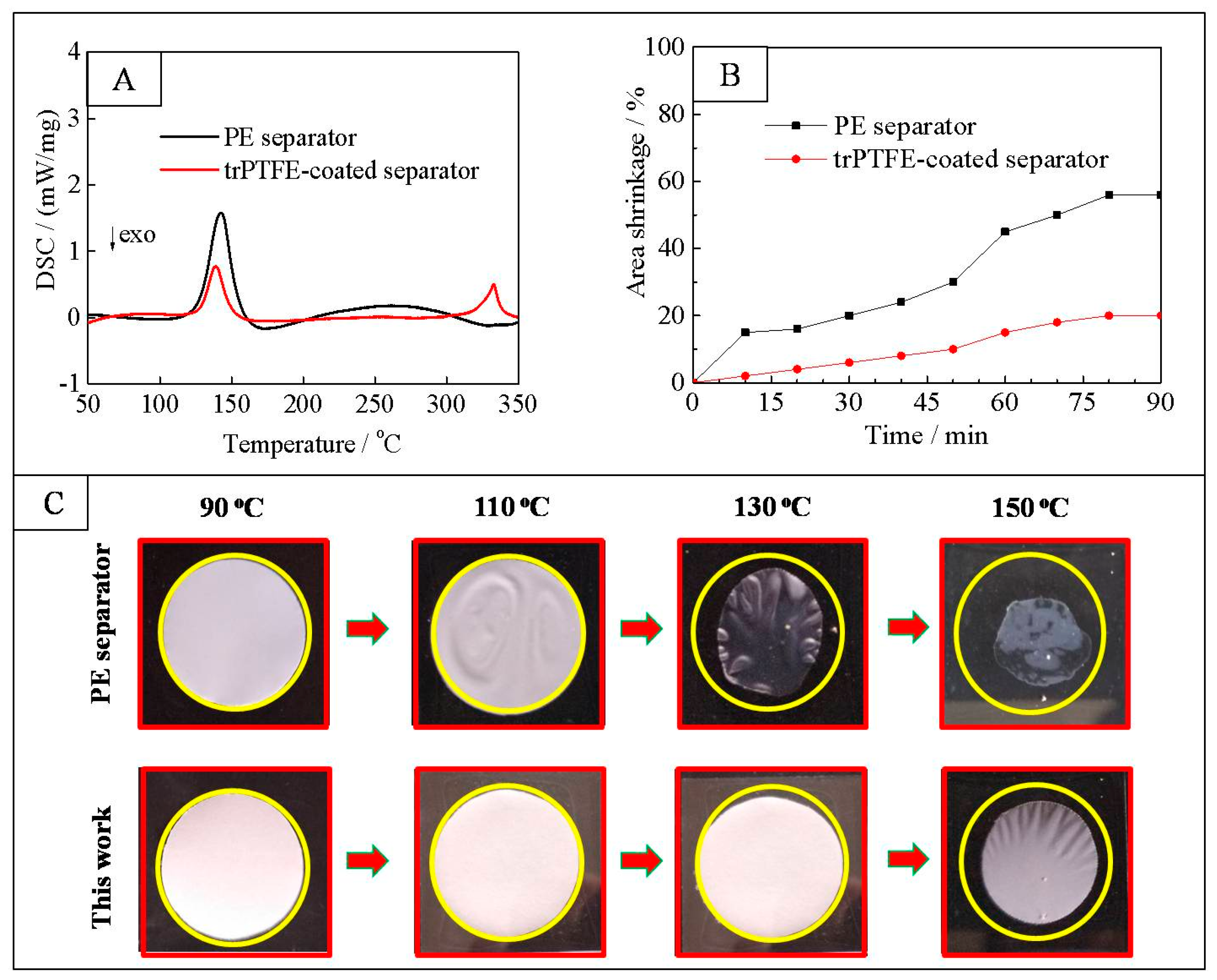
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jiang, F.; Yin, L.; Yu, Q.; Zhong, C.; Zhang, J. Bacterial cellulose nanofibrous membrane as thermal stable separator for lithium-ion batteries. J. Power Sources 2015, 279, 21–27. [Google Scholar] [CrossRef]
- Gu, Y.J.; Guo, Z.; Liu, H.Q. Structure and electrochemical properties of Li4Ti5O12 with Li excess as an anode electrode material for Li-ion batteries. Electrochim. Acta 2014, 123, 576–581. [Google Scholar] [CrossRef]
- Bai, X.; Li, T.; Qi, Y.X.; Gao, X.P.; Yin, L.W.; Li, H.; Zhu, H.L.; Lun, N.; Bai, Y.J. Simple fabrication of TiO2/C nanocomposite with enhanced electrochemical performance for lithium-ion batteries. Electrochim. Acta 2015, 169, 241–247. [Google Scholar] [CrossRef]
- Repp, S.; Harputlu, E.; Gurgen, S.; Castellano, M.; Kremer, N.; Pompe, N.; Wörner, J.; Hoffmann, A.; Thomann, R.; Emen, F.M.; et al. Synergetic effects of Fe3+ doped spinel Li4Ti5O12 nanoparticles on reduced graphene oxide for high surface electrode hybrid supercapacitors. Nanoscale 2018, 10, 1877–1884. [Google Scholar] [CrossRef] [PubMed]
- Genc, R.; Alas, M.O.; Harputlu, E.; Repp, S.; Kremer, N.; Castellano, M.; Colak, S.G.; Ocakoglu, K.; Erdem, E. High-capacitance hybrid supercapacitor based on multi-colored fluorescent carbon-dots. Sci. Rep. 2017, 7, 11222. [Google Scholar] [CrossRef]
- Liu, L.; Xu, S.D.; Yu, Q.; Wang, F.Y.; Zhu, H.L.; Zhang, R.L.; Liu, X. Nitrogen-doped hollow carbon spheres with a wrinkled surface: Their one-pot carbonization synthesis and supercapacitor properties. Chem. Commun. 2016, 52, 11693–11696. [Google Scholar] [CrossRef]
- Cho, T.H.; Tanaka, M.; Ohnish, H.; Kondo, Y.; Yoshkazu, M.; Nakamura, T.; Sakai, T. Composite nonwoven separator for lithium-ion battery: Development and characterization. J. Power Sources 2010, 195, 4272–4277. [Google Scholar] [CrossRef]
- Xiao, W.; Gong, Y.; Wang, H.; Zhao, L.; Liu, J.; Yan, C. Preparation and electrochemical performance of ZrO2 nanoparticle-embedded nonwoven composite separator for lithium-ion batteries. Ceram. Int. 2015, 41, 14223–14229. [Google Scholar] [CrossRef]
- Xiao, W.; Wang, J.; Wang, H.; Gong, Y.; Zhao, L.; Liu, J.; Yan, C. Hollow mesoporous silica sphere-embedded composite separator for high-performance lithium-ion battery. J. Solid State Electrochem. 2016, 20, 2847–2855. [Google Scholar] [CrossRef]
- Zhang, S.S.; Xu, K.; Jow, T.R. An inorganic composite membrane as the separator of Li-ion batteries. J. Power Sources 2005, 140, 361–364. [Google Scholar] [CrossRef]
- He, M.; Zhang, X.; Jiang, K.; Wang, J.; Wang, Y. Pure inorganic separator for lithium ion batteries. ACS Appl. Mater. Interfaces 2015, 7, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Cho, T.; Tanaka, M.; Onishi, H.; Kondo, Y.; Nakamura, T.; Yamazaki, H.; Tanase, S.; Sakai, T. Battery performances and thermal stability of polyacrylonitrile nano-fiber-based nonwoven separators for Li-ion battery. J. Power Sources 2008, 181, 155–160. [Google Scholar] [CrossRef]
- Huang, X. A facile approach to make high performance nano-fiber reinforced composite separator for lithium ion batteries. J. Power Source 2016, 323, 17–22. [Google Scholar] [CrossRef]
- Jeon, H.; Yeon, D.; Lee, T.; Park, J.; Ryou, M.; Lee, Y.M. A water-based Al2O3 ceramic coating for polyethylene-based microporous separators for lithium-ion batteries. J. Power Sources 2016, 315, 161–168. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, P.; Chen, L.; Yang, P.; Zhao, J. Effect of a thin ceramic-coating layer on thermal and electrochemical properties of polyethylene separator for lithium-ion batteries. J. Power Sources 2014, 270, 547–553. [Google Scholar] [CrossRef]
- Shi, C.; Dai, J.; Shen, X.; Peng, L.; Li, C.; Wang, X.; Zhang, P.; Zhao, J. A high-temperature stable ceramic-coated separator prepared with polyimide binder/Al2O3 particles for lithium-ion batteries. J. Membr. Sci. 2016, 517, 91–99. [Google Scholar] [CrossRef]
- Choi, J.; Jung, Y.; Lee, Y.; Kim, D. High performance separator coated with amino-functionalized SiO2 particles for safety enhanced lithium-ion batteries. J. Membr. Sci. 2017, 535, 151–157. [Google Scholar] [CrossRef]
- Prasanna, K.; Kim, C.; Chang, W. Effect of SiO2 coating on polyethylene separator with different stretching ratios for application in lithium ion batteries. Mater. Chem. Phys. 2014, 146, 545–550. [Google Scholar] [CrossRef]
- Lee, T.; Kim, W.K.; Lee, Y.; Ryou, M.H.; Yong, M.L. Effect of Al2O3 coatings prepared by RF sputtering on polyethylene separators for high-power lithium ion batteries. Macromol. Res. 2014, 22, 1190–1195. [Google Scholar] [CrossRef]
- Catalá-Icardo, M.; Torres-Cartas, S.; Meseguer-Lloret, S.; Gómez-Benito, C.; Carrasco-Correa, E.; Simo-Alfonso, E.F.; Ramis-Ramos, G.; Herrero-Martínez, J.M. Preparation of organic monolithic columns in polytetrafluoroethylene tubes for reversed-phase liquid chromatography. Anal. Chim. Acta 2017, 960, 160–167. [Google Scholar] [CrossRef]
- Li, J.; Zhong, Q.; Yao, Y.; Bi, S.; Zhou, T.; Guo, X.; Wu, M.; Feng, T.; Xiang, R. Electrochemical performance and thermal stability of the electrospun PTFE nanofiber separator for lithium-ion batteries. J. Appl. Polym. Sci. 2018, 135, 46508. [Google Scholar] [CrossRef]
- Löhbach, C.; Bakowsky, U.; Kneuer, C.; Jahn, D.; Graeter, T.; Schäfers, H.; Lehr, C. Wet chemical modification of PTFE implant surfaces with a specific cell adhesion molecule. Chem. Commun. 2002, 21, 2568–2569. [Google Scholar] [CrossRef]
- Oh, S.W.; Myung, S.T.; Oh, S.M.; Woon, C.S.; Amine, K.; Sun, Y.K. Polyvinylpyrrolidone-assisted synthesis of microscale C-LiFePO4 with high tap density as positive electrode materials for lithium batteries. Electrochim. Acta 2010, 55, 1193–1199. [Google Scholar] [CrossRef]
- Plaimer, M.; Breitfuß, C.; Sinz, W.; Heindl, S.F.; Ellersdorfer, C.; Steffan, H.; Wilkening, M.; Hennige, V.; Tatschl, R.; Geier, A.; et al. Evaluating the trade-off between mechanical and electrochemical performance of separators for lithium-ion batteries: Methodology and application. J. Power Sources 2016, 306, 702–710. [Google Scholar] [CrossRef]
- Li, X.F.; He, J.J.; Wu, D.Z.; Zhang, M.Z.; Meng, J.W.; Ni, P.H. Development of plasma-treated polypropylene nonwoven-based composites for high-performance lithium-ion battery separators. Electrochim. Acta 2015, 167, 396–403. [Google Scholar] [CrossRef]
- Choi, J.; Kim, S.; Kim, D. Enhancement of thermal stability and cycling performance in lithium-ion cells through the use of ceramic-coated separators. J. Power Sources 2010, 195, 6192–6196. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, S. Closely packed SiO2 nanoparticles/poly vinylidene fluoride-hexafluoropropylene layers-coated polyethylene separators for lithium-ion batteries. J. Power Sources 2010, 196, 6716–6722. [Google Scholar] [CrossRef]




| PE Separator | PTFE-Coated Separator | trPTFE-Coated Separator | |
|---|---|---|---|
| Thickness (μm) | 12 | 18 | 18 |
| Porosity (coating layer/PE separator) (%/%) | -/45 | 65/45 | 66/45 |
| Average pore size (nm) | 43 | 40 | 40 |
| Contact angle (°) | 43.4 | 48.5 | 33.4 |
| Electrolyte uptake (%) | 110.7 | 171.5 | 190.6 |
| Puncture resistance (gf) | 320.5 | 368.6 | 365.8 |
| Tensile strength (kgf) | 3.2 | 3.6 | 3.6 |
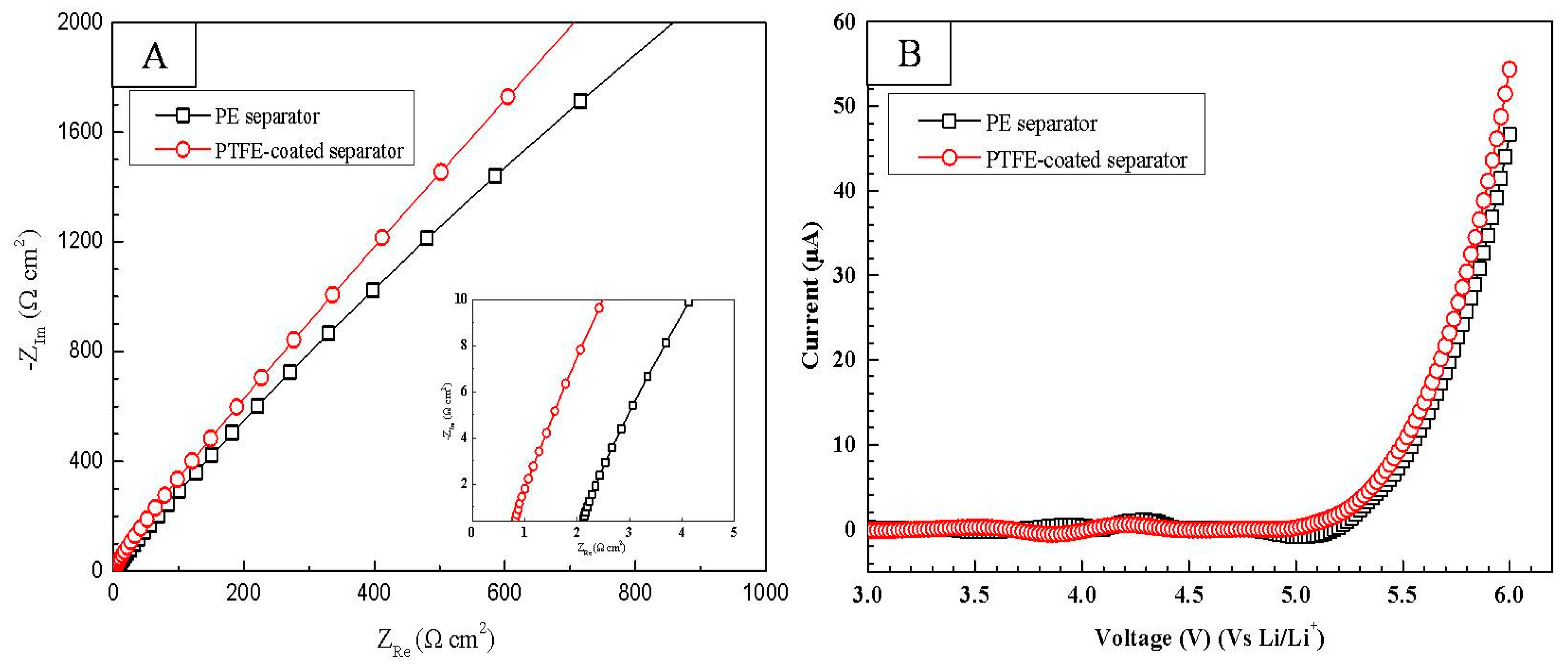
| Rb (Ω) | Rsei (Ω) | Rct (Ω) | |
|---|---|---|---|
| PE separator-No cycle | 2.5 | 93.1 | 341.5 |
| PE separator-5th cycle | 3.1 | 101.4 | 370.2 |
| PE separator-100th cycle | 5.4 | 280.5 | 733.2 |
| trPTFE-coated separator-No cycle | 1.1 | 80.6 | 290.3 |
| trPTFE-coated separator-5th cycle | 1.5 | 85.2 | 304.2 |
| trPTFE-coated separator-100th cycle | 4.1 | 166.5 | 441.7 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, K.; Xiao, W.; Liu, J.; Yan, C. A Novel Self-Binding Composite Separator Based on Poly(tetrafluoroethylene) Coating for Li-Ion Batteries. Polymers 2018, 10, 1409. https://doi.org/10.3390/polym10121409
Zhang K, Xiao W, Liu J, Yan C. A Novel Self-Binding Composite Separator Based on Poly(tetrafluoroethylene) Coating for Li-Ion Batteries. Polymers. 2018; 10(12):1409. https://doi.org/10.3390/polym10121409
Chicago/Turabian StyleZhang, Kaiyue, Wei Xiao, Jianguo Liu, and Chuanwei Yan. 2018. "A Novel Self-Binding Composite Separator Based on Poly(tetrafluoroethylene) Coating for Li-Ion Batteries" Polymers 10, no. 12: 1409. https://doi.org/10.3390/polym10121409