1. Introduction
Polythene (PE) and polypropylene (PP), as representatives of aliphatic polyolefins, and poly(ethylene terephthalate) (PET), as a representative of aromatic polymers, are the three most common commercialized synthetic polymers, owing to their excellent overall performance and affordable price. The yield of the three polymers in China reached more than 14, 20, 40 million tons in 2016, respectively. PET can be categorized into three types with different intrinsic viscosities (IV) or molecular weights according to the application field: fiber grade, bottle grade and industrial yarn, whose IV are about 0.65 dL/g, 0.85 dL/g and 1.0 dL/g and whose corresponding aims are to be applied in the field of textiles, containers bottles and belts, respectively [
1]. Occasionally, these three polymers can be used simultaneously in one product, for example, in beverage bottles. It also can be seen that recycled PET from different sources is basically a mixture of PETs with different properties [
2].
The blending of polyolefins and PET can be an alternative to improve cost-effectiveness for industrial applications [
3]. However, the thermodynamically immiscible nature of blend roots with large differences in their polarities, which leads to insufficient interfacial adhesion between blend components, result in poor mechanical properties [
4,
5,
6]. Therefore, the polyolefin/PET compatibilization has already attracted a great deal of attention in the past decades.
Generally, the compatibilization of two immiscible polymers can be achieved by implementing a suitable grafting modification of polyolefin chains with reactive groups. The modification is achieved by chemical reactions between polar functional groups and polymer chains in melting blending [
7]. By enhancing the interactions between grafted functional groups of polyolefin and PET end groups, the phase dispersion and adhesion at the interface can be improved [
8]. For example, the functional groups like maleic anhydride (MAH) or glycidyl methacrylate (GMA) can be easily grafted onto polyolefins, and advantageously react with the carboxyl and hydroxyl end groups of PET. This method presents an excellent compatibilization effect with respect to chemical interactions between polyolefin and PET [
9,
10]. However, the conversion of functional groups grafted onto polyolefins is usually insufficient even though some residual active ingredients get involved in crosslinking reactions [
11]. More importantly, it is not difficult to imagine that this route goes against the large-scale application due to its high cost. From this perspective, the grafted functional groups of polyolefins can be used as compatibilizers, with a small amount added into the PET/polyolefin blend during extrusion processes, then the desirable compatibilization effect can be obtained. The compatibilizer is concentrated at the interface of two phases in the blending system, and the co-crystallization/molecular chains’ entanglement between the polymer component of the compatibilizer and polymer matrix would be generated to facilitate the interfacial adhesion between two immiscible polymers. Consequently, two immiscible blending components are effectively compatible by forming a dispersed structure in one unity [
12,
13,
14].
In the previous literature, GMA, MAH, acrylic acid (AA), ethylene-vinyl acetate copolymer (EVA), and maleimide (MI) are the common functional groups that can be either grafted onto polyolefins or copolymerized into compatibilizer. Recently, Jazani et al. [
15] improved the mechanical properties of bottle grade PET by grafting GMA onto PP with the aid of styrene (St) comonomer, and found that the increase of impact strength was attributed to the enhanced interaction between epoxy groups of GMA and carboxyl end groups of PET, as well as the hydrogen bonds formed between PP-
g-GMA and PET. In yet another approach, a compatibilizer composed of styrene–ethylene–butylene–styrene (SEBS) grafted with MAH was used as the compatibilizer in the blending of PP and PET spun fibers [
16]. Results showed that the compatibilizer significantly affected the elongation at break of the blend, while no relevant effect could be observed in the anisotropically-oriented fibers. Using PP-
g-AA as a compatibilizer, a good interaction between the PP and PET surfaces was achieved as PET islands was deformed in micro-fibers after hot stretching [
17].
In most published works, PP is the dominant continuous phase in the blend system of polyolefins/PET. There is little research focusing on PE/PET blends, let alone on the compatibilizer, by grafting the functional groups onto PE. Lei et al. [
18] studied the compatibility between PET and HDPE using PE-
g-MAH, SEBS-
g-MAH and E–GMA, respectively. It was found that the compatibilizing effectiveness of E–GMA was the best. Raffa et al. [
19] indicated the chemical reactions among polymer and additives had a significant effect on the ultimate melt rheology and mechanical properties of recycled PET/polyolefin blends.
In most cases, a variety of nanomaterials as a third component are added into two immiscible polymers to improve the compatibilizing effect [
20]. As a matter of fact, the functional groups grafted onto polyolefins have been commercialized and applied gradually, such as PP-
g-MAH and PE-
g-MAH, which offer the best convenience for designing polymer blend systems.
Based on the successful surface functionalization of polypropylene/polystyrene and their application in polymer blends, as described in our previous work [
7,
21,
22,
23,
24], the motivation has turned to developing possible industrialization polymer blends by exploring the relationships between the structure and properties in the processable polymers. Consequently, this work concentrates on the comparative investigation of the blending of PP and LDPE with different IV of PET using commercialized polyolefin-
g-MAH as a compatibilizer. A systematic performance investigation of the polymer blends with or without compatibilizer was performed to elucidate the processable correlation between several major polymer commodities.
2. Materials and Methods
2.1. Materials
Three poly(ethylene terephthalate) (PET) chips with different intrinsic viscosities (IV) of 0.7 dL/g, 0.85 dL/g and 1.0 dL/g, were purchased from Zhejiang Guxiandao Polyester Dope Dyed Yarn Co., Ltd. (Shaoxing, China). The moisture and carboxyl end group content of the PET chips are also listed as
Table 1. LDPE (F401) and PP (DNDB7441) were purchased from Sinopec Yangzi Petrochemical Co., Ltd. (Nanjing, China), LDPE-
g-MAH and PP-
g-MAH were purchased from Fine-blend Compatibilizer Jiangsu Co., Ltd. (Nantong, China).
In order to decrease the hydrolytic decomposition, the PET chips were dried at 130 °C for 9 h in a vacuum oven before being blended into either LDPE or PP. The polymer blends were processed in a RM-200C torque rheometer (Hapro, Haerbin, China). The processing temperatures were set to 260 °C for all blends in the rheometer, and the processing time was set to 5~10 min and the rotor speed was set to 50 r/min. The total weight of polymer blend was no more than 50 g. The weight ratio of the components in the blending are listed in
Table 2. The compatibilizer usually accounts for five percent of the total weight of the two polymers (5 wt %). The blank control experiments were also carried out in the absence of compatibilizer.
2.2. Measurements
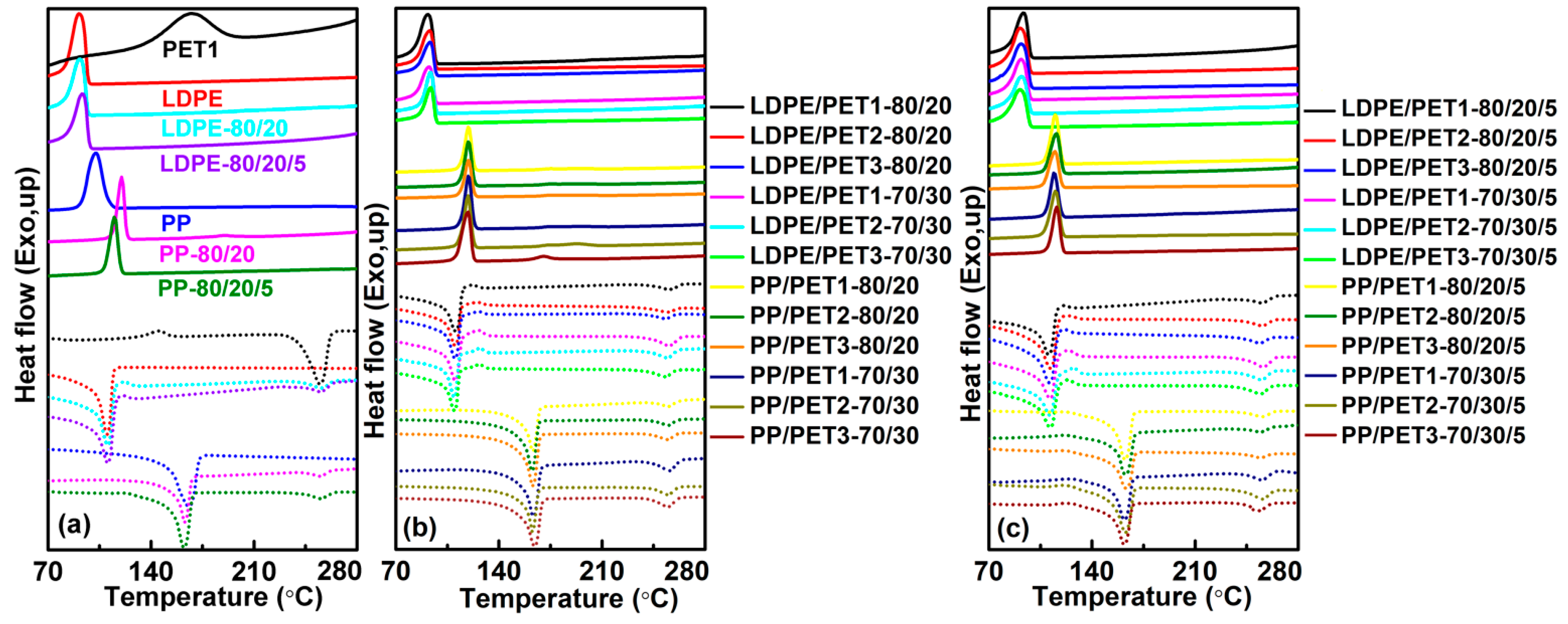
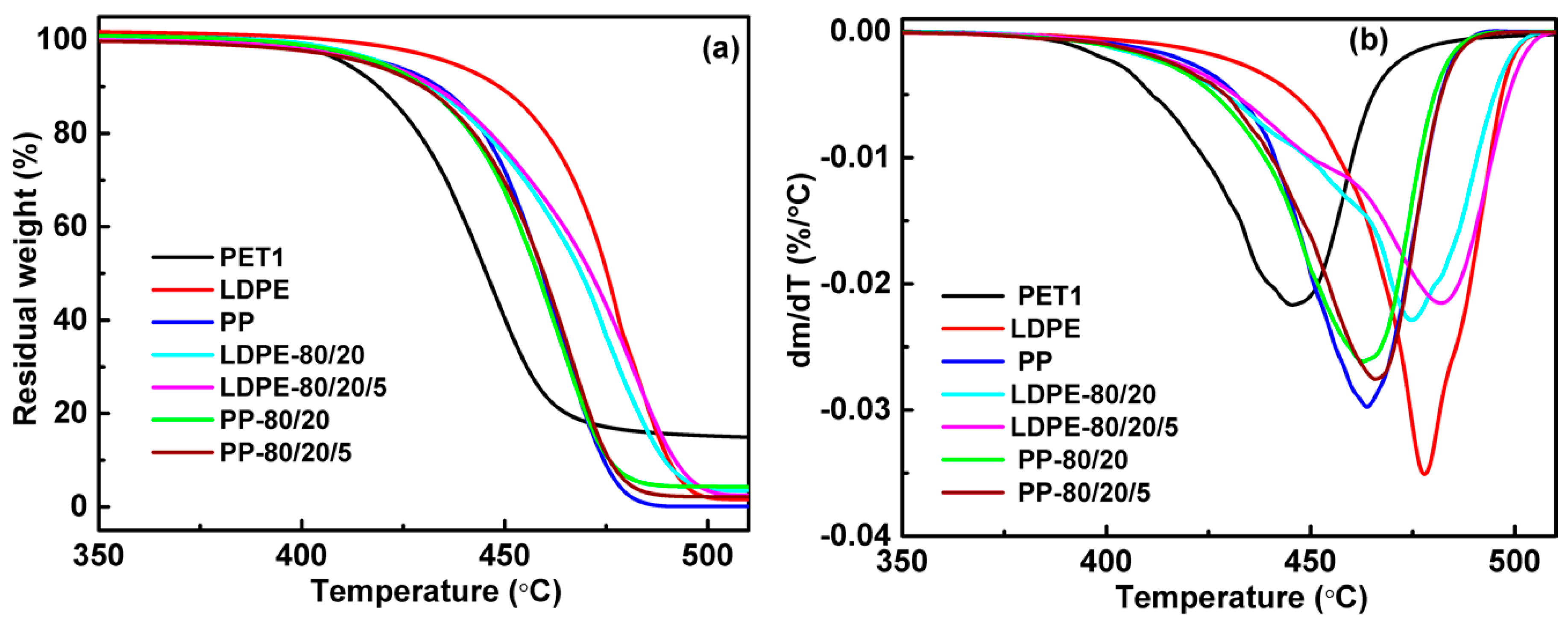
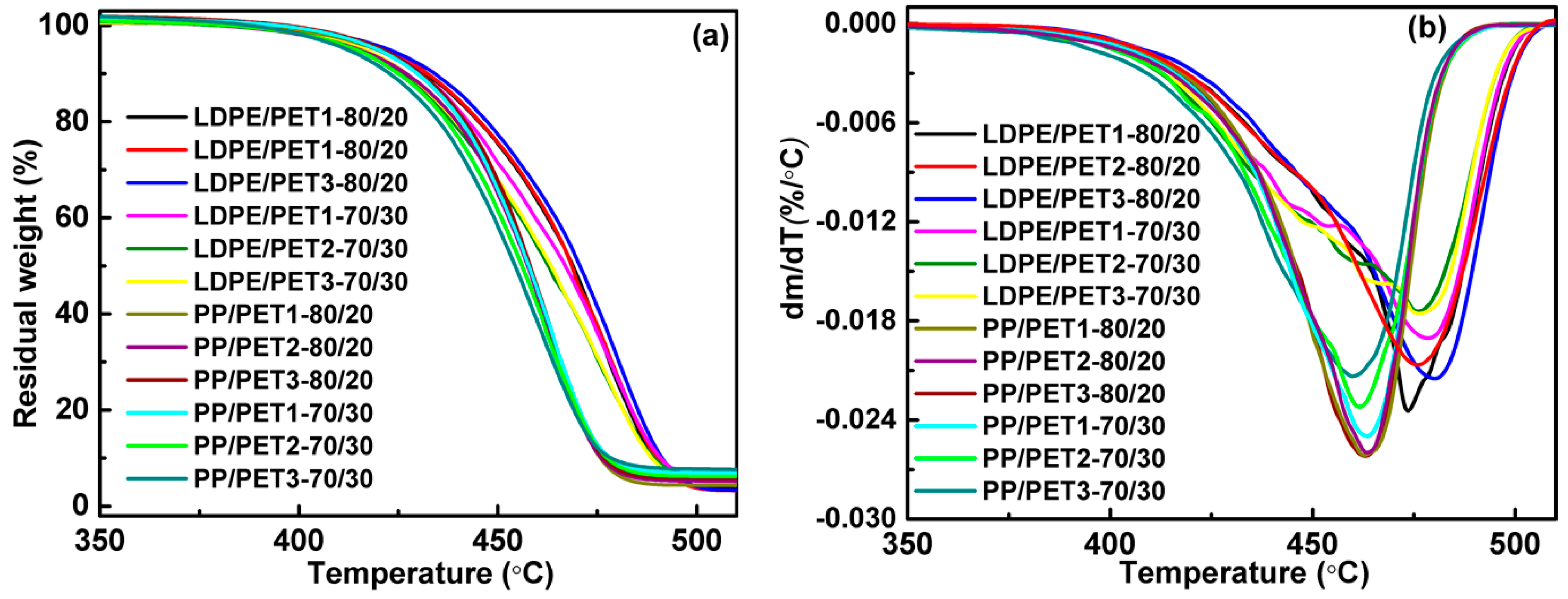
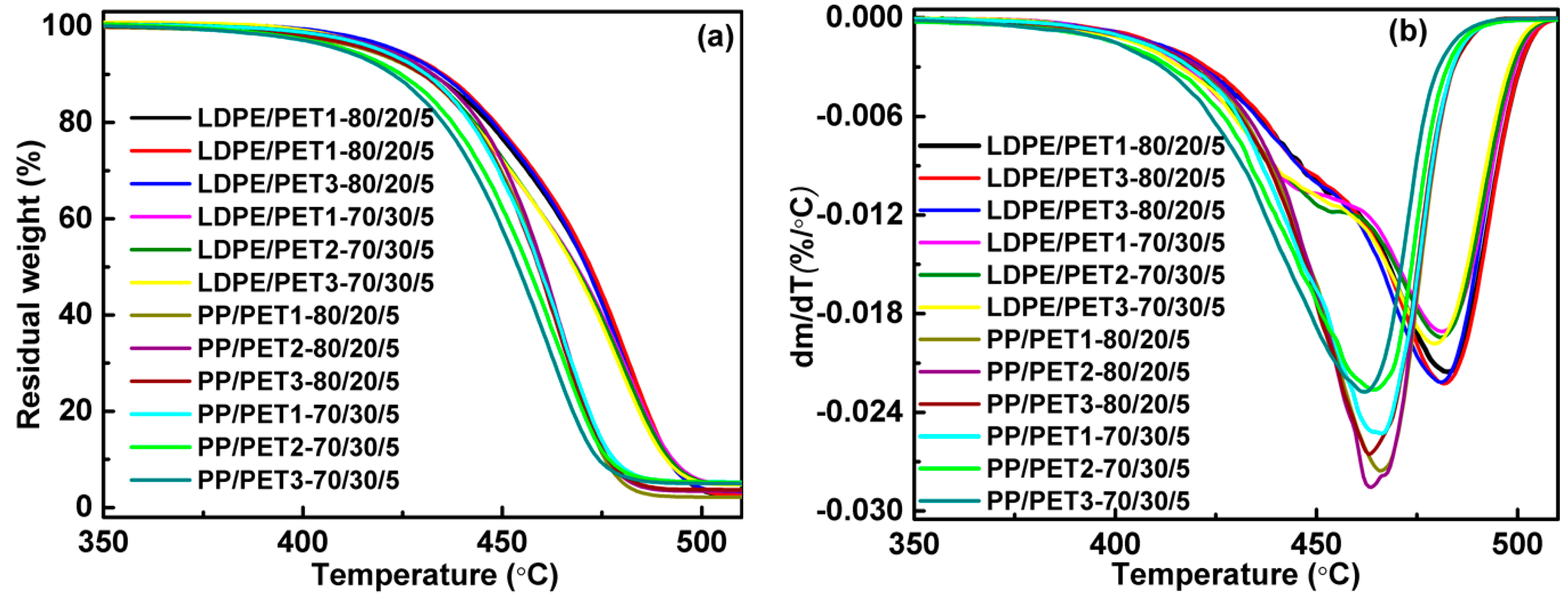
The thermal behavior of the blends was investigated using differential scanning calorimeter (DSC1, Mettler Toledo, Schwerzenbach, Switzerland) and thermogravimetric analyzer (TGA, Mettler Toledo, Schwerzenbach, Switzerland) under nitrogen atmosphere. The drying samples were initially heated from 25 to 300 °C at a rate of 50 °C/min. After that, the samples were kept at the final temperature for 3 min to eliminate the thermal history, and then cooled to 50 °C at a rate of 20 °C/min to determine the crystallization temperature (Tc) and before being kept for 3 min. Finally, the samples were heated to 300 °C at a rate of 10 °C/min to determine the melt point (Tm). About 5 mg of the samples was heated from 25 to 600 °C at a rate of 10 °C/min with a nitrogen flow rate of 45 mL/min to determine the decomposition temperature (thermal gravimetric rate 5%, T5%).
The rheological properties of the polymer blends as functions of angular frequency were investigated via plate-plate geometry (plate radius = 25 mm; gap = 1 mm) using a stress-controlled rheometer (MCR 301, Anton Paar, Graz, Austria) at 260 °C. The measurements of the granules and sheet samples were performed in air atmospheres. The loaded frequency sweep was undirectional from the angular frequency of ω = 500 1/s to 0.1 1/s.
The banded samples of polymers were prepared from a blending system so that the morphological characteristics of the polymer blends could be examined using an ULTRATM 55 field-emission scanning electron microscopy (SEM Ultra 55, Zeiss, Jena, Germany) at an acceleration voltage of 1 kV. The samples were cooled in liquid nitrogen for 15 min and then taken out quickly to be brittle fractured. The fracture surface of PET was etched by using the mixed solution of phenol and tetrachloroethane (1/1 (wt %)), then the fracture surface was gilded because of the poor electrical conductivity.
To test the mechanical property of the polymer blends, the round rod samples with diameter of 1.5 mm were stretched on a universal testing machine (Instron 3367, Instron, Canton, USA) at speed of 10 mm/min. Generally, the effective stretch length was about 2 mm, and then the tensile strength and elongation at break could be achieved.
4. Conclusions
The blending of LDPE and PP with different intrinsic viscosities (IV) of PET was performed in the absence or presence of compatibilizer. The impact of the blending components on the thermal, rheological, morphological and mechanical properties was discussed in detail, and the following conclusions were drawn from the discussion:
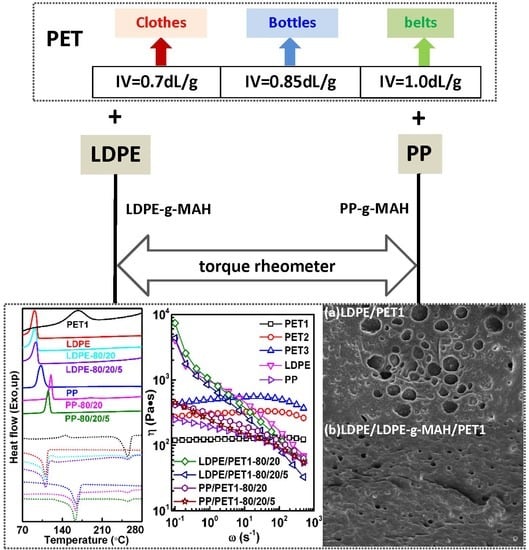
(1) The blending leads to a higher crystallization temperature of the polyolefin component, yet the melting peak both of the polyolefin component and of the PET component in the blends are little different from their pure polymer. While the thermal stability of polymer blends is generally better than PET but worse than LDPE, and blending of PP with PET had no significant effect on the thermal stability of PP. The thermal performance of polyolefin/PET was slightly decreased by either the increase of the IV of PET or the decrease of PET content. It was found that the compatibilizer can improve the thermal stability of the blending system by enhancing the interface adhesion.
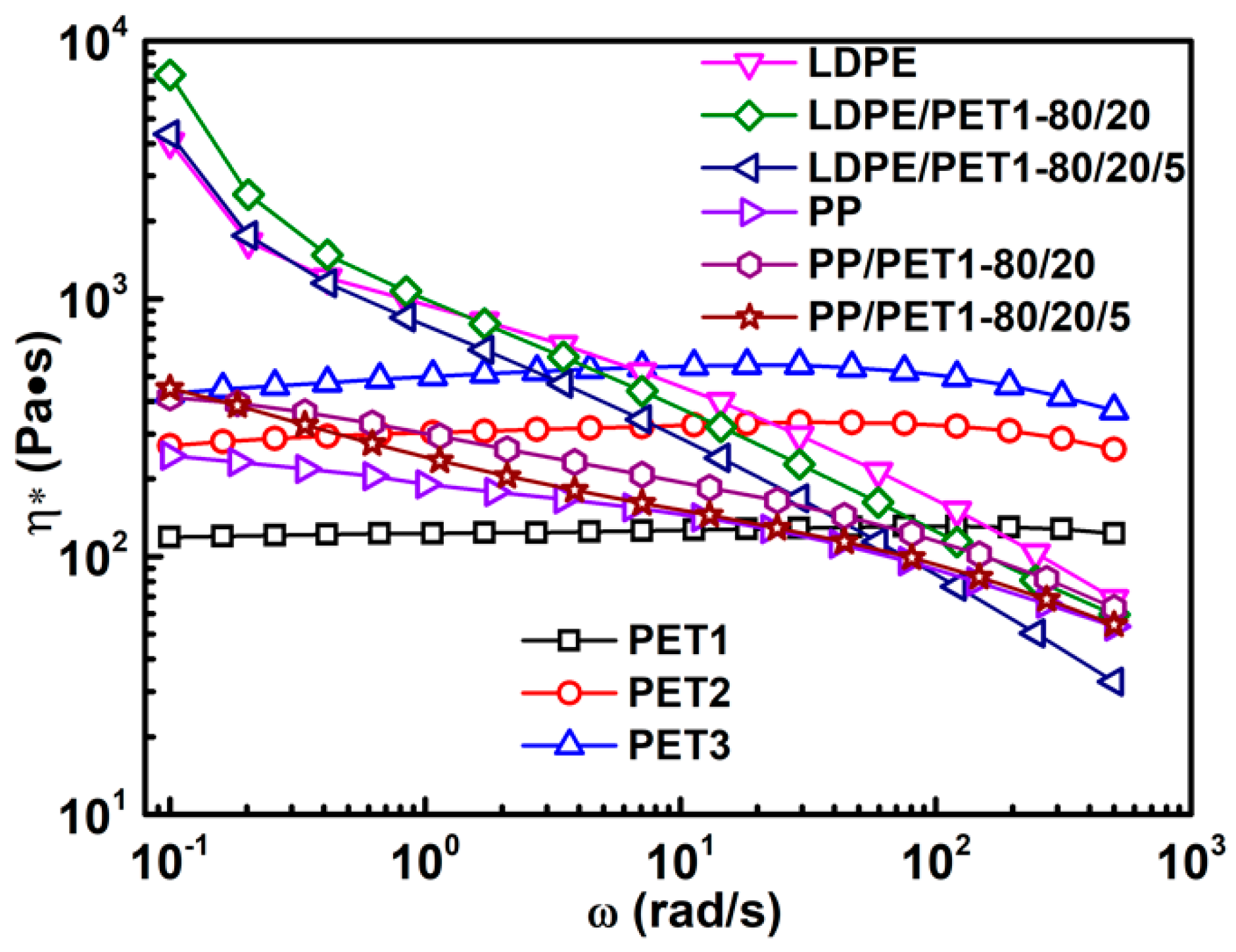
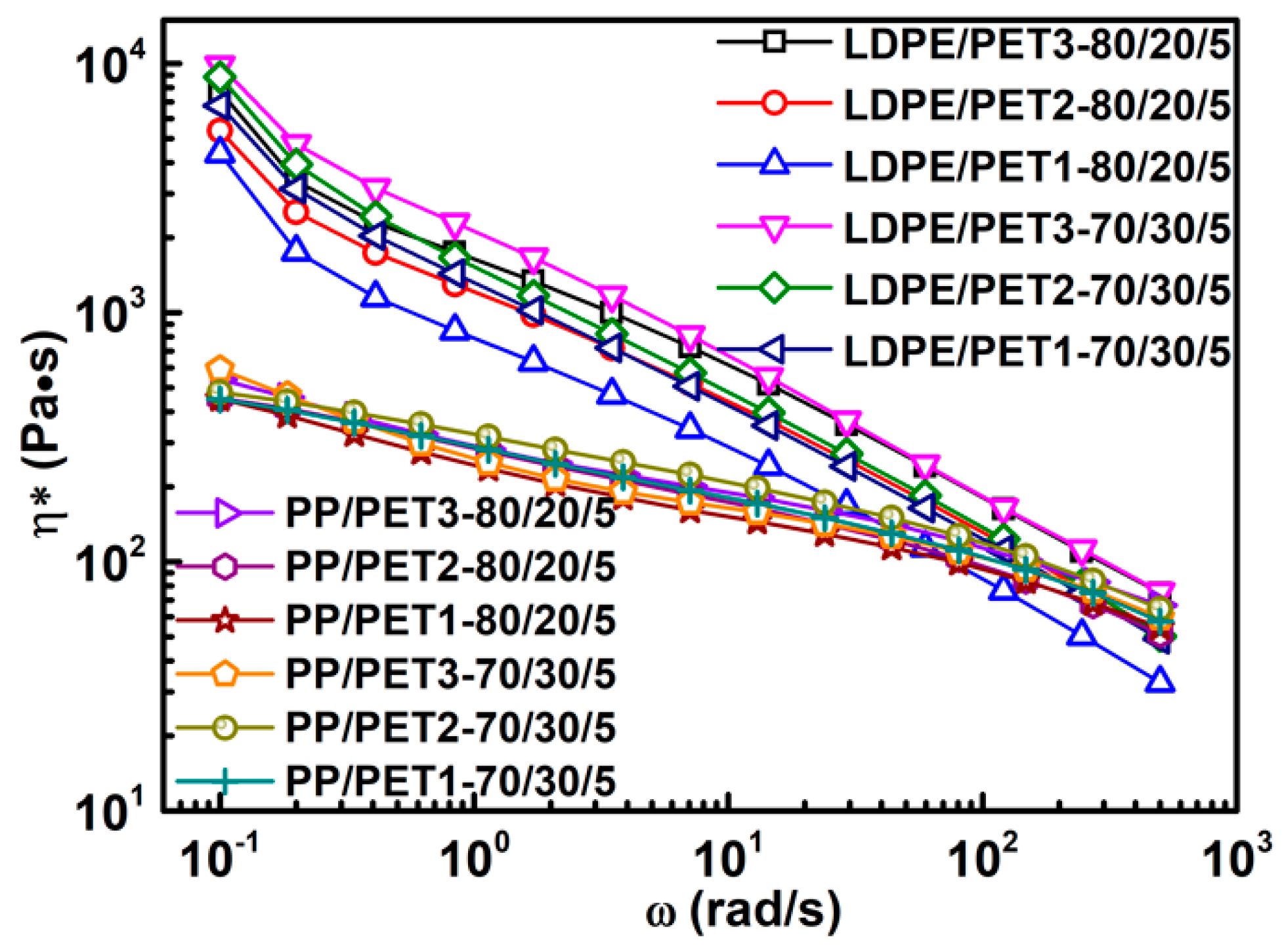
(2) The complex viscosity of incompatible polymer blends is slightly higher than that of compatibilized blends. The results show that it is increased with the increase of the IV of PET, yet decreased with the increase of PET content increasing in blends. The complex viscosity of the blend with compatibilizer can be similarly increased by either increasing the IV of PET or increasing the PET content in blend.
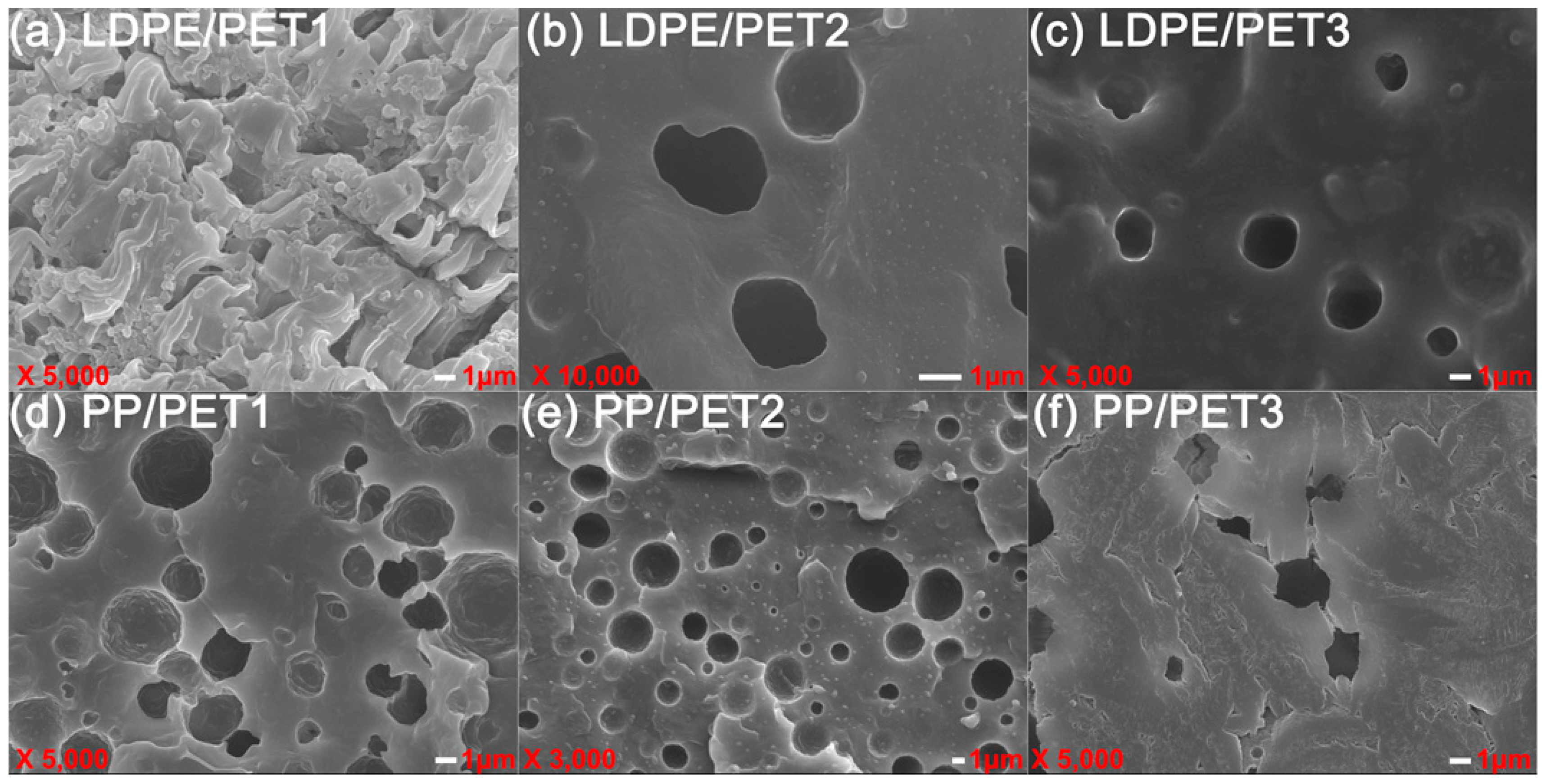
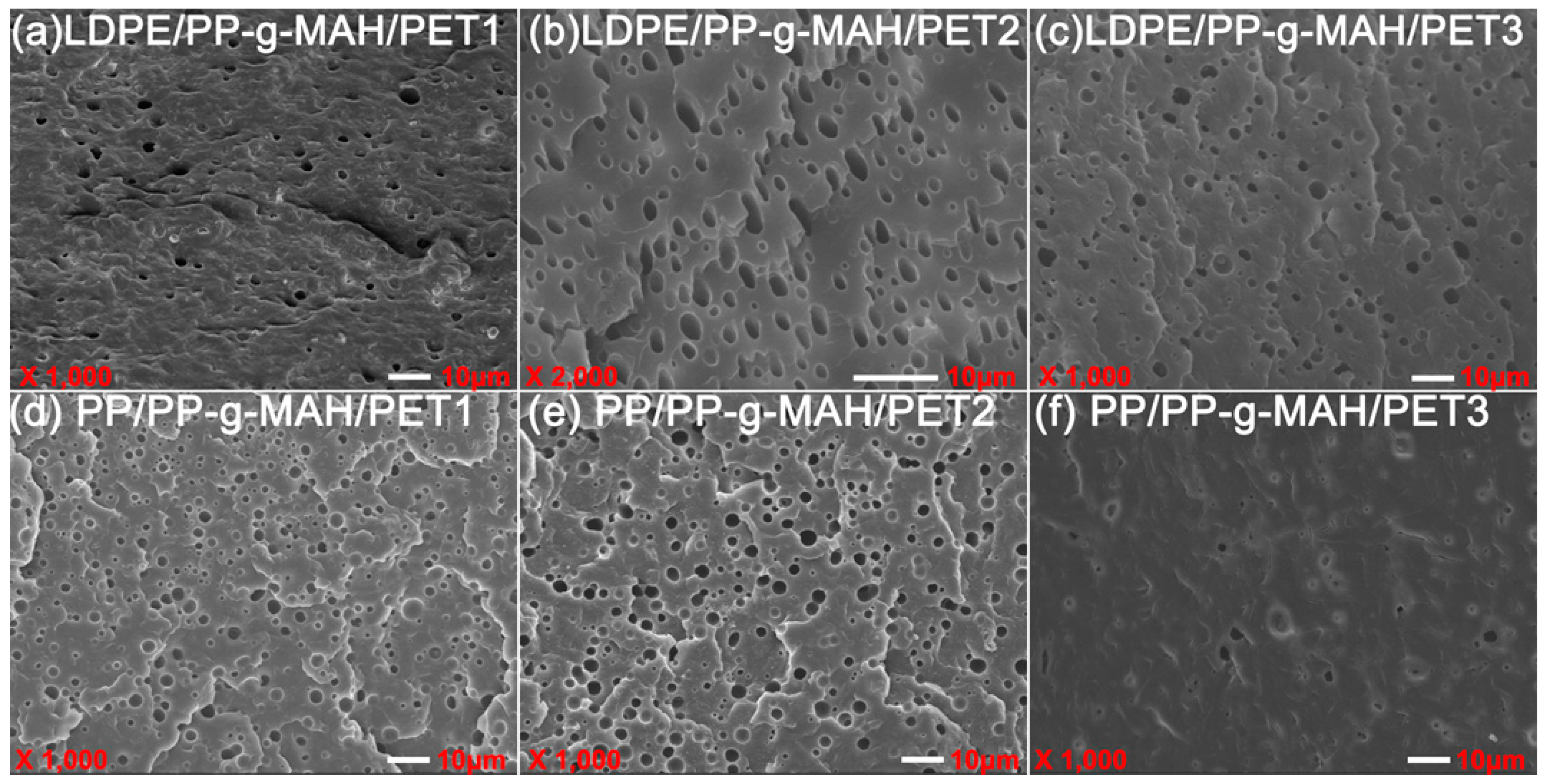
(3) PET disperses nonuniformly in LDPE in the form of granules; the cross-section of the incompatible blend shows brittle fracture. The number of holes in the blend increases with the increase of PET content. In contrast, the cross-section tends to be indistinct in the presence of LDPE-g-MAH as a compatibilizer. As the IV of the PET increases in the polymer blends, PET can be easily gathered to enlarge the scale of the dispersed phase, resulting in worse nonuniform dispersity, while it can be improved by adding compatibilizer.
(4) It was demonstrated that with the increase of the IV of PET, the elongation at break of the polymer blend decreases slightly and the tensile strength of the LDPE/PET blend also decreased accordingly, while the tensile strength of the PP/PET blend gradually increased. It is worth noting that the higher content of PET in the blend results in a smaller elongation at break and lower tensile strength. When the compatibilizer was added to the blends, an increase of elongation at break and a decrease of tensile strength could be seen simultaneously, and the difference of tensile strength caused by either changing the IV of PET or changing the PET content declined.