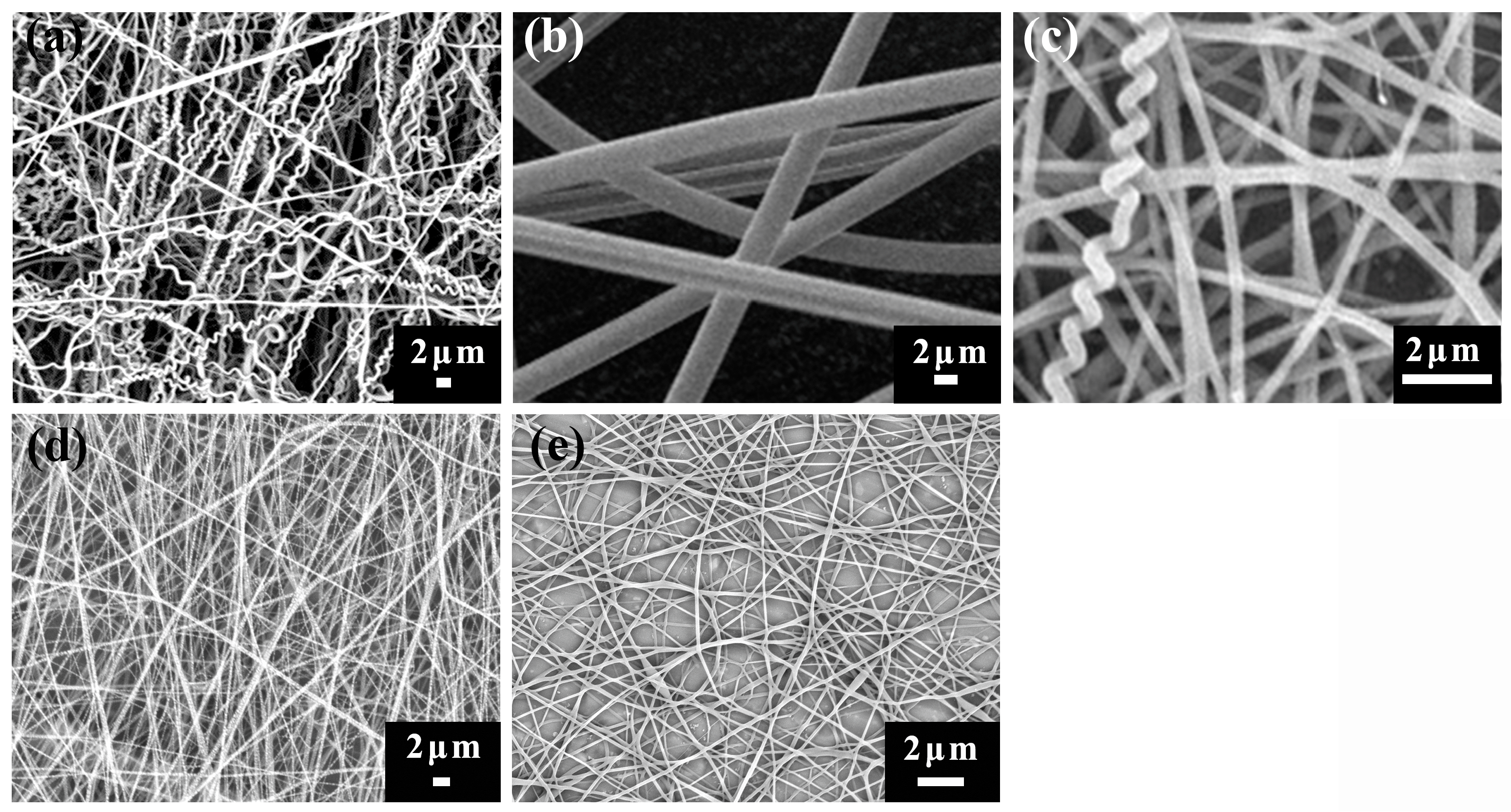
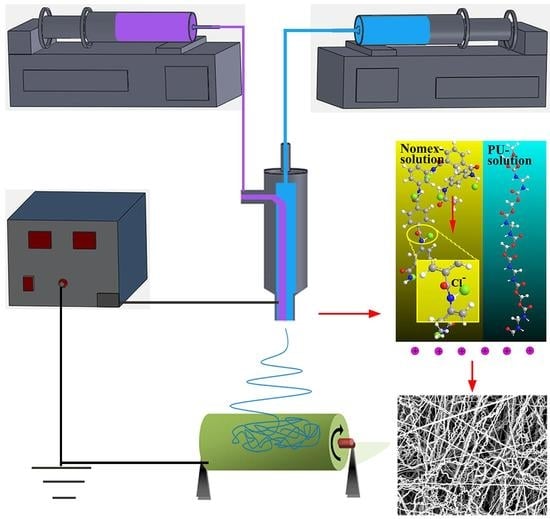
In this study, the fabrication of helical nanofibers is based on the combination of an elastomeric with a stiff component in co-electrospinning. In our experiments, TPU is used as the elastomeric component, while Nomex, PAN and PS are chosen as the stiff part. An interfacial interaction is introduced between the two components due to the different mechanical properties they display. In the following, focusing on the interfacial interaction induced by polymer structure and intrinsic properties, we try to explore the effect of polymer type on the formation of helical fibers.
3.1. Rigidity of Polymer Chain
In the co-electrospinning process, the longitudinal compressive stress arising from the resilience of the flexible component (i.e., TPU) and the rigidity of the stiff component (i.e., Nomex, PS and PAN) is fundamental for the formation of helical structures. It can be predicted that the more significant the rigidity (or flexibility) differential of the two components, the greater the potential for the component system to generate helical structures in co-electrospinning due to the greater interfacial stress between the components.
The glass transition temperature of a polymer,
Tg, is an important intrinsic property that influences both the physical and mechanical properties including strength, toughness and stiffness. Typically, polymers with high chain rigidity have higher
Tg [
16,
17]. DSC analysis is one of the convenient methods for determining the glass transition temperature of the polymer.
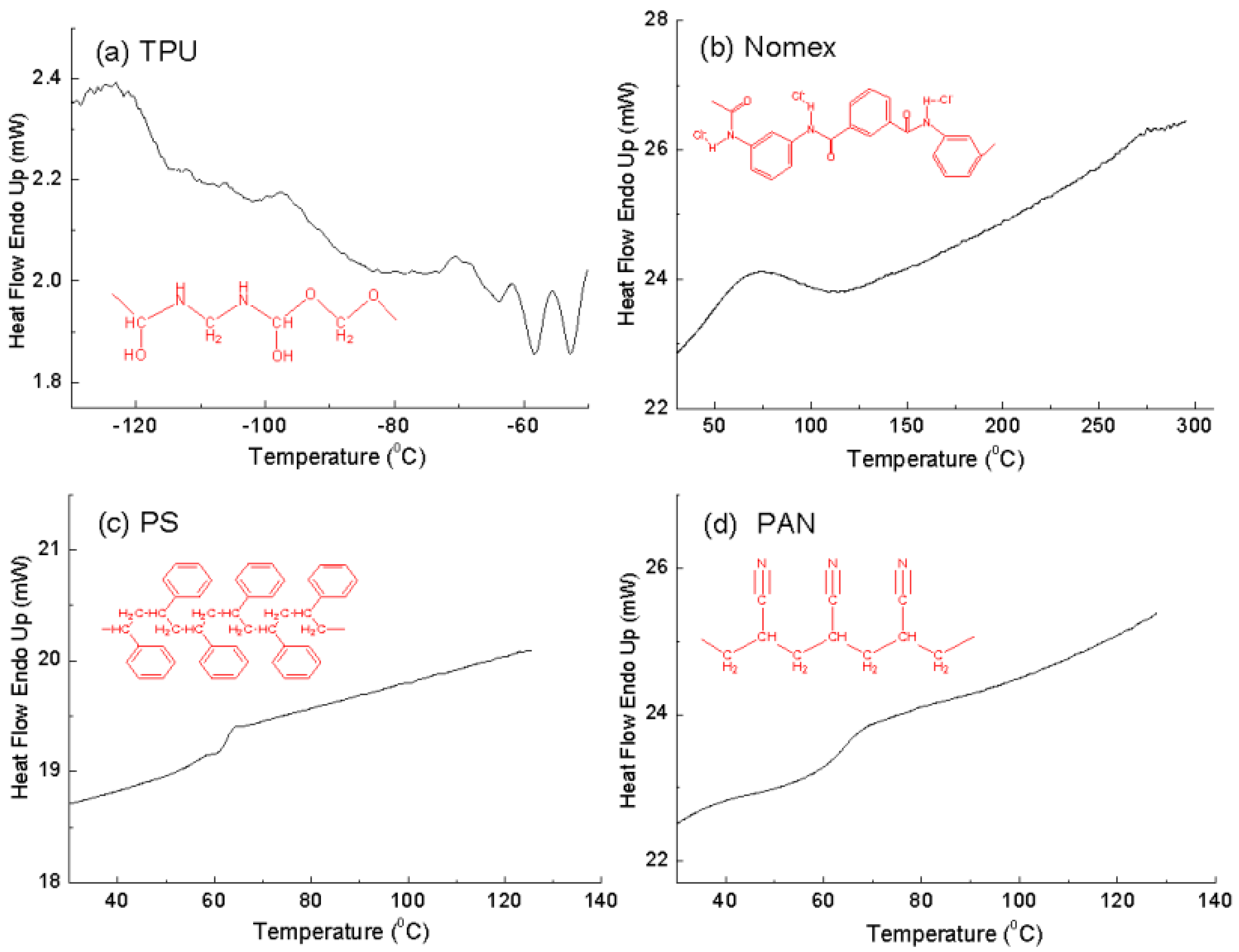
Figure 2 shows the DSC thermograms of the components used in the study, TPU, Nomex, PS and PAN. As is known, TPU has a
Tg of about −85 °C, indicating a quite flexible polymer chain (
Figure 2a). We turned our attention to the three stiff components shown in
Figure 2b–d. Nomex has a higher
Tg (about 110 °C) than PS and PAN (about 70 °C), indicating the greater chain rigidity of Nomex. Also shown in
Figure 2 are the molecular formulas of the polymers. It can be seen that the stiff chain backbone arising from the benzene ring groups contributed to the rigidity of Nomex, while the flexible backbone contributed to the flexibility of PAN. As to the chain of PS, although it contains stiff side groups (benzene ring groups), the flexible backbone determines the lesser rigidity of PS. The larger chain rigidity differential of the Nomex and TPU leads to a larger interfacial interaction between them, and consequently provides a greater potential for the Nomex/TPU component system to produce helical fibers in co-electrospinning, as shown in the experimental results.
3.2. Miscibility Behavior and Hydrogen Bonding in Blends
Co-electrospinning involves two polymer solutions which are blended in the outlet of the spinneret. When stretched by the electric field, the interfacial tension occurs between the two components. Immiscible blends may result in the failure of the fabrication of bicomponent fibers due to less interaction between the two components. At the opposite extreme of the immiscible case is the completely miscible case, where the interfacial interaction may vanish in a one-phase system of the completely miscible blend. Helical fibers can hardly be fabricated in these two cases. It is expected that the extent of miscibility in blends should be properly arranged to fabricate helical fibers effectively. To obtain polymer blends with good miscibility, it is usually necessary to ensure that hydrogen bonding exists between two base components. In this study, FTIR and DSC are used to analyze the miscibility behavior and hydrogen bonding in the blends of Nomex/TPU, PS/TPU and PAN/TPU systems. Blends with different component pairs were prepared by solution blending. Blend solutions were stirred for 6–8 h, and were allowed to evaporate slowly at 50 °C for one day. Films of the blends were then dried at 80 °C for two days to ensure the total elimination of solvents.
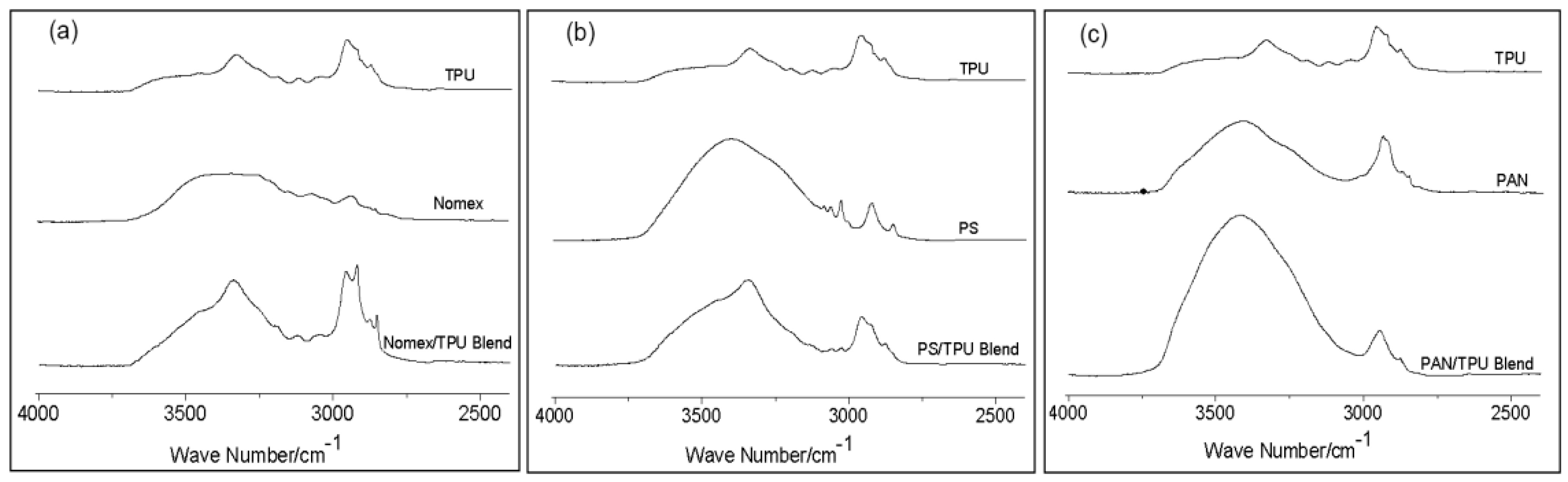
Figure 3 shows infrared spectra in the range of 2500–4000 cm
−1 of the three systems.
Figure 3a shows a broad band centered at 3306 cm
−1 for pure TPU and Nomex, corresponding to the hydrogen-bonded –NH. Although the broad band of the Nomex/TPU blend keeps at 3306 cm
−1, the intensity of the –NH group increases, indicated the formation of a new hydrogen bonding between the –NH in TPU and the oxygen in Nomex. As for the case of the PS/TPU system shown in
Figure 3b, a broad band, corresponding the –CH group, centered at 3400 cm
−1 for pure PS. In the PS/TPU blend, the position of this band shifts to a slightly lower wavenumber, which is closer to pure TPU. Meanwhile, the intensity of the band for the PS/TPU blend is in the range between the intensities for the two pure components, suggesting that there is no evidence of hydrogen bonding between PS and TPU. We turned our attention to the PAN/TPU system.
Figure 3c shows a band with significantly increased intensity centered at 3418 cm
−1 for the PAN/TPU blend. This phenomenon can be attributed to the hydrogen-bonding interactions between the –NH in TPU and the nitrogen in PAN. These data from
Figure 3 suggest that the extent of miscibility is different for the three component systems. Nomex is partially miscible with TPU due to the formation of hydrogen bonding between their polymer chains. PS and TPU are immiscible due to the absence of the specific intermolecular interaction. PAN is completely miscible with TPU and the blend becomes a one-phase system due to strong hydrogen bonding between their chains.
The miscibility of the polymer blends can be further analyzed by the DSC technique. If the two components are completely incompatible with each other, then they have two
Tgs that correspond to the two components. If the two components are partially compatible, then the blends have two
Tgs shifted towards each other when the compositions of blends are out of the range of compatibility. If the components in binary blends are fully compatible, then the blends display a single
Tg within the full range of compositions [
18].
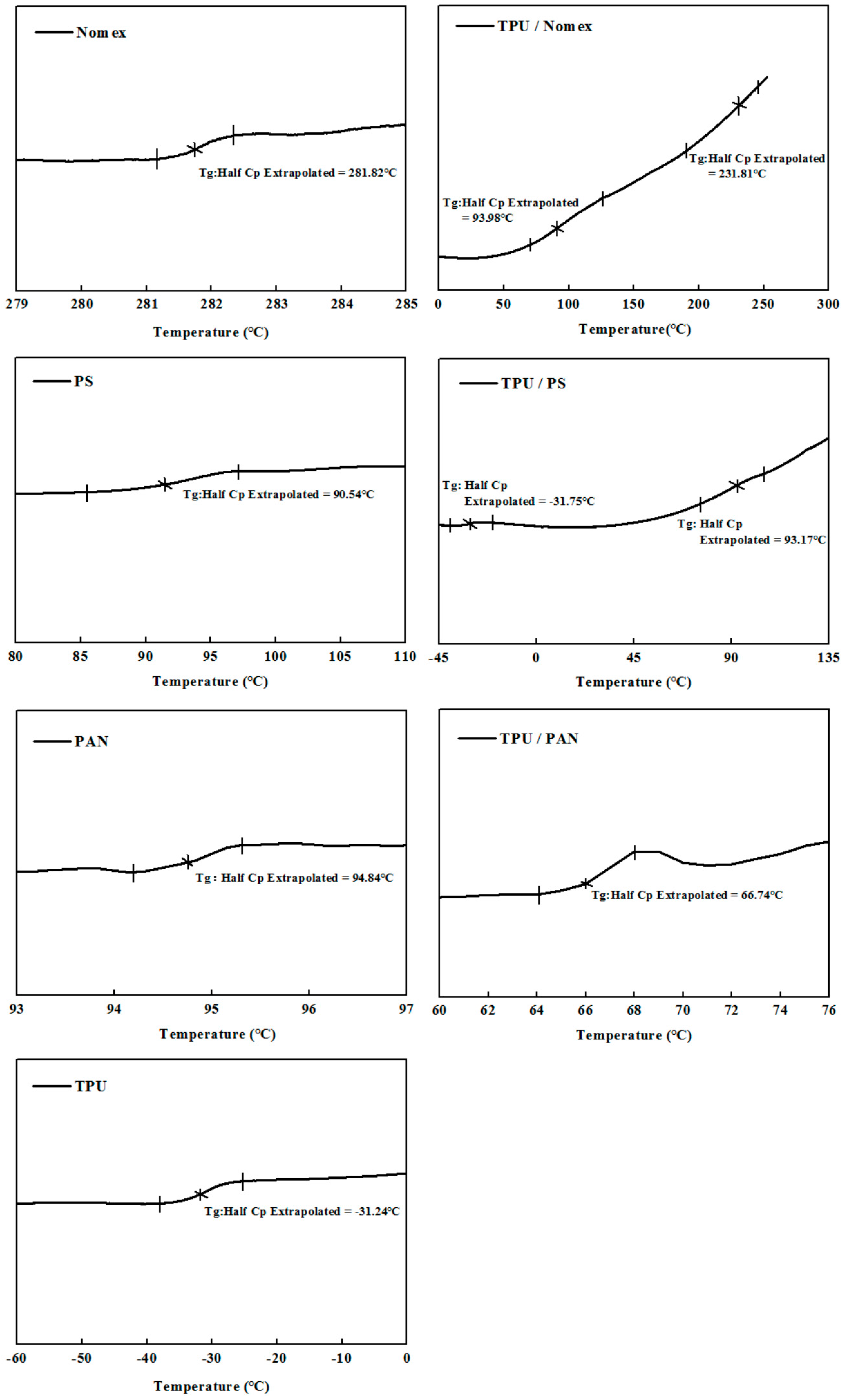
Figure 4 shows the DSC thermograms of the Nomex/TPU, PS/TPU and PAN/TPU systems. Here we need to emphasize that the components employed in this analysis were prepared by TPU, Nomex, PS and PAN solutions, rather than the chopped fibers or pellets used in the DSC analysis for polymer chain rigidity (
Figure 2).
Figure 4 illustrates that in the Nomex/TPU blend, there are two
Tgs (93.98 and 231.81 °C) located between the
Tgs of the two individual polymers (−31.24 °C for pure TPU and 281.82 °C for pure Nomex), which gives an indication of partial miscibility in the blend. Although there are two
Tgs shown in the PS/TPU blend, unlike the partial miscibility observed in
Figure 4a, the
Tgs (−31.75 and 93.17 °C) of the blend are out of the range of those of pure TPU and pure PS (−31.24 and 90.54 °C). This phenomenon indicates an immiscibility behavior of the PS/TPU blend. As can be seen in the PAN/TPU blend curve, a single
Tg (66.74 °C) strongly suggests that the PAN/TPU is fully miscible and exist as a one-phase system.
By analyzing the miscibility of the three systems, we believe that the partially miscible Nomex/TPU system tends to generate helical structures due to the intensified interfacial interaction attributed to hydrogen bonding. The PS/TPU system can hardly form helical fibers in coelectrsopinning because when there is no compatibility between the two blends, the interfacial interaction is too weak to support the helical interfacial stress, while if the two polymers are fully compatible, like the PAN/TPU system, the rigidity differential of the two components disappears, which also makes it difficult to meet the helical fiber formation conditions. This analysis coincides with the experimental results.
3.3. Coulumb Force between Molecules and Solution Surface
When preparing the polymer solutions for co-electrospinning, we found that PS, PAN and TPU can be dissolved in the pure solvents DMF, DMAc and DMF/THF, respectively. However, Nomex is difficult to dissolve in pure solvents due to its highly structured molecular arrangement. The dissolution of Nomex in DMAc/LiCl, the mechanism of which was introduced in our previous study [
15], leads to the combination of the negatively charged chloride ions with the polymer chain of Nomex. In this study, we used Zeta potential analyzer for the quantification of the magnitude of the charge contained within the polymer molecules.
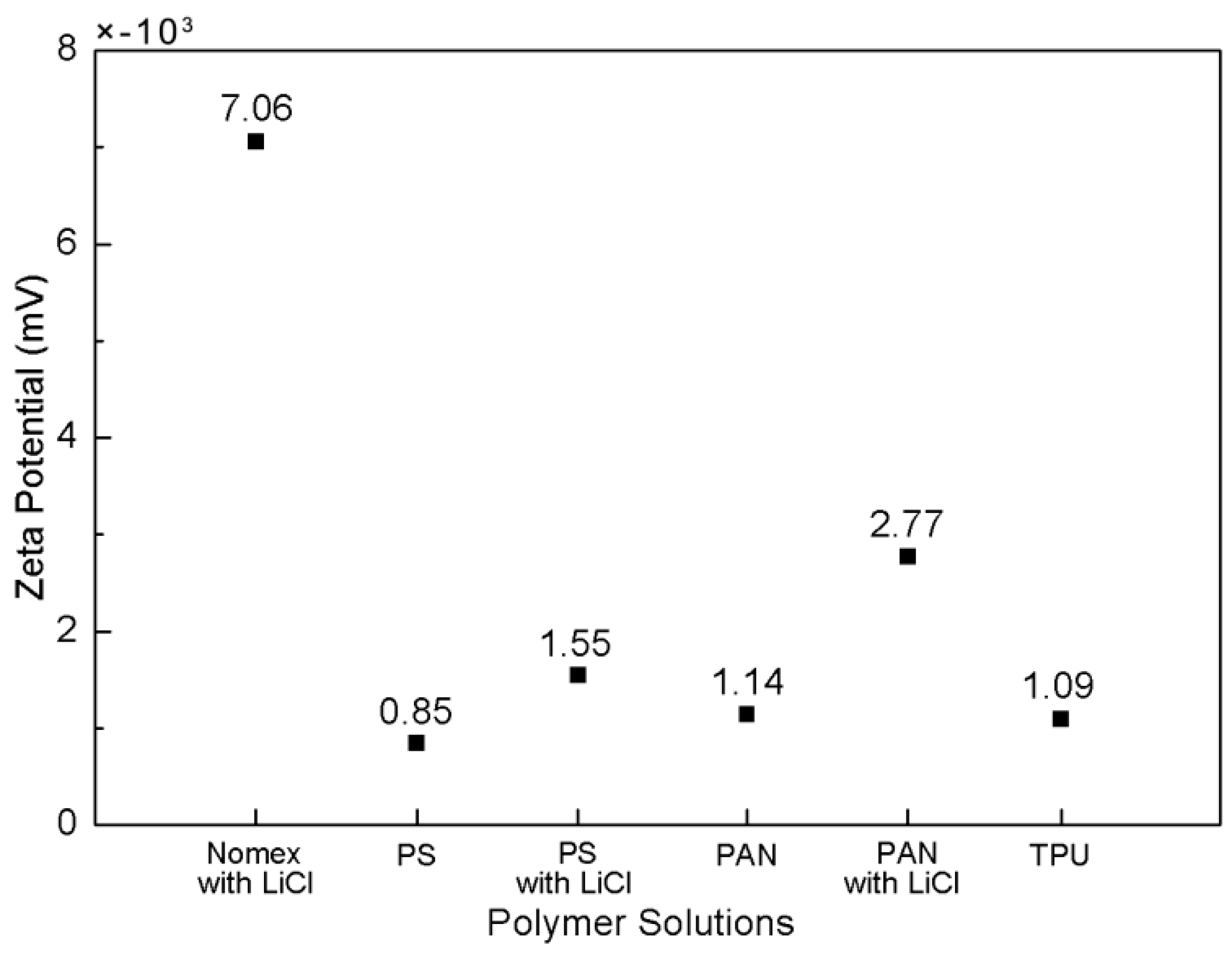
Figure 5 shows the obtained Zeta potentials (negative) for the Nomex/DMAc with LiCl, PS/DMF, PAN/DMAc, and TPU/(DMF/THF) solutions. For comparison, the Zeta potentials of the PS and PAN solutions with LiCl are also measured and shown in the figure. The amount of LiCl used in the three types of solutions are all 1.8 wt % in order to exclude the influence of the solution conductivity. It can be seen that the Zeta potential for the PAN, PS and TPU (without LiCl) are very low, indicating that few free charges are contained in the molecules. In contrast, the Zeta potential for Nomex is much higher. Although the Zeta potentials increase a bit for the PS and PAN solutions when adding LiCl, they are still much lower than that of the Nomex solution. The small magnitude of Zeta potential for the PS and PAN solutions with LiCl indicate that the chloride ions are dispersed in the solutions, rather than accumulated in the polymer chain. On the contrary, the great magnitude of Zeta potential for the Nomex solution indicates that the negative charges are contained in the molecules as expected.
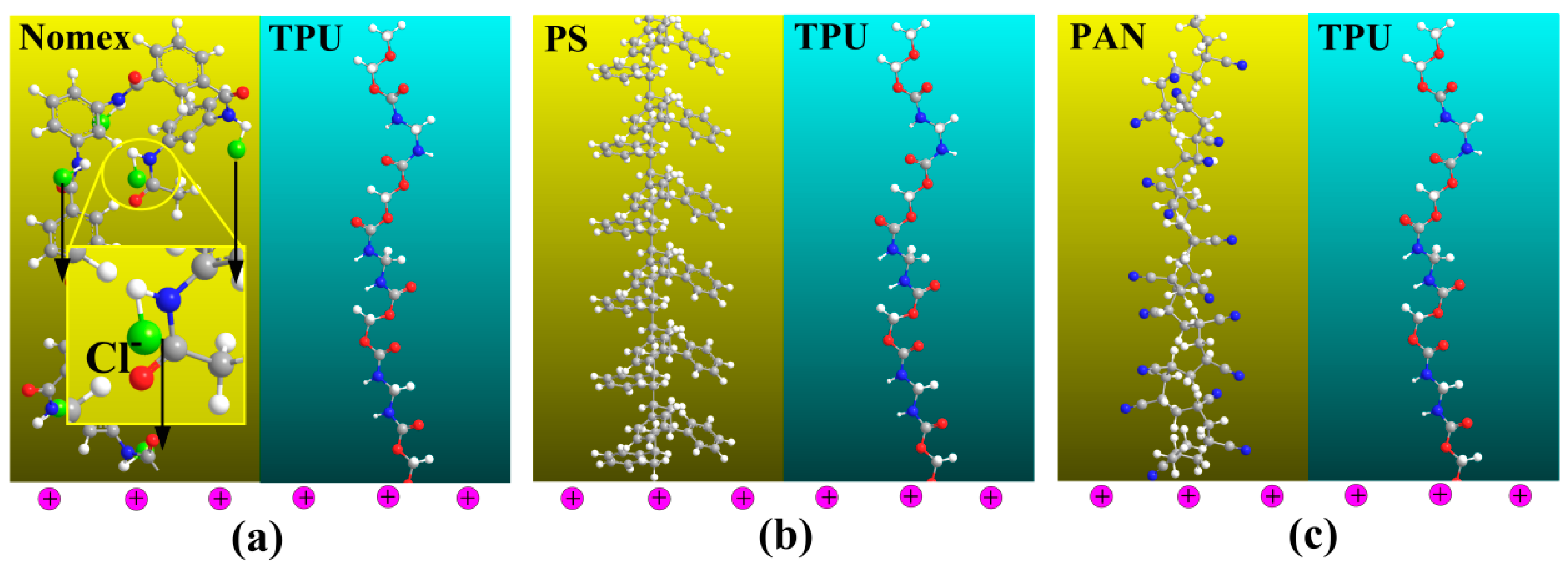
When the polymer solutions are charged by the applied high voltage, the free charges are accumulated on the surface of the solution. It can be predicted that in the Nomex solution, there will be a Coulomb force of attraction generated between the positive charges at the solution surface and the negative charges carried by chloride ions in the polymer chain. In contrast, due to the lack of free charges contained in the molecules, the situation is different for the other three components. In other words, the Coulomb force between the charges on the solution surface and within the molecules will not generate for the TPU, PS and PAN solutions. A schematic illustration of the composite solutions for the three component systems being charged is shown in
Figure 6 [
15]. It can be seen that the Coulomb force for the Nomex solution generates a longitudinal interfacial stress between the Nomex and TPU solutions, which helps to form helical structures (
Figure 6a).
Figure 6b,c illustrates that the interfacial interaction arising from the Coulomb force cannot be observed for the PS/TPU and PAN/TPU systems.
From the above analyses of the three aspects, it can be summed up that among the three component systems, the Nomex/TPU system has the most potential to produce helical nanofibers via co-electrospinning, which is consistent with the experiments.